The helminth parasite fauna of Profundulus punctatus in 7 localities from Oaxaca State, Mexico, was studied. A total of 132 fish were analyzed. Fourteen helminth taxa were recorded, including 9 digeneans (Paracreptotrema blancoi, Paracreptotrema profundulusi, Phyllodistomum sp., Culuwiya cf. cichlidorum, Clinostomum sp., Diplostomidae gen. sp., Posthodiplostomum minimum, Ascocotyle (Ascocotyle) felippei and Centrocestusformosanus), 2 monogeneans (Gyrodactylus sp. and Salsuginus sp.), and 3 nematodes (Spinitectus humbertoi, Rhabdochona salgadoi, and Eustrongylides sp.). Helminth parasite communities showed low values of richness (14 species) and diversity (Shannon-Wiener and Brillouin indices ranged between 0.359 to 2.083 and 0.358 to 2.042 bits/ind.) in the 7 localities, and some were dominated by 3 helminth species (Ascocotyle (Ascocotyle) felippei, Centrocestus formosanus, and Rhabdochona salgadoi). With the current data, we concluded that P. punctatus is parasitized by a particular group of helminth species (e. g., Paracreptotrema blancoi, Paracreptotrema profundulusi, Spinitectus humbertoi, and Rhabdochona salgadoi) exclusive to this fish family that are not shared with other Central American fish species.
Se estudió la helmintofauna de Profundulus punctatus de 7 localidades del estado de Oaxaca, México. Se examinó un total de 132 peces. El registro helmintológico de este pez consta de 14 taxa de helmintos, los cuales incluyen 9 taxa de digéneos (Paracreptotrema blancoi, Paracreptotrema profundulusi, Phyllodistomum sp., Culuwiya cf. cichlidorum, Clinostomum sp., Diplostomidae gen. sp., Posthodiplostomum minimum, Ascocotyle (Ascocotyle) felippei y Centrocestus formosanus), 2 taxa de monogéneos (Gyrodactylus sp. y Salsuginus sp.) y 3 taxa de nemátodos (Spinitectus humbertoi, Rhabdochona salgadoi y Eustrongylides sp.). Las comunidades de helmintos mostraron bajos valores de riqueza (14 especies) y diversidad (índices de Shannon-Wiener y Brillouin mostraron valores entre 0.359 a 2.083 y 0.358 a 2.042 bits/ind.) en las 7 localidades y algunas de estas fueron dominadas por 3 especies de helmintos (esto es, Ascocotyle (Ascocotyle) felippei, Centrocestus formosanus y Rhabdochona salgadoi). Nuestros datos sugieren que Profundulus punctatus es parasitado por un grupo particular de helmintos (esto es, Paracreptotrema blancoi, Paracreptotrema profundulusi, Spinitectus humbertoi y Rhabdochona salgadoi) especies exclusivas de esta familia de peces y al parecer no se encuentran en otras especies de peces de Centroamérica.
Members of Profundulidae Hoedeman and Bronner are distributed along the Atlantic and Pacific Ocean slopes of southern Mexico, Guatemala, and Honduras (Miller, 1955, 2005; Doadrio et al., 1999; Matamoros and Schaeffer, 2010). This group shows low levels of richness with 7 known species classified in a single genus (Profundulus Hubbs) and all their members represent an endemic lineage, that probably inhabited this region since the Pliocene and perhaps even the Miocene (Miller, 1955; Doadrio et al., 1999; González-Diaz et al., 2005; Nelson, 2006; Frose and Pauly, 2010; Matamoros and Schaeffer, 2010).
In Mexico, the freshwater fish species of Profundulus (Teleostei: Profundulidae) inhabit the hydrological basins of the southern part of the country; the previous helminthological record for these fishes comprises the adult nematodes Spinitectus humbertoiMandujano-Caspeta and Moravec, 2000; Rhabdochona salgadoiCaspeta-Mandujano and Moravec, 2000; and Spinitectus mariaisabelaeCaspeta-Mandujano, Cabañas-Carranza and Salgado-Maldonado, 2007, all found in the intestine of Profundulus labialis and P. punctatus (Caspeta-Mandujano and Moravec, 2000; Caspeta-Mandujano et al., 2007); the metacercariae of Clinostomum sp., the larvae of the nematodes Contracaecum sp. and Eustrongylides sp., and the adult cestode Bothriocephalus acheilognathi Yamaguti, 1934 recorded from P. punctatus, P. labialis, and the endangered fish P. hildebrandi (Salgado-Maldonado et al., 2011a; Velázquez-Velázquez et al., 2011). In addition, a new species of digenean, Paracreptotrema profundulusi Salgado-Maldonado, Caspeta-Manduajano and Martínez-Ramírez, 2011, was described from P. punctatus and P. balsanus from Oaxaca, Mexico (Salgado-Maldonado et al., 2011b). However, the helminth fauna of Profundulus is far from complete due to their wide distribution range in Mexico; therefore, in the current research a total of 132 fish belonging Profundulus punctatus were collected in 7 localities from Oaxaca State, Mexico with the aims of (1) presenting the helminthological record in this area of distribution, in terms of taxonomic composition and (2) describing for the first time the richness and diversity of helminth communities in P. punctatus by examining the similarity among the structures in fishes from all these localities.
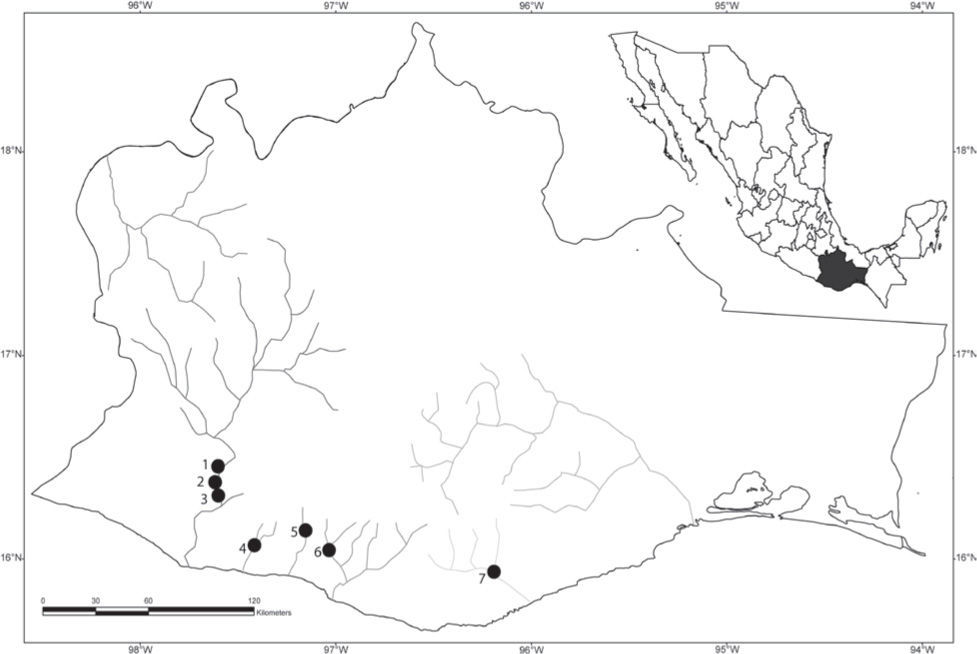
Materials and methodsLocalities and sampling. The nomenclature for host species follows Martínez-Ramírez et al. (2004) and Doadrio et al. (1999). A total of 132 fish specimens from 7 localities in Oaxaca were examined for the presence of helminth parasites between March 2007 and March 2010 (Table 1, Fig. 1): 1) La Soledad Carrizo River, Santiago Tetepec (16°25'00.4” N, 97°40'12.9” W, altitude 733m) (n=25); 2) San José de las Flores River, Santiago Jamiltepec (16°24'21.5” N, 97°44'22.6” W, altitude 619m) (n=20); 3) Santa Cruz Flores Magón River, Santiago Jamiltepec (16°21'06.1” N, 97°45'38.3” W, altitude 275m) (n=18); 4) Pichuaca River, Santiago Jocotepec (16°05'34.2” N, 97°24'18.1” W, altitude 139m) (n=22); 5) La Reforma River, San Juan Lachao, Manialtepec (16°8'33.5” N, 97°8'41.6” W, altitude 517m) (n=20); 6) Pueblo Viejo River, San Gabriel Mixtepec (16°06'22.3” N, 97°03'47.8” W, altitude 522m) (n=20), and 7) Santa María Huatulco River, Pochutla (15°50'14.2” N, 96°19'30.8” W, altitude 199m) (n=7).
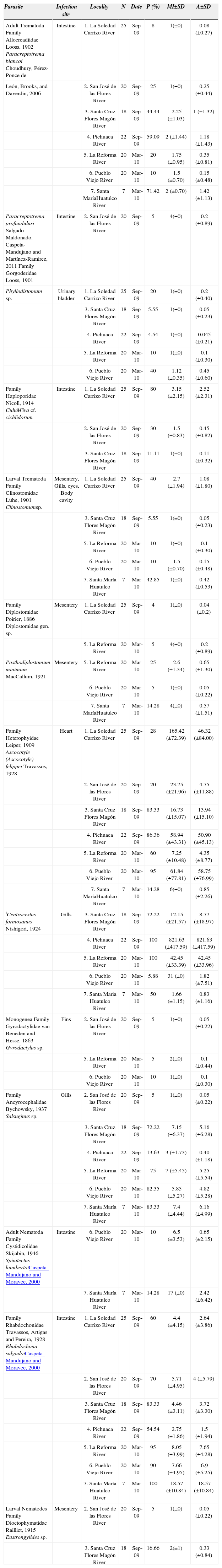
Helminth parasites collected in Profundulus punctatus from 7 localities from Oaxaca, Mexico. Parasite, number of hosts examined (N), prevalence (P), mean intensity (MI), and abundance (A), i= introduced
| Parasite | Infection site | Locality | N | Date | P (%) | MI±SD | A±SD |
|---|---|---|---|---|---|---|---|
| Adult Trematoda Family Allocreadiidae Looss, 1902 Paracreptotrema blancoi Choudhury, Pérez-Ponce de | Intestine | 1. La Soledad Carrizo River | 25 | Sep-09 | 8 | 1(±0) | 0.08 (±0.27) |
| León, Brooks, and Daverdin, 2006 | 2. San José de las Flores River | 20 | Sep-09 | 25 | 1(±0) | 0.25 (±0.44) | |
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 44.44 | 2.25 (±1.03) | 1 (±1.32) | ||
| 4. Pichuaca River | 22 | Sep-09 | 59.09 | 2 (±1.44) | 1.18 (±1.43) | ||
| 5. La Reforma River | 20 | Mar-10 | 20 | 1.75 (±0.95) | 0.35 (±0.81) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 10 | 1.5 (±0.70) | 0.15 (±0.48) | ||
| 7. Santa MaríaHuatulco River | 7 | Mar-10 | 71.42 | 2 (±0.70) | 1.42 (±1.13) | ||
| Paracreptotrema profundulusi Salgado-Maldonado, Caspeta-Mandujano and Martínez-Ramirez, 2011 Family Gorgoderidae Looss, 1901 | Intestine | 2. San José de las Flores River | 20 | Sep-09 | 5 | 4(±0) | 0.2 (±0.89) |
| Phyllodistomum sp. | Urinary bladder | 1. La Soledad Carrizo River | 25 | Sep-09 | 20 | 1(±0) | 0.2 (±0.40) |
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 5.55 | 1(±0) | 0.05 (±0.23) | ||
| 4. Pichuaca River | 22 | Sep-09 | 4.54 | 1(±0) | 0.045 (±0.21) | ||
| 5. La Reforma River | 20 | Mar-10 | 10 | 1(±0) | 0.1 (±0.30) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 40 | 1.12 (±0.35) | 0.45 (±0.60) | ||
| Family Haploporidae Nicoll, 1914 CuluM'iva cf. cichlidorum | Intestine | 1. La Soledad Carrizo River | 25 | Sep-09 | 80 | 3.15 (±2.15) | 2.52 (±2.31) |
| 2. San José de las Flores River | 20 | Sep-09 | 30 | 1.5 (±0.83) | 0.45 (±0.82) | ||
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 11.11 | 1(±0) | 0.11 (±0.32) | ||
| Larval Trematoda Family Clinostomidae Lühe, 1901 Clinostomumsp. | Mesentery, Gills, eyes, Body cavity | 1. La Soledad Carrizo River | 25 | Sep-09 | 40 | 2.7 (±1.94) | 1.08 (±1.80) |
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 5.55 | 1(±0) | 0.05 (±0.23) | ||
| 5. La Reforma River | 20 | Mar-10 | 10 | 1(±0) | 0.1 (±0.30) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 10 | 1.5 (±0.70) | 0.15 (±0.48) | ||
| 7. Santa María Huatulco River | 7 | Mar-10 | 42.85 | 1(±0) | 0.42 (±0.53) | ||
| Family Diplostomidae Poirier, 1886 Diplostomidae gen. sp. | Mesentery | 1. La Soledad Carrizo River | 25 | Sep-09 | 4 | 1(±0) | 0.04 (±0.2) |
| 5. La Reforma River | 20 | Mar-10 | 5 | 4(±0) | 0.2 (±0.89) | ||
| Posthodiplostomum minimum MacCallum, 1921 | Mesentery | 5. La Reforma River | 20 | Mar-10 | 25 | 2.6 (±1.34) | 0.65 (±1.30) |
| 6. Pueblo Viejo River | 20 | Mar-10 | 5 | 1(±0) | 0.05 (±0.22) | ||
| 7. Santa MaríaHuatulco River | 7 | Mar-10 | 14.28 | 4(±0) | 0.57 (±1.51) | ||
| Family Heterophyidae Leiper, 1909 Ascocotyle (Ascocotyle) felippei Travassos, 1928 | Heart | 1. La Soledad Carrizo River | 25 | Sep-09 | 28 | 165.42 (±72.39) | 46.32 (±84.00) |
| 2. San José de las Flores River | 20 | Sep-09 | 20 | 23.75 (±21.96) | 4.75 (±11.88) | ||
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 83.33 | 16.73 (±15.07) | 13.94 (±15.10) | ||
| 4. Pichuaca River | 22 | Sep-09 | 86.36 | 58.94 (±43.31) | 50.90 (±45.13) | ||
| 5. La Reforma River | 20 | Mar-10 | 60 | 7.25 (±10.48) | 4.35 (±8.77) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 95 | 61.84 (±77.81) | 58.75 (±76.99) | ||
| 7. Santa MaríaHuatulco River | 7 | Mar-10 | 14.28 | 6(±0) | 0.85 (±2.26) | ||
| iCentrocestus formosanus Nishigori, 1924 | Gills | 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 72.22 | 12.15 (±21.57) | 8.77 (±18.97) |
| 4. Pichuaca River | 22 | Sep-09 | 100 | 821.63 (±417.59) | 821.63 (±417.59) | ||
| 5. La Reforma River | 20 | Mar-10 | 100 | 42.45 (±33.39) | 42.45 (±33.96) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 5.88 | 31 (±0) | 1.82 (±7.51) | ||
| 7. Santa Maria Huatulco River | 7 | Mar-10 | 50 | 1.66 (±1.15) | 0.83 (±1.16) | ||
| Monogenea Family Gyrodactylidae van Beneden and Hesse, 1863 Gvrodactylus sp. | Fins | 2. San José de las Flores River | 20 | Sep-09 | 5 | 1(±0) | 0.05 (±0.22) |
| 5. La Reforma River | 20 | Mar-10 | 5 | 2(±0) | 0.1 (±0.44) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 10 | 1(±0) | 0.1 (±0.30) | ||
| Family Ancyrocephalidae Bychowsky, 1937 Salsuginus sp. | Gills | 2. San José de las Flores River | 20 | Sep-09 | 5 | 1(±0) | 0.05 (±0.22) |
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 72.22 | 7.15 (±6.37) | 5.16 (±6.28) | ||
| 4. Pichuaca River | 22 | Sep-09 | 13.63 | 3 (±1.73) | 0.40 (±1.18) | ||
| 5. La Reforma River | 20 | Mar-10 | 75 | 7 (±5.45) | 5.25 (±5.54) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 82.35 | 5.85 (±5.27) | 4.82 (±5.28) | ||
| 7. Santa María Huatulco River | 7 | Mar-10 | 83.33 | 7.4 (±4.44) | 6.16 (±4.99) | ||
| Adult Nematoda Family Cystidicolidae Skijabin, 1946 Spinitectus humbertoiCaspeta-Mandujano and Moravec, 2000 | Intestine | 6. Pueblo Viejo River | 20 | Mar-10 | 10 | 6.5 (±3.53) | 0.65 (±2.15) |
| 7. Santa María Huatulco River | 7 | Mar-10 | 14.28 | 17 (±0) | 2.42 (±6.42) | ||
| Family Rhabdochonidae Travassos, Artigas and Pereira, 1928 Rhabdochona salgadoiCaspeta-Mandujano and Moravec, 2000 | Intestine | 1. La Soledad Carrizo River | 25 | Sep-09 | 60 | 4.4 (±4.15) | 2.64 (±3.86) |
| 2. San José de las Flores River | 20 | Sep-09 | 70 | 5.71 (±4.95) | 4 (±5.79) | ||
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 83.33 | 4.46 (±3.11) | 3.72 (±3.30) | ||
| 4. Pichuaca River | 22 | Sep-09 | 54.54 | 2.75 (±1.86) | 1.5 (±1.94) | ||
| 5. La Reforma River | 20 | Mar-10 | 95 | 8.05 (±3.99) | 7.65 (±4.28) | ||
| 6. Pueblo Viejo River | 20 | Mar-10 | 90 | 7.66 (±4.95) | 6.9 (±5.25) | ||
| 7. Santa María Huatulco River | 7 | Mar-10 | 100 | 18.57 (±10.84) | 18.57 (±10.84) | ||
| Larval Nematodes Family Dioctophymatidae Railliet, 1915 Eustrongylides sp. | Mesentery | 2. San José de las Flores River | 20 | Sep-09 | 5 | 1(±0) | 0.05 (±0.22) |
| 3. Santa Cruz Flores Magón River | 18 | Sep-09 | 16.66 | 2(±1) | 0.33 (±0.84) |
Sampling sites of specimens of Profundulus punctatus collected in 7 localities from Oaxaca, México. 1, La Soledad Carrizo River; 2, San José de las Flores River; 3, Santa Cruz Flores Magón River; 4, Pichuaca River; 5, La Reforma River; 6, Pueblo Viejo River; 7, Santa María Huatulco River.
In each locality, fish were caught by electrofishing, transported live to a laboratory, and necropsied immediately after capture. All the external surfaces, viscera, and musculature of each host were examined under a stereomicroscope; all the helminths were counted in situ. Digenean larvae, monogeneans, and nematodes were fixed in hot 4% neutral formalin. Some monogeneans were mounted in ammonium picrate (Ergens, 1969), and mounted unstained in Gray-Wess fixative (Vidal-Martínez et al., 2001) for study of sclerotized structures. Digeneans and monogeneans used for morphological studies of whole mounts were stained with Mayer's paracarmine, dehydrated using a graded alcohol series, cleared in methyl salicylate, and mounted in Canada balsam. Nematodes were cleared with glycerine for light microscopy and stored in 70% ethanol. Voucher specimens of each helminth species were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico. Prevalence (% infected), mean intensity of infection (number of parasites per parasitized fish), and mean abundance were used as proposed by Bush et al. (1997).
Community structure. Analyses were carried out at the component community level (Holmes and Price, 1986) i.e., considering all helminth species in the sample of P. punctatus in each particular site of collection. To determine if sample size was sufficient to produce an accurate estimate of the pool of parasites in each sampled locality, a species accumulation curve and the species richness estimators Clench equation were used for each component community (Magurran, 1988). A final value of the slope of the species accumulation curve no higher than 0.1 species per sample was used as the criterion for adequate sampling because empirically this final slope of accumulation curve indicates that at least 70% of the species in the component have been sampled already (Jiménez-Valverde and Hortal, 2003). The attributes at component community level structure calculated were the total number of helminth species per locality (richness); the total number of individuals (abundance) (Bush et al., 1990); diversity (Shannon-Wiener index and Brillouin index) and evenness (Simpson's reciprocal index); and the numerical dominance at the component community level, determined using the Berger-Parker dominance index, all calculated following Magurran (1988). The possible relation among the number of fish examined and the number of helminth species was analyzed using the Spearman rank correlation coefficient.
The similarity between each pair of localities was determined qualitatively (Jaccard similarity index) and quantitatively using the percent similarity index separately, following Esch et al. (1988), and the mean of all possible pair combinations was obtained. Additionally, species were categorized as allogeneic (completing their life cycle in terrestrial hosts, such as birds and mammals) or autogenic (developing the full life cycle in aquatic environments) (Esch et al., 1988).
ResultsGeneral descriptions. A total of 7 freshwater bodies were sampled (Table 1, Fig. 1) and 132 individual fish were examined. A total of 14 helminth species belonging to 13 genera and 11 families were collected (Table 1). Eight helminth species were recorded for the first time in this host species from Mexico. The helminthological record comprises 9 digenean species: Paracreptotrema blancoi Choudhury, Pérez-Ponce de León, Brooks, and Daverdin, 2006, Paracreptotrema profundulusi Salgado-Maldonado, Caspeta-Mandujano and Martínez-Ramírez, 2011, Phyllodistomum sp., Culuwiya cf. cichlidorum, and the metacercariae of Clinostomum sp., Diplostomidae gen. sp., Posthodiplostomum minimum MacCallum, 1921, Ascocotyle (Ascocotyle) felippei Travassos, 1928, and Centrocestusformosanus Nishigori, 1924; 2 monogeneans: Gyrodactylus sp. and Salsuginus sp.; 3 nematodes: Spinitectus humbertoiCaspeta-Mandujano and Moravec, 2000, Rhabdochona salgadoiCaspeta-Mandujano and Moravec, 2000, and the larval forms of Eustrongylides sp. The most abundant group was the digeneans (4 adults and 5 metacercariae), followed by the nematodes (2 adults and 1 larval form), and 2 monogeneans. The adult nematode Rhabdochona salgadoi and the metacercaria of Ascocotyle (Ascocotyle) felippei reached the highest levels of prevalence and mean abundance in the 7 localities. Helminth parasite taxa, infection site, number of infected fish, prevalence, mean intensity, and abundance for each helminth species are shown in Table 1. Eight of these species are autogenics, completing their life cycle in water and incapable of crossing land barriers between freshwater bodies. Six more species found as larvae are generalist allogenics, using birds as definitive hosts. Prevalence, mean intensity, and abundance between autogenics and allogenics species were highly variable (Table 1). The number of helminth taxa varied among localities, from a minimum of 6 species in the Pichuaca River to a maximum of 10 species in La Reforma River and Pueblo Viejo River. The number of individuals was also variable between localities, with a minimum of 196 in San José de las Flores River and a maximum of 19,265 in Pichuaca River. The total number of taxa was not significantly correlated with the number of fish examined (r=−0.47, p>0.05). Ascocotyle (A.) felippei was the most abundant species in all samples sites.
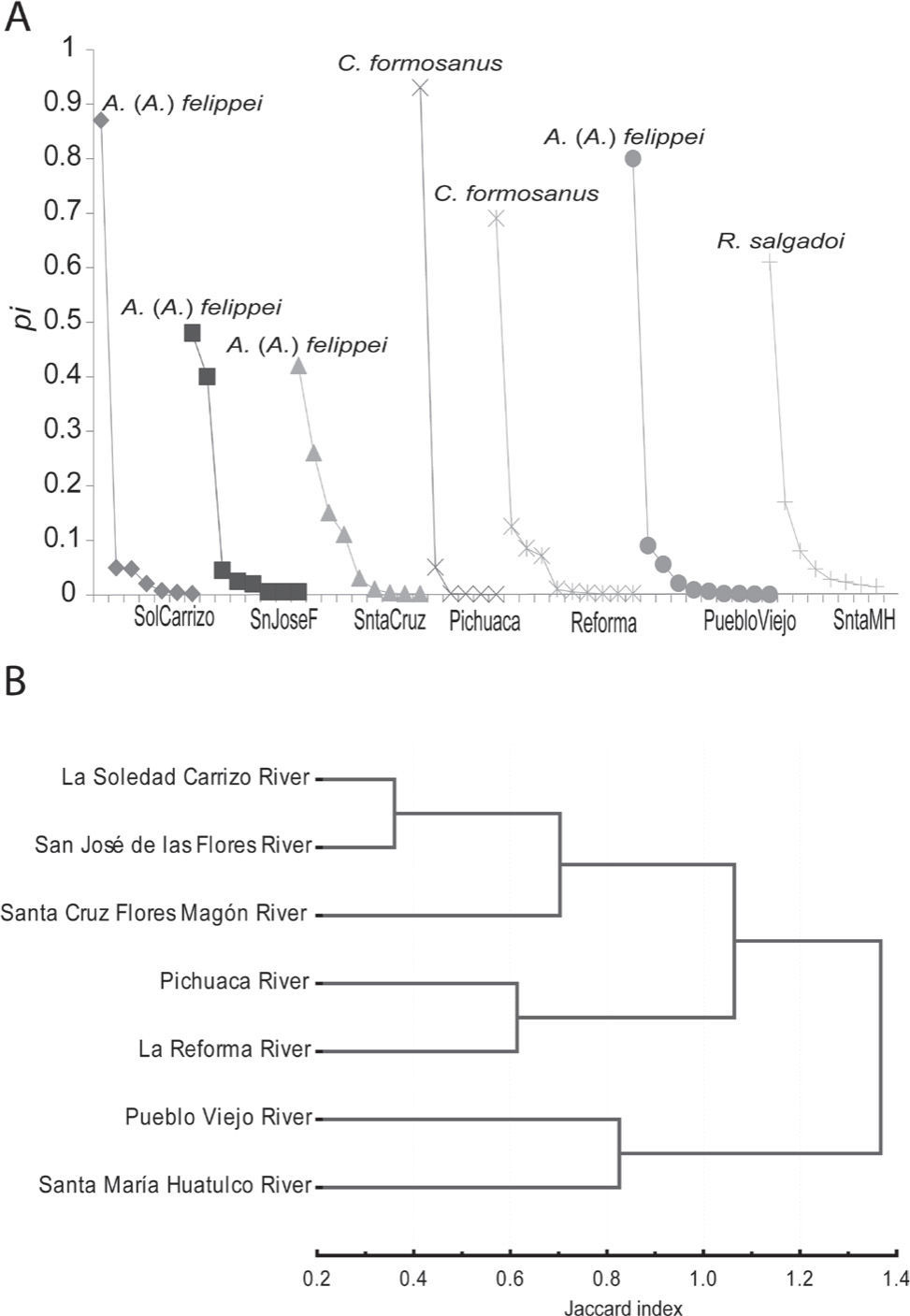
Community structure. The sample size was sufficient in 6 of 7 sampled localities. Table 2 shows the richness estimated by the Clench model a/b and the slope of the last point of the curve a/(1+b*n)2. The analysis of species accumulation curves shows that the inventory of helminth species of the component communities was almost complete for 6 of 7 sampled localities; the slope of the last point of each curve calculated from the Clench model was <0.1, indicating a good coverage of helminth species recovered from each component community (Table 2). Santa María Huatulco River was the only locality where the asymptote in the curve is not reached. The total number of helminth species oscillated among 6 to 10 in fish from the 7 sampled localities (Table 3). The abundance varied between 196 and 19,265 in San José de las Flores River and Pichuaca River, respectively. The Shannon-Wiener and Brillouin diversity values ranged from 0.359 to 2.083 and 0.358 to 2.042 in the 7 sampled localities, with the evenness and numerical dominance values also ranging from 0.151 to 0.390, and 0.42 to 0.93, respectively. The matacercariae of Ascocotyle (A.) felippei were numerically dominant in 4 localities; 2 localities were dominated by Centrocestusformosanus, and 1 locality by the nematode Rhabdochona salgadoi (Table 3; Fig. 2A). Likewise, qualitative similarity among the 7 component communities evaluated was heterogeneous. Table 4 shows that several localities shared a relatively high number of helminth species, resulting in a Jaccard value that ranged 0.33 to 0.81; the percent similarity index ranged from 0.05 to 0.86. Helminth communities from Pueblo Viejo River and La Reforma River consistently showed the highest levels of qualitative similarity (0.81) with respect to other localities; San José de las Flores River and Santa María Huatulco River showed the lowest values of qualitative similarity (0.33) (Fig. 2B).
Richness estimated by the Clench model for all localities
| Localities | No. of fish examined | Total no. of species (Sobs) | Correlation coefficient with the Clench model (R2) | Clench model parameters | Richness estimated by the Clench model a/b | Slope of the last point of the curve in the Clench model a/(1+b*n)2 | |
|---|---|---|---|---|---|---|---|
| a | b | ||||||
| La Soledad Carrizo River | 25 | 7 | 0.99 | 3.152852 | 0.419106 | 7.5 | 0.023 |
| San José de las Flores River | 20 | 8 | 0.98 | 1.534902 | 0.150576 | 10.1 | 0.095 |
| Santa Cruz Flores Magón River | 18 | 9 | 0.96 | 5.259541 | 0.555026 | 9.47 | 0.043 |
| Pichuaca River | 22 | 6 | 0.95 | 5.339126 | 0.880555 | 6.06 | 0.012 |
| La Reforma River | 20 | 10 | 0.95 | 4.395730 | 0.406227 | 10.8 | 0.052 |
| Pueblo Viejo River | 20 | 10 | 0.96 | 3.504056 | 0.302658 | 11.57 | 0.092 |
| Santa Maria Huatulco River | 7 | 6 | 0.98 | 6.134194 | 0.619753 | 9.87 | 0.27 |
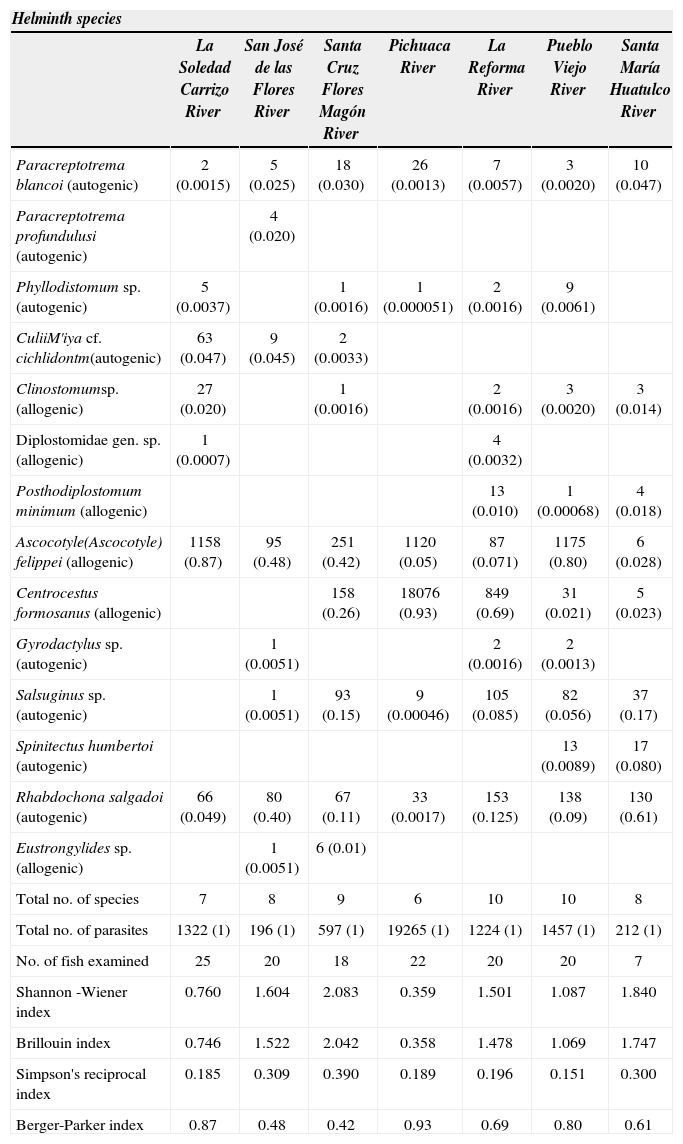
Relative abundance of each helminth species as a proportion (pi) of the total number of all helminths and diversity characteristics in Profiindidiispiinctatus in 7 localities of the Oaxaca
| Helminth species | |||||||
|---|---|---|---|---|---|---|---|
| La Soledad Carrizo River | San José de las Flores River | Santa Cruz Flores Magón River | Pichuaca River | La Reforma River | Pueblo Viejo River | Santa María Huatulco River | |
| Paracreptotrema blancoi (autogenic) | 2 (0.0015) | 5 (0.025) | 18 (0.030) | 26 (0.0013) | 7 (0.0057) | 3 (0.0020) | 10 (0.047) |
| Paracreptotrema profundulusi (autogenic) | 4 (0.020) | ||||||
| Phyllodistomum sp. (autogenic) | 5 (0.0037) | 1 (0.0016) | 1 (0.000051) | 2 (0.0016) | 9 (0.0061) | ||
| CuliiM'iya cf. cichlidontm(autogenic) | 63 (0.047) | 9 (0.045) | 2 (0.0033) | ||||
| Clinostomumsp. (allogenic) | 27 (0.020) | 1 (0.0016) | 2 (0.0016) | 3 (0.0020) | 3 (0.014) | ||
| Diplostomidae gen. sp. (allogenic) | 1 (0.0007) | 4 (0.0032) | |||||
| Posthodiplostomum minimum (allogenic) | 13 (0.010) | 1 (0.00068) | 4 (0.018) | ||||
| Ascocotyle(Ascocotyle) felippei (allogenic) | 1158 (0.87) | 95 (0.48) | 251 (0.42) | 1120 (0.05) | 87 (0.071) | 1175 (0.80) | 6 (0.028) |
| Centrocestus formosanus (allogenic) | 158 (0.26) | 18076 (0.93) | 849 (0.69) | 31 (0.021) | 5 (0.023) | ||
| Gyrodactylus sp. (autogenic) | 1 (0.0051) | 2 (0.0016) | 2 (0.0013) | ||||
| Salsuginus sp. (autogenic) | 1 (0.0051) | 93 (0.15) | 9 (0.00046) | 105 (0.085) | 82 (0.056) | 37 (0.17) | |
| Spinitectus humbertoi (autogenic) | 13 (0.0089) | 17 (0.080) | |||||
| Rhabdochona salgadoi (autogenic) | 66 (0.049) | 80 (0.40) | 67 (0.11) | 33 (0.0017) | 153 (0.125) | 138 (0.09) | 130 (0.61) |
| Eustrongylides sp. (allogenic) | 1 (0.0051) | 6 (0.01) | |||||
| Total no. of species | 7 | 8 | 9 | 6 | 10 | 10 | 8 |
| Total no. of parasites | 1322 (1) | 196 (1) | 597 (1) | 19265 (1) | 1224 (1) | 1457 (1) | 212 (1) |
| No. of fish examined | 25 | 20 | 18 | 22 | 20 | 20 | 7 |
| Shannon -Wiener index | 0.760 | 1.604 | 2.083 | 0.359 | 1.501 | 1.087 | 1.840 |
| Brillouin index | 0.746 | 1.522 | 2.042 | 0.358 | 1.478 | 1.069 | 1.747 |
| Simpson's reciprocal index | 0.185 | 0.309 | 0.390 | 0.189 | 0.196 | 0.151 | 0.300 |
| Berger-Parker index | 0.87 | 0.48 | 0.42 | 0.93 | 0.69 | 0.80 | 0.61 |
A, relative abundance of each helminth species as a proportion (pi) of the total number of all helminths of all species found in Profundulus punctatus in 7 localities; B, dendrogram of Jaccard similarity index of total helminth communities between pairs of localities for the 7 locations.
Similarity between helminth communities of P. punctatus from 7 localities
| San José de las Flores River | Santa Cruz Flores Magón River | Pichuaca River | La Reforma Rive | Pueblo Viejo River | Santa María Huatulco River | |
|---|---|---|---|---|---|---|
| La Soledad Carrizo River | 0.36/0.58* | 0.45/0.47 | 0.44/0.06 | 0.54/0.12 | 0.41/0.86 | 0.36/0.09 |
| San José de las Flores River | 0.54/0.57 | 0.40/0.06 | 0.38/0.20 | 0.38/0.58 | 0.33/0.46 | |
| Santa Cruz Flores Magón River | 0.66/0.32 | 0.58/0.54 | 0.58/0.59 | 0.54/0.35 | ||
| Pichuaca River | 0.60/0.75 | 0.60/0.08 | 0.55/0.05 | |||
| La Reforma River | 0.81/0.25 | 0.63/0.28 | ||||
| Pueblo Viejo River | 0.80/0.21 |
The helminth parasite fauna of Profundulus punctatus in the 7 studied localities from Oaxaca, Mexico includes 14 helminth species: 9 digeneans, 2 monogeneans, and 3 nematodes. Most of the helminth species found in this study have been previously recorded from diverse freshwater fish species in southwest Mexico (Salgado-Maldonado, 2006; Salgado-Maldonado et al., 2011a). Profundulus punctatus from Oaxaca acts as definitive host for 8 species, namely, P. blancoi, P. profundulusi, Phyllodistomum sp.,Culuwiya cf. cichlidorum, Gyrodactylus sp., Salsuginus sp., S. humbertoi, and R. salgadoi. In addition, it serves as an intermediate/paratenic host for 6 helminth taxa (Clinostomum sp., Diplostomidae gen. sp., P. minimum, Ascocotyle (A.) felippei, C. formosanus, and Eustrongylides sp.). Previous studies on helminth communities of freshwater fishes in Mexico recorded a higher number of larvae in their taxonomic composition than reported here (see Salgado-Maldonado and Kennedy, 1997; Martinez-Aquino et al., 2007, 2009, 2011; Salgado-Maldonado et al., 2014). The low larval richness in our work can be attributed to the food habits of P. punctatus (see below), and/or the absence of suitable intermediate-definitive hosts in the studied areas. Nevertheless, our ecological results convey a rather static picture of the helminth species richness of P. punctatus for a short period of time. Therefore, further long-term parasitological studies are necessary to understand the helminth community dynamics, and assess variation in the helminth species richnes of P. punctatus.
Four of these taxa, 2 digenean (P. blancoi and P. profundulusi), and 2 nematodes, (S. humbertoi and R. salgadoi), are commonly found in profundulid fishes, and have been considered as a part of the core fauna for this fish family (Caspeta-Mandujano and Moravec, 2000; Caspeta-Mandujano et al., 2007; Salgado-Maldonado et al., 2011b). Paracreptotrema blancoi lives sympatrically with P. profundulusi as a parasite of the fish P. punctatus in San José de las Flores River. Co-occurrence of trematode species in the same profundulid species may be attributed to recent contact among different host populations, but the geology of the area is not well known, which prevents establishing the biological significance of these data (Salgado-Maldonado et al., 2011b). Other helminth species recorded herein as adults were Phyllodistomum sp., and Culuwiya cf. cichlidorum. The findings of Phyllodistomum sp. in the present study represent a new record in profundulid fishes. In Mexico, 6 nominal species of Phyllodistomum have been recorded so far, 4 of them in marine or brackish water fishes: Phyllodistomum carangis Manter, 1947, P. marinae Bravo-Hollis and Manter 1957, P. mirandai Lamothe, 1969, and P. centropomi Mendoza-Garfias and Pérez-Ponce de León 2005, and 2 in freshwater fishes: P. lacustri (Loewen, 1929) Lewis, 1935, as a parasite of ictalurid and cichlid fishes, and P. inecoli Razo-Meldivil, Pérez-Ponce de León and Rubio-Godoy, 2013, found in Heterandria bimaculata (Razo-Mendivil et al., 2013). Considering the host associations and geographical distribution of the species of Phyllodistomum in freshwater fishes of North and Central America, we may speculate that our specimens represent an undescribed species.
The trematode Culuwiya cf. cichlidorum found in this research represents a new record in profundulid fishes. Culuwiya cichlidorum was described from the intestine of the black-belt cichlid Vieja maculicauda (= Cichlasoma maculicauda (Regan)) from the Atlantic coast of Nicaragua (Aguirre-Macedo and Scholz, 2005). However, the taxonomy of these digeneans is complicated for several reasons: its morphology (external and internal) is very complicated and tends to change during fixation and staining processes (Overstreet and Curran, 2005). Further analyses using both morphological and molecular data, will allow us to identify with accuracy this digenean.
Of the 14 taxa found, 42.85% were larval forms of generalist species that use freshwater fish as intermediate or paratenic hosts. The taxonomic composition of the helminth parasite fauna of P. punctatus comprises 5 generalist species:, Clinostomum sp., Diplostomidae gen. sp., Posthodiplostomum minimum (MacCallum, 1921) Dubois, 1936, Ascocotyle (Ascocotyle) felippei Travassos, 1928, and Eustrongylides sp., which are widely distributed among freshwater fish in several localities in Mexico (Salgado-Maldonado, 2006). Centrocestus formosanus (Nishigori, 1924) was introduced to Mexican water bodies from Asia (Scholz and Salgado-Maldonado, 2000); the current study represents the first report of this species in P. punctatus. The 2 monogenean, taxa, Gyrodactylus sp., and Salsuginus sp., collected from the fins and gills of their hosts, have been recently recorded from diverse freshwater fishes in central and northern Mexico (Mendoza-Palmero, 2007; Mendoza-Palmero et al., 2009); further studies will enable us to establish the taxonomic identity and potential host specificity of Gyrodactylus and Salsuginus species occurring in Mexican freshwater fishes. The helminth fauna found for this host species included digeneans, monogeneans, and nematodes, but no tapeworms or acanthocephalans were recorded. This could be because the diet of this fish species does not include crustaceans, which act as intermediate host for these 2 groups of helminths.
Prevalence of species recovered from P. punctatus was variable and mean abundances were generally low for the fish sampled in the 7 locations (Table 1). The high variability in the infection parameters of most species suggests that the host is subject to the effect of wide environmental variability, the presence and abundance of P. punctatus appears to be associated aquatic ecosystems with little human disturbance, where the physical and chemical characteristics of the water and habitat are good (Velázquez-Velázquez et al., 2011). The community structure of the helminth parasites of P. punctatus is consistent with the pattern that shows a poor parasite fauna with respect to Cichlasoma urophthalmus inhabiting freshwater localities in the Neotropical part of Mexico (Salgado-Maldonado and Kennedy, 1997). However, the helminth fauna composition and the community structure of P. punctatus was more diverse than parasite fauna of goodeid fishes in the Nearctic part of Mexico, such as of Characodon audax Smith and Miller (parasitized by 8 species), Xenotaenia resolanae Turner (infected by 6 species), and Zoogoneticus purhepechus Domínguez-Domínguez (7 species) (Martínez-Aquino et al., 2007; 2009; 2011). The richness and component communities of P. punctatus were similar to helminth communities of Heterandria bimaculata from the upper Río La Antigua basin, east-central Mexico (parasitized by 12 species) (Salgado-Maldonado et al., 2014). Previous studies showed that P. punctatus feeds primarily on clams and snails (Melaneidae); its habitat is characterized by environments with sandy substrate, silt, mud, gravel, rock, and boulders (Miller, 2005), which facilitates contact with mollusks. Both factors determine the helminth community structure herein described, and are in accordance with those described in the aforementioned studies (in this case, feeding habits and omnivory). Parasite communities of P. punctatus in the 7 localities have low diversity (Shannon-Wiener index= 0.359 to 2.083 and Brillouin index= 0.358 to 2.042). Pichuaca River was the locality with the lowest values (Shannon- Wiener index= 0.359 and Brillouin index= 0.358), and Santa Cruz Flores Magón River had the highest diversity (Shannon-Wiener index= 2.083 and Brillouin index= 2.042). In the Pichuaca River, the diversity was low due to high dominance of Centrocestus formasanus, which caused a heterogeneous distribution of the proportional abundance. Our values of diversity (Shannon-Wiener) were higher than those reported by Salgado-Maldonado et al. (2014) which ranged from 0.75 to 1.358, establishing the helminth communities of P. punctatus as more diverse than those of Heterandria bimaculata. Also, our diversity values obtained for the Brillouin index are higher than those obtained for goodeid fishes in the Nearctic part of Mexico (Martínez-Aquino et al., 2009). The similarity between the 7 component communities (qualitative and quantitative), was low, due to the heterogeneity of infection between fishes from different localities; San José de las Flores River and Santa María Huatulco River showed the lowest values of similarity (Jaccard index= 0.33); our data suggest that the similarity in species composition decreases among remote locations, as suggested for many communities of parasites (similarity vs. distance) (Poulin and Morand, 1999; Salgado-Maldonado et al., 2014). Based on the results of the species accumulation curves and the species richness estimators, we are confident about the accuracy of species richness and community structure patterns herein described. Likewise, our analysis confirmed that the number of host analyzed represents a sufficient sample size to recover most members of the parasite community. Our data revealed that P. punctatus is parasitized by 14 helminth species, 9 digeneans, 2 monogeneans, and 3 nematodes, and that Paracreptotrema blancoi, Paracreptotrema profundulusi, Spinitectus humbertoi, and Rhabdochona salgadoi represent the core fauna of this species of fish host.
We are grateful to Marly Martínez, David Hernández Mena, Jesús Hernández Orts, and Mirza Patricia Ortega Olivares for their help during field work. CDPP thanks Conacyt and the Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México for the scholarships.