Due to the quick technological evolution, orthodontic product’s manufacturers continuously develop adhesives to satisfy the needs of the specialist, who trusts the published virtues. Due to the fact that clinics often choose products based on marketing or maybe by habit, it is necessary to analyze orthodontic products and assess their physical properties in order to make better choices of one product over another. During the last two decades investigators have taken as a parameter the Transbond XT resin, however, recently other companies have launched to the market new products. It is for this reason that the objective of this study was to determine some of the physical characteristics of the most widely used bracket adhesives in Orthodontic graduate programs of public and private teaching institutions of Mexico: Transbond XT (TB), Enlight (IN), Super C-Ortho (SC) and Fuji LC (FJ).
MethodThe sorption and solubility (n=10) in accordance with ISO 404 were calculated and the film thickness was measured (n=10) attending to the norm ISO 11405. The ANOVA and Test of Tukey was used to determine the statistical differences.
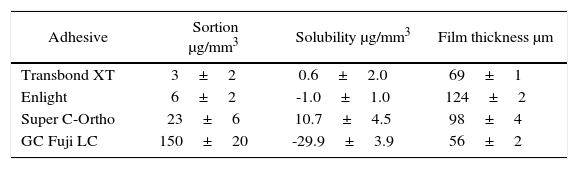
ResultsSorption (μg/mm3): TB (3±2), EN (6±2), SC (23±6), FJ (150±20). Solubility (μg/mm3): TB (0.6±2.0), EN (-1.0±1.0), SC (10.7±4.5), FJ (-29.9±3.9). Film thickness (um): TB (68±1), EN (124±2), SC (98±4), FJ (56±2). This study was financed by UNAM-DEGAPA-PAPIIT IT 201612.
Debido a la rápida evolución tecnológica, los fabricantes de productos ortodóncicos desarrollan continuamente adhesivos para satisfacer las necesidades del especialista, confiando éste en las bondades publicitadas. Debido a que el clínico elige los productos con base en la mercadotecnia o por costumbre, es necesario analizarlos y comprobar sus propiedades físicas para hacer una elección certera de un producto sobre otro. Durante las dos últimas décadas, los investigadores han tomado como parámetro a Transbond XT; sin embargo, recientemente otras casas comerciales han sacado al mercado otros productos. Es por esto que el objetivo de este estudio es determinar algunas de las características físicas de los adhesivos para brackets más utilizados en instituciones de enseñanza de la Especialidad en Ortodoncia a nivel estatal y privado de México: Transbond XT (TB), Enlight (EN), Super C-Ortho (SC) y Fuji LC (FJ).
MétodosSe calculó la sorción y solubilidad (n=10) de acuerdo con ISO 4049 y se midió el espesor de película (n=10) atendiendo a ISO 11405. Se usó ANOVA y prueba de Tukey para determinar diferencias estadísticas.
ResultadosSorción μg/mm3): TB (3±2), EN (6±2), SC (23±6), FJ (150±20). Solubilidad (μg/ mm3): TB (0.6±2.0), EN (-1.0±1.0), SC (10.7±4.5), FJ (-29.9±3.9). Espesor de película (pm): TB (68±1), EN (124±2), SC (98±4), FJ (56±2). Este estudio fue financiado por la Universidad Nacional Autónoma de México - DEGAPA-PAPIIT IT 201612.
With the discovery of acid etching1 and enamel adhesion,2 banded orthodontic treatments fell into disuse, providing the operator with less working time and greater patient comfort, thus the technological development in adhesives for orthodontics began to evolve rapidly.It began in the 70’s with the use of acrylic resins (Super C-Ortho);3 in the 80’s, the two-step and two consistencies autocuring resins appeared (Concise),4,5 in this same decade the resin modified glass ionomercement appeared. In the 90’s when the light-cured resin modified glass ionomer cement appeared (GC Fuji Ortho LC)6–10 it did so looking for specific adhesion to the tooth and a sustained fluoride release, which helped in reducing even more chairtime adhesion for appliance bonding. Later on, single-step light-cured resins were developed as the ones currently used (Transbond XT).9–11
The orthodontist requires an adhesive that in addition to the decrease in chair time, is easy to manipulate, allows sufficient time to position the appliances, that has the needed fluidity to keep the bracket over the tooth surface while it is being light-cured, that penetrates in the retentions created on the tooth as well as in the ones in the bracket, that has minimal water sorption and minimal film thickness to respect the system’s prescription; easy identification and removal of resin excess, that does not solubilize in order to avoid microfiltration, decrease the risk of developing lesions under the bracket and the premature debonding of the appliances; that has dimensional stability, and the sufficient resistance to debonding to withstand orthodontic biomechanics.
Some authors emphasize that during bonding at the end of the treatment precaution is recommended to avoid harm to the enamel.12–15
Due to the rapid technological development orthodontic product’s manufacturers are continuously developing orthodontic adhesives to meet the needs of the specialist, who trusts the published virtues. Since the clinician chooses products based on marketing or by habit the provided information is limited (usually: handling instructions and data regarding the resistance to debonding), their physical properties need to be analyzed to make an informed choice of one product over another.
Since the creation of adhesives for bonding, the traditional method of assessment has been resistance to debonding (Figure 1); during the past three decades in the investigation lines focused on bonding adhesives, Transbond XT resin has been taken as a parameter. Some authors have been using the methods of International Norms for evaluating adhesive materials in response to ISO 11405.16
The polymers used in the manufacture of composite resins for restoration and dental implants are composed of mono or di acrylates of chemical structure nearly identical to orthodontic adhesives, therefore the reaction of orthodontic polymers should be similar to that of restoration materials. The influence of aqueous environments over them has been observed: it affects their mechanical behavior, dimensional stability and the useful life of the dental restorations.17–19
Therefore, it is necessary to determine some physical characteristics (other than the resistance to the debonding) of the bonding adhesives most commonly used in some of Orthodontics specialty programs in public and private educational institutions of Mexico.
Materials and methodsWe selected four adhesive systems (Figure 2): Transbond XT Light Cure Adhesive (3M UNITEK Monrovia California, Batch: 7YF2009-11); Enlight Light Cure Adhesive (ORMCO Corporation, located in Glendora, Ca, USA. Batch: 2693221 2009-08); Super C-Ortho (AMCO Manufacturing Philadelphia, PA, USA Batch: not specified); GC Fuji Ortho LC light-cured bonding orthodontic adhesive (GC Corporation Tokyo, Japan. Batch: 0704041).
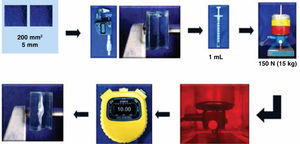
The Norm ISO 404920 indicates that all tests must be carried out in a laboratory with controlled temperature and humidity (23 °C, 60% RH) by a single operator with gloves free of dust. 30 Specimens were prepared for each group; we used a stainless steel mold of 15 mm in diameter and adjusted the depth to 0.5±0.1 mm. It was lubricated with oil silicon. It was overfilled with the adhesive and Mylar tape and a glass tile (100×25×1.6 mm, with a weight of 10 g) were placed above. On top of this a 500 g weight was placed for a minute.
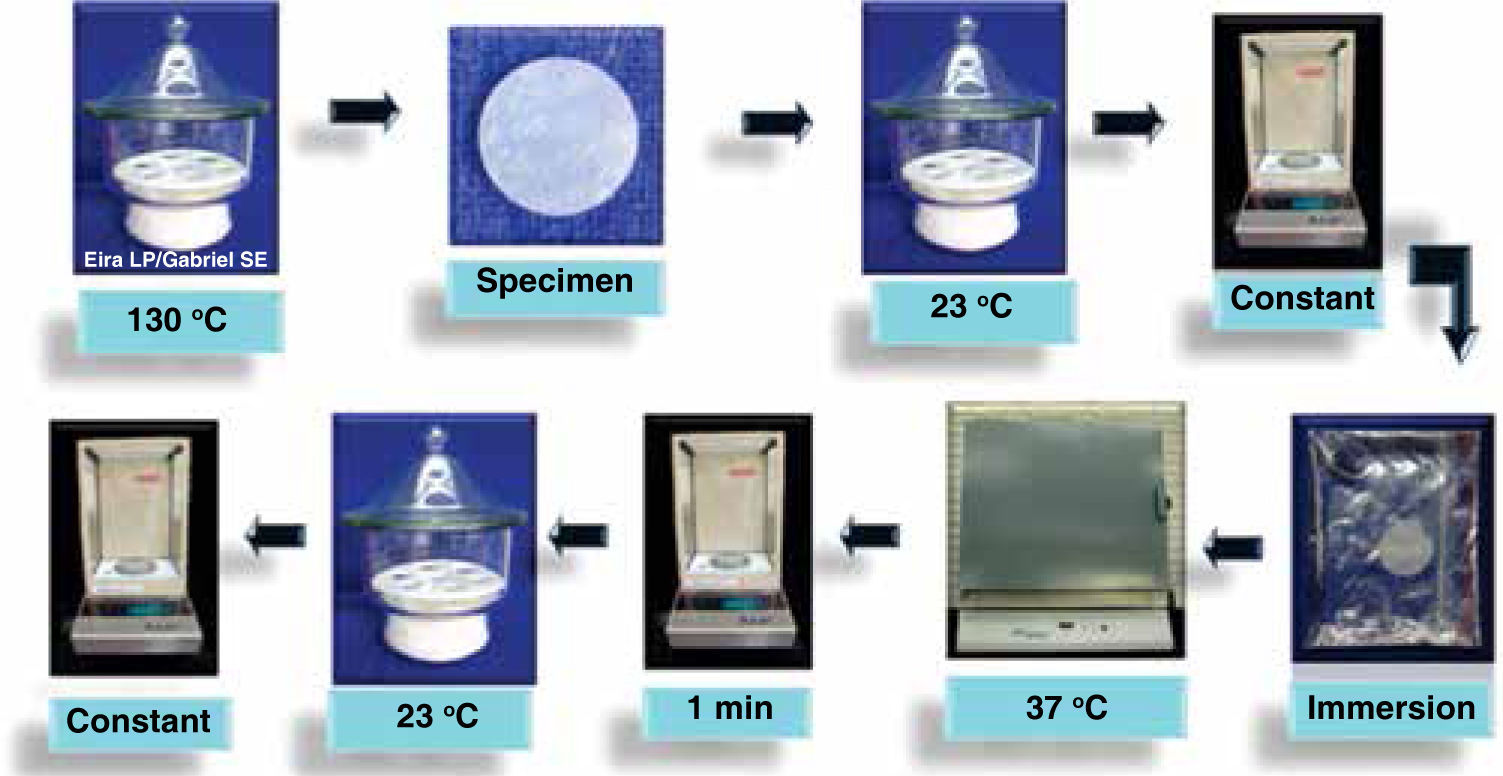
To polymerize specimen groups 1, 2 and 4, a light-curing lamp was used (Visilux 2, 3M ESPE Monrovia Calif, USA), checking its function previously with a curing radiometer (model 100, Optilux Radiometer Measures Demetron Research Corporation. Danbury, CT. USA) resulting in a value of 350 mW/cm2, and with the thermal radiometer (model 200, Heat/Glare Demetron Research Corporation. Danbury, CT. USA). A value of 25 mW/cm2 by was obtained by placing the tip in five sites on the tile: on the center and to the four sides for 10 seconds each time. In the case of group 3, for being autocurable, it was left to polymerize for 10 minutes due to the fact that the manufacturer recommends loading the brackets after this time. In all of the cases the tile and the Mylar tapewere withdrawn to dislodge the specimen; once removed, they were placed within a desiccator at 23 °C with silica which was previously dehydrated during five hours at 130 °C. After 24 hours, they were weighed in an analytical balance (OHAUS) with an accuracy of±0.2mg repeating this cycle until a constant mass was obtained in a period of 24 hours. This measurement was reported as M1.
The specimens were submerged in distilled water in hermetically sealed containers at 37 °C for seven days within an incubator (Microwave Felisa, Mexico). After this time, they were removed from the containers, dried with a paper towel until they were apparently moisture-free, agitated in the air for 15 seconds. After a minute of having being removed from the water, they were weighed in the analytical balance and reported as M2.
The specimens were reconditioned in the desiccator, until they showed a non-variable constant weight of±0.2 mg. This was reported as M3 (Figure 3).
The diameter (D) and thickness (h) of each specimen was measured to calculate volume (V) in cubic millimeters: V=(π/4) (D2h).
For the aqueous sorption test expressed in μ/mm3, the formula: AS=(M2-M3)/Vwas employed.
Solubility: Ws μg/mm3, it was calculated for each one of the ten specimens with the equation: Ws=(M1-M3)/V.
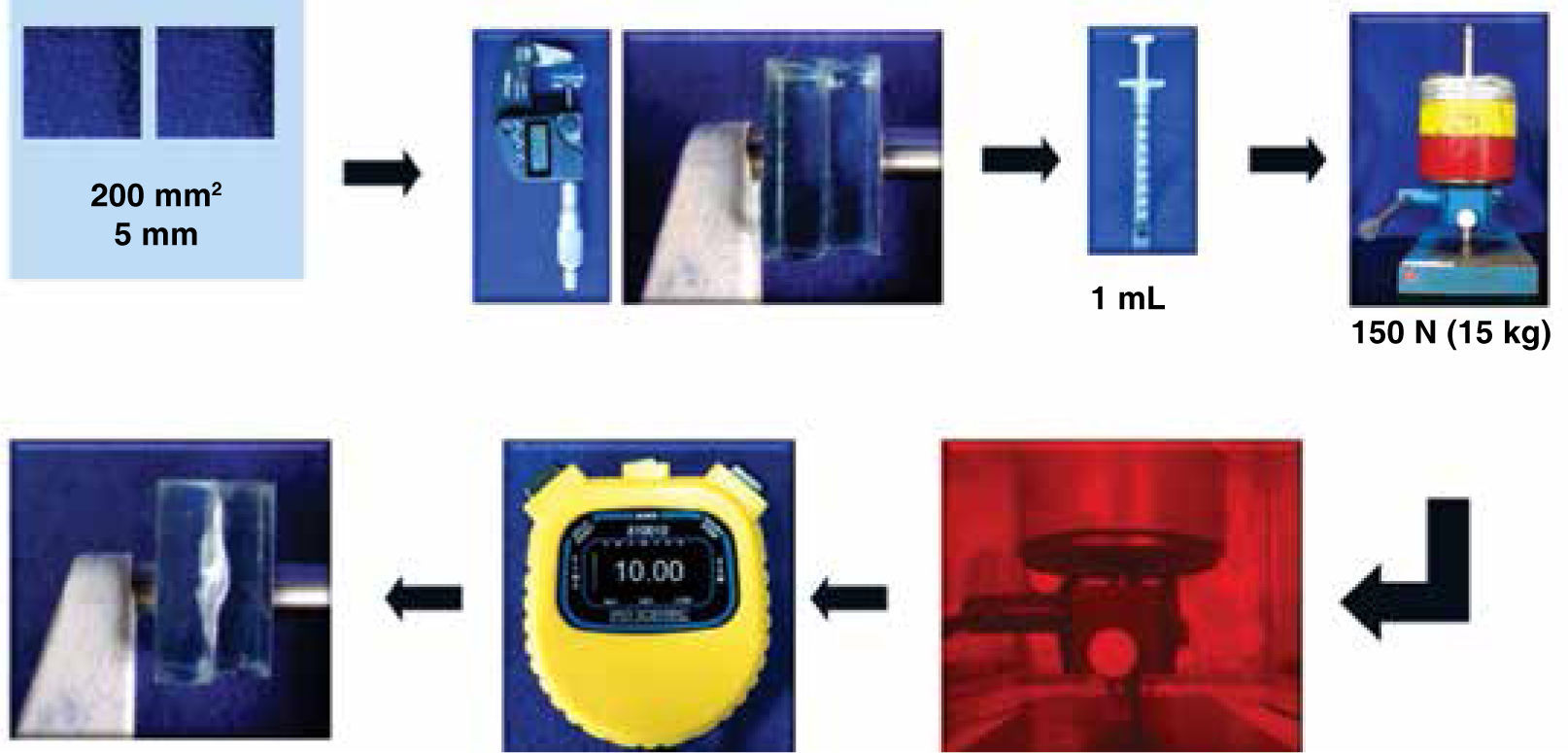
According to the Norm ISO 1140562 a test was performed to determine film thickness within an orange transparent chamber (51cm×35cm×36cm) to filter light and avoid photopolymerization of the adhesives. Two glass plates, with a contact surface of 200 mm2 and 5 mm thick were employed. They were superimposed one on top of the other, and measured with a Micrometric Screw (Mitutoyo Coolant Proof). This was reported (reading A). The was removed top glass, 0.1 mL of adhesive is placed in the center of the bottom plate and the top glass was placed once again in the same position in which the first measurement was made. They were placed centered beneath the charging device, previously calibrated and 150 N (15 kg) of vertical force was applied. After 10 minutes, the load (force) was removed and measured again, registering this measure (reading B) (Figure 4).
To report the film thickness, the thickness difference of the plates was recorded with and without adhesive film E=(reading B-reading A).
The statistical analysis was carried out using ANOVA (p=0.05) and the Tukey test (SPSS v.20).
ResultsThe results are contained in table I. Statistical analyses show that there is a statistically significant difference between the four groups.
GC Fuji Ortho LC presented higher sorption, Transbond XT and Enlight, absorbed less. (sig: 0.001)
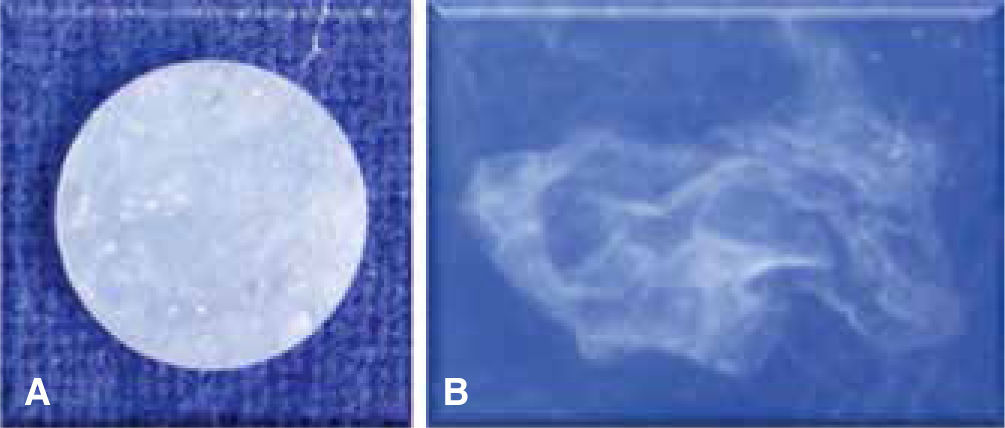
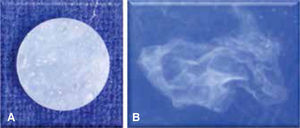
The negative solubility of GC Fuji Ortho LC and Enlight meant that they gained water. The specimens immersed in distilled water for six months at 37 °C of GC FujiOrtho LCdissolved (Figure 5) in contrast to the others. Enlight presented more film thickness, followed by Super C-Ortho and Transbond XT; being GC Fuji Ortho LC the one with the less film thickness (sig: 0.001).
DiscussionIn the line of research on orthodontic adhesives, the majority of the studies have been focused on performing only adhesion tests (resistance to debonding).3–26,28,32–61 None of these studies compares the results with the physical characteristics of the adhesive.
It was found that physical properties such as sorption, solubility and film thickness must have a direct effect on the mechanical behavior of orthodontic adhesives because when assessing only resistance to debonding it is not possible to determine which adhesive system is preferable to use on each clinical situation. It is necessary to know the chemical composition of these agents and relate it to their physical behavior. Those values must be interpreted because in general, there are discrepancies between the results of different studies regarding resistance to bracket debonding using resin reinforced glass ionomers with and without acid etching of the enamel.
GC Fuji LC presents more sorption because it has polyacrylic acid, a material that absorbs water. Super C-Ortho manufactured for the most part with polymethyl-methacrylate does not absorb much. Transbond XT and Enlight are formulated with diacrylates, so they absorb less. Clinically it would be expected that intraoral behavior is more stable in the last two adhesives.
The negative solubility of GC Fuji LC and Enlight is consistent due to its sorption, it does not dissolve in the short term, but after six months in immersion at 37 °C in distilled water, GC Fuji Ortho LC was solubilized almost entirely. Clinically, the loss of material might decrease the area of adhesion favoring debonding and accumulation of dental plaque increasing the tissue’s susceptibility to develop white lesions.
The film thickness of Enlight might indicate greater particle size. Super C-Ortho by being autocurable might not be consistent with the results since during manipulation, the polymerization starts, so it requires ability to manipulate. Working time, since the adhesive is autocurable, is short and hardens quickly, varying the thickness of film and predisposing to errors of bracket location during cementation and altering the prescription of the system.
The results show that it is preferable to use Transbond XT in closed meshes, Enlight when bracket retention is greater, Super C-Ortho for acrylic restorations and plastic brackets, and GC Fuji Ortho LC for patients with previous surface defects in the enamel, short treatments and need to release fluoride.
ConclusionsTransbond XT presented the lowest sorption, solubility and film thickness, being the most stable adhesive.
Enlight exhibited minimum sorption, negative solubility and greater film thickness, so it is preferred to use on brackets with wide retentions (Figure 6).
GC Fuji Ortho LC presented the highest sorption, negative solubility and less film thickness, so that it could be used in short treatments or need for fluoride release (Figure 7).
Super C-Ortho presented higher sorption, greater solubility and high film thickness, so it is preferred to use it bonding plastic brackets (Figure 6) or acrylic crowns (Figure 8).