Mineral trioxide aggregate (MTA) is a cement mainly used to seal tooth perforations; this is due to the fact that it hardens when in presence of humidity. It is composed of Portland cement and Bismuth trioxide.
ObjectiveTo analyze and compare with PIXE, DSC, TGA and DRX elementary chemical and phase composition of MTA Angelus® cement with a white Portland cement (WPC).
Material and methodsMTA Angelus® white and a white Portland cement were analyzed with PIXE in a particle accelerator, phase analyses were conducted with XRD contrasting peaks with those in the ICDD database. DSC was conducted in a calorimeter up to 900 oC.
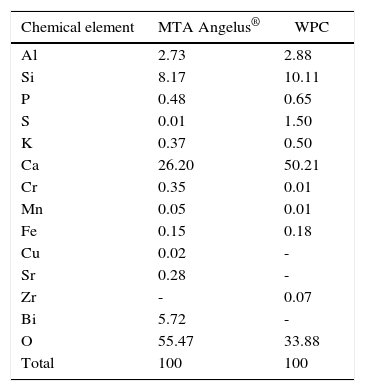
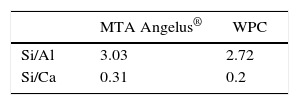
ResultsPIXE detected the following as greater percentage elements: aluminum, silica and calcium for both cements. Differences were found with sulfur percentages; Bismuth was only detected in MTA Angelus®. Trace elements of copper and strontium were detected in MTA Angelus® and zirconium in WPC. Relationship between silica-calcium and silica-aluminum was similar in both cements. In both cements, three crystalline phases were detected: dicalcium silicate, tricalcium silicate and tricalcium aluminate. Nevertheless, Bismite was identified in MTA Angelus® and calcium sulfate in the form of gypsum in WPC, this was corroborated with DSC technique.
ConclusionsIn MTA Angelus®, low gypsum amounts were observed by means of calorimetry. In both cements, crystalline phases and elemental chemical composition were similar.
El mineral trióxido agregado (MTA) es un cemento usado principalmente para sellar perforaciones en órganos dentales debido a que endurece en presencia de humedad, está compuesto por cemento Portland y trióxido de bismuto.
ObjetivoAnalizar y comparar por medio de PIXE, DSC, TGA y DRX la composición química elemental y de fases del cemento MTA Angelus® y de un cemento Portland blanco (CPB-CA).
Material y métodosMTA Angelus® blanco y un cemento Portland blanco fueron analizados con PIXE en un acelerador de partículas; el análisis de fases cristalinas se realizó por medio de DRX y contrastado los picos con los de base de datos del ICDD, el DSC se realizó en un calorímetro hasta 900 oC.
ResultadosPIXE detectó como elementos de mayor porcentaje fueron aluminio, silicio y calcio para ambos cementos; habiendo diferencias en los porcentajes de azufre; el bismuto sólo se detectó en MTA Angelus®. Se detectaron como elementos traza cobre y estroncio en el MTA Angelus®, además de zirconio en CPB-CA. La relación entre silicio-calcio y silicio-aluminio en los dos cementos es similar. Se identificaron tres fases cristalinas en ambos cementos, silicato dicálcico, silicato tricálcico y aluminato tricálcico; sin embargo, se identificó Bismita en el MTA Angelus® y sulfato de calcio en forma de yeso en CPB-CA, que se logró corroborar con la ayuda de la técnica DSC.
ConclusionesSe logró observar la baja cantidad de yeso en MTA Angelus® por medio de la calorimetría. Tanto las fases cristalinas como la composición química elemental son similares en ambos cementos.
Particle Induced X ray Emission
Scanning Differential Calorimetry
Thermogravimetry
X Ray Diffraction
Mineral Trioxide Aggregate
Emisión de Rayos X inducido por partículas
Calorimetría diferencial de barrido
Termogravimetría
Difracción de Rayos X
Mineral Trióxido Agregado
In 1995, Torabinejad1 first studied MTA as a material to be used to seal perforations within root and bifurcation canals in teeth. Other authors2,3 have studied MTA with respect to its physiochemical and mechanical properties, as well as its clinical applications4,5 and biological behavior.6 Since it has been reported that MTA composition is 80% Portland cement and 20% bismuth trioxide,7 many studies have been undertaken to compare physical, chemical and mechanical properties as well as clinical applications of MTA with Portland cement, considering a possible use in dentistry.8,9
One of the characterization techniques that have been used to conduct chemical elemental analysis of this material is Energy Dispersion Spectroscopy (EDS) also called X Ray Energy Dispersion Analysis (EDAX) adapted to a scanning electron microscope.9–13 This technique provides the elements that compose the material, this is achieved with x ray emission after having been irradiated by an electron beam. One of the disadvantages of this technique is that it is a punctual analysis thus, several readings at several locations of the sample must be achieved; moreover, detection limits are low.14
In 2009, Belio-Reyes and Bucio15 studied the element composition of MTA ProRoot by means of Particle Induced X-rays Emission (PIXE). Since this is a technique used to identify trace elements it counts with a detection limit of 0.1-1ppm. This technique is based on the detection of X-ray spectrum characteristic peaks and their quantification according to their different intensities. One of the advantages of this technique is that it represents a non-destructive, multi-elemental analysis.16
Phase analysis by means of X-ray Diffraction (XRD) is widely used to study crystalline materials. The technique is based on obtaining a diffraction pattern for an individual crystalline phase; where a set of peaks corresponds to a given intensity and 2θ specific diffractions.17 Several authors have used this technique in their studies of comparison between MTA and Portland cements.8,10,13,15,18
Many studies conducted on MTA are achieved with MTA ProRoot.10,12,15,18,19 Another brand is commercially available: MTA Angelus; this brand has not been the subject of many studies. The aim of the present study was to analyze and compare by means of PIXE, DSC, TGA, and XRD the elemental chemical composition and phases of MTA Angelus® (MTA-A) and a white Portland cement (WPC).
MATERIALS AND METHODSFor the present study MTA Angelus® was used (Angelus, Dental Products Manufacturer, Londrina, Brazil, lot 12394) and a white Portland cement (Cruz Azul, Mexico, Lot 033442).
Chemical element analysisPIXE was conducted in an Pelletron NEC accelerator (National Electrostatics Corp, Middletown WI) with a proton beam of 1mm diameter and 3MeV. The sample was placed in front of both detectors, one of Si(Li) for light elements and one of LEGe for trace and heavy elements, for a period of 10minutes. To gauge equipment, certified Portland cement was used: NISTSRM 1880a (National Institute of Standards and Technology).
Both results were processed with software (AXIL) tailored to detect all x-ray peaks characteristic of each element as well as conduct their quantification.
Crystalline phase analysisX-ray diffraction by means of the powder method was achieved in a Brunker D8 Advance (Brunker D8 Advance (Brunker AXS GmbH, Karlsruhe, Germany) radiation Cu Kα, λ=1.5405Å) data were obtained from 2θ=8 to 80 degrees. Afterwards, according to element analysis, obtained difractograms were compared to PDF (Power Diffraction Files) database so as to find the group of peaks which would coincide among experimental data and some known phase within ICDD (International Center of Diffraction Data).20
Thermal analysisDifferential scanning calorimetry (DSC) and thermogravimetry (TGA) were conducted in TA calorimeter (Instrument Calorimetry (SDT Q600, United States), from a room temperature up to 900o, with a rate of heating of 10 oC/min in an air atmosphere. Samples were placed in an alumina crucible, so as to obtain suitable calcium sulfate identification based on hydration degree, pure Naica gypsum was used as a reference.
RESULTSFigure 1andTable I present PIXE results. This analysis reveals that in both cements, elements found in greater percentage are aluminum, silica and calcium. Among them notable difference in Sulfur percentages was found, whereas Bismuth was only detected in MTA Angelus®. As trace elements, Copper and Strontium were found in MTA Angelus® and Zirconium in WPC. Relationship between Silica-Calcium and Aluminum -Calcium was obtained from a coefficient among percentages of each one of these elements (Table II).
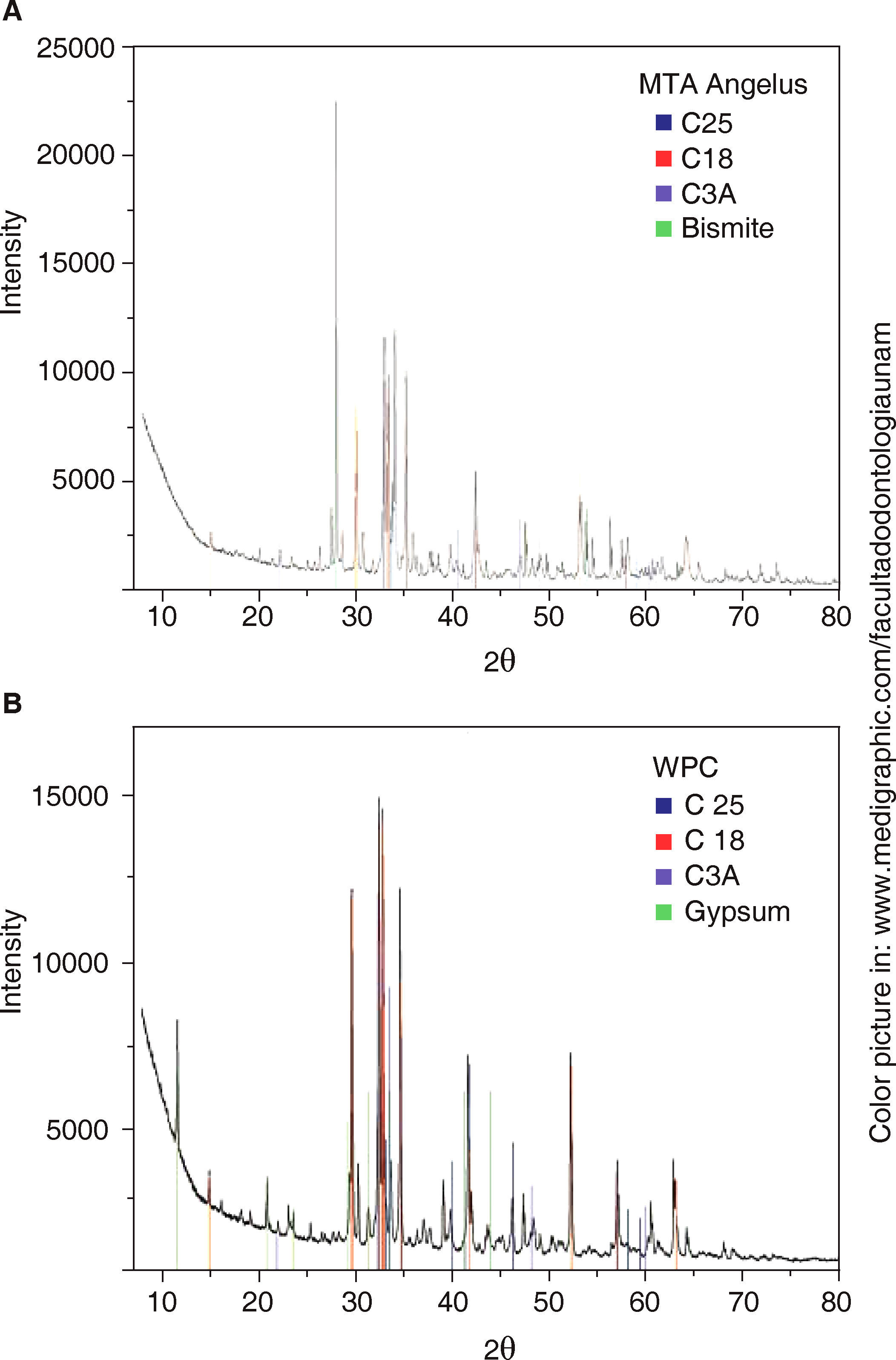
Obtained diffraction patterns are presented in figure 2.
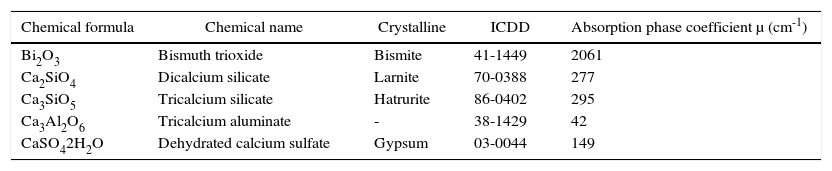
Analysis of MTA-A and WPC phases indicates they are mainly composed of three similar phases: dicalcium silicate «C2S» (PDF: 70-0388) with the highest reflections of angles 2θ 32.07, 32.2, 32.62 and 34.41; tricalcium silicate «C3S» (PDF: 86:38-1429) with highest peaks at 29.35, 32.19 34.35 and 41.29; and the last phase, tricalcium aluminate «C3A» (PDF: 38-1429) with highest peaks at 33.16, 47.62 and 59.27. Bismite phase (PDF: 41-1449) with angles 2θ at 26.92, 27.37 and 33.03 was only detected in MTA-A; on the contrary, calcium phosphate in the form of gypsum (PDF: 030044) was detected in WPC, with more pronounced reflections at 11.64, 20.73 and at 43.47. Table III shows absorption coefficients of detected mineral phases.
Absorption coefficient of components identified in studied cements.
| Chemical formula | Chemical name | Crystalline | ICDD | Absorption phase coefficient μ (cm-1) |
|---|---|---|---|---|
| Bi2O3 | Bismuth trioxide | Bismite | 41-1449 | 2061 |
| Ca2SiO4 | Dicalcium silicate | Larnite | 70-0388 | 277 |
| Ca3SiO5 | Tricalcium silicate | Hatrurite | 86-0402 | 295 |
| Ca3Al2O6 | Tricalcium aluminate | - | 38-1429 | 42 |
| CaSO42H2O | Dehydrated calcium sulfate | Gypsum | 03-0044 | 149 |
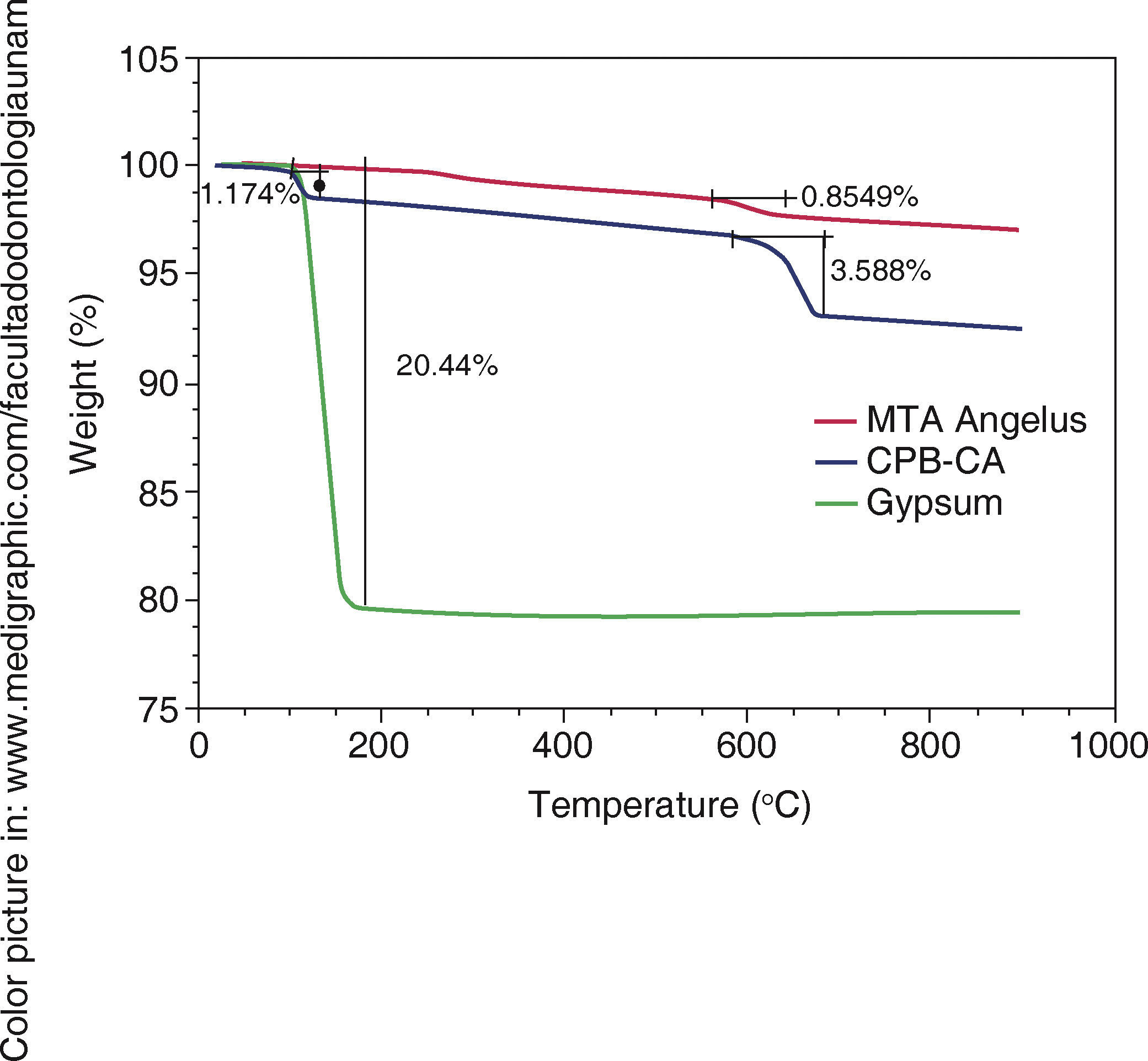
TGA results in WPC indicated weight loss at 100 oC, this loss could be attributed to water evaporation of bi-hydrated calcium sulfate or gypsum. Samples were compared with Naica pure gypsum (Figure 3).
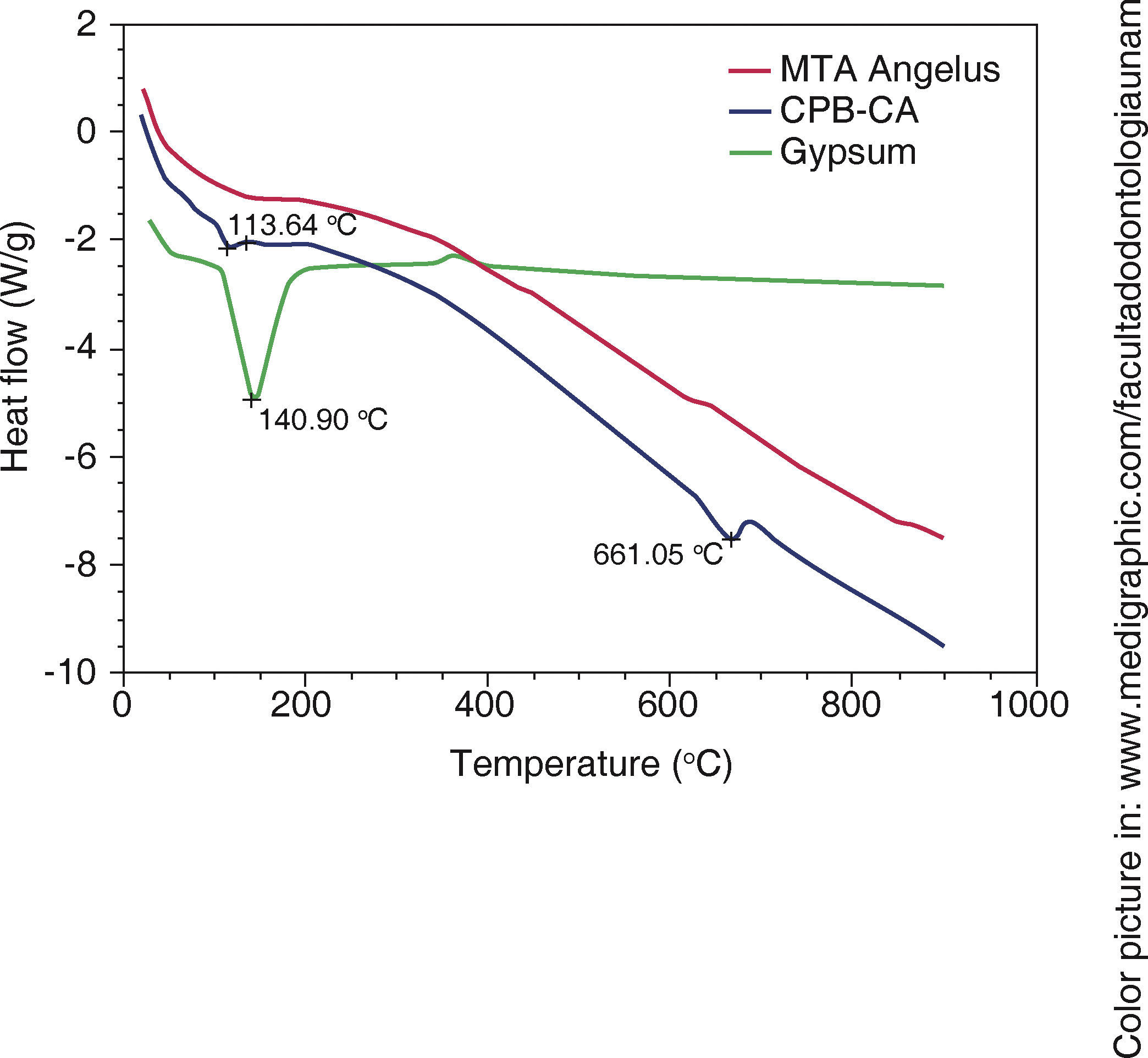
DSC showed thermal reaction at around 113 oC in WPC and at 141o in Naica gypsum, nevertheless, this endothermic flow was not observed in MTA Angelus®, therefore it was concluded it did not contain calcium sulfate (Figure 4).
DISCUSSIONWithin the realm of analyses performed with EDS, Oliveira9 found that, when comparing ProRoot MTA, MTA Angelus® and a Portland cement, chemical elements were very similar, with minimal variations, with exception of Bismuth which was only detected in MTA. This behavior was equally observed in our study. It must be remembered that Bismuth is only aggregated with the aim of manufacturing a radioopaque material which can be clinically detected in the practice of dentistry. In a similar manner, and following this technique, Asgary12 conducted a comparison of components amongst different types of MTA and Portland cements. In that study it was observed that trace element concentrations were very varied with respect to amounts of Al2O2, MgO and FeO. Use of nuclear techniques for analyses of materials such as PIXE allows the rapid identification of low concentration elements, thus presence of Chrome, Manganese, Strontium, Copper and Zirconium was detected, with precise quantification in all of them. In the case of MTA Angelus, when using PIXE it was difficult to identify presence of Sulfur, this is due to the fact that sulfur's X-rays K α peaks (2.3 keV) overlap to Bismuth's Mα (2.4keV) peaks.
Camilleri13 and García Aranda21 analyzed Portland cements for a probable dental use; results of that research revealed that in all cements, the main elements are Calcium, Silica and Aluminum, with some traces of magnesium, potassium and sodium. Meanwhile, García Aranda and Rodríguez,22 when studying MTA cements, detected trace elements such as phosphorus, chrome, chlorine and iron, even though the cements were white. These results concur with results of the present study, since, when using PIXE as technique for element analysis, the following elements were detected: chrome, zirconia, iron, and manganese in WPC and MTA Angelus® besides copper and strontium in this latter one. Presently, many of the materials used in dentistry have received an aggregation of ZrO2 as filling material with the aim of improving their mechanical properties, or as radio-opaque material.24 This leads the way for further studies to find relationship between the presence of this element and the improvements of some properties of the cement.
Belio-Reyes and Bucio15 conducted an XRD analysis; they detected the following as main phases through a Rietveld refining technique: Bismite, Hatrurite, Larnite and Anhydrite in respective percentages of 19.8, 51.9, 23.2 and 1.3%. The three first phases were also detected in our analysis for MTA-A. Calcium sulfate phase in Portland cement was different, since gypsum was found as part of the basic composition. Following this technique, Camillieri13 also studied different Portland cements; he observed that main phases detected in white Portland cement were tricalcium silicate (31-0301) and dicalcium silicate (310299). The present study concurs with those detected mineral phases, nevertheless, they presented different crystalline structure; additionally, we observed absence of calcium sulfate in MTA Angelus®, and this must be reflected in the cement's setting time,23,24 since it is known that presence of gypsum in cements avoids the reaction known as «flash set» or «rapid setting». XRD is an excellent technique to identify mineral phases in poly-crystalline ceramic materials. In an ideal situation, this study would be supplemented with a quantification of found phases performed with the Rietveld method.
CONCLUSIONWith the methodology used in the present study it can be concluded that both materials possessed similar chemical composition; Calcium, Silica and Aluminum were the elements found in higher percentage. Detected Iron amounts were very low, since both materials were white cements; ferrite phase was not present as would be the case in grey cements. Differing from WPA, very low amounts of Sulfur were detected in MTA Angelus®.
By means of XRD it was observed that the most noticeable differences were absence of calcium sulfate in MTA Angelus® as well as bismite phase in WP. Mineral phases present in both cements were dicalcium silicate, tricalcium silicate and tricalcium aluminate.
Resident, PhD of Dental Biomaterials Laboratory Science of the National University of Mexico (UNAM).
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam




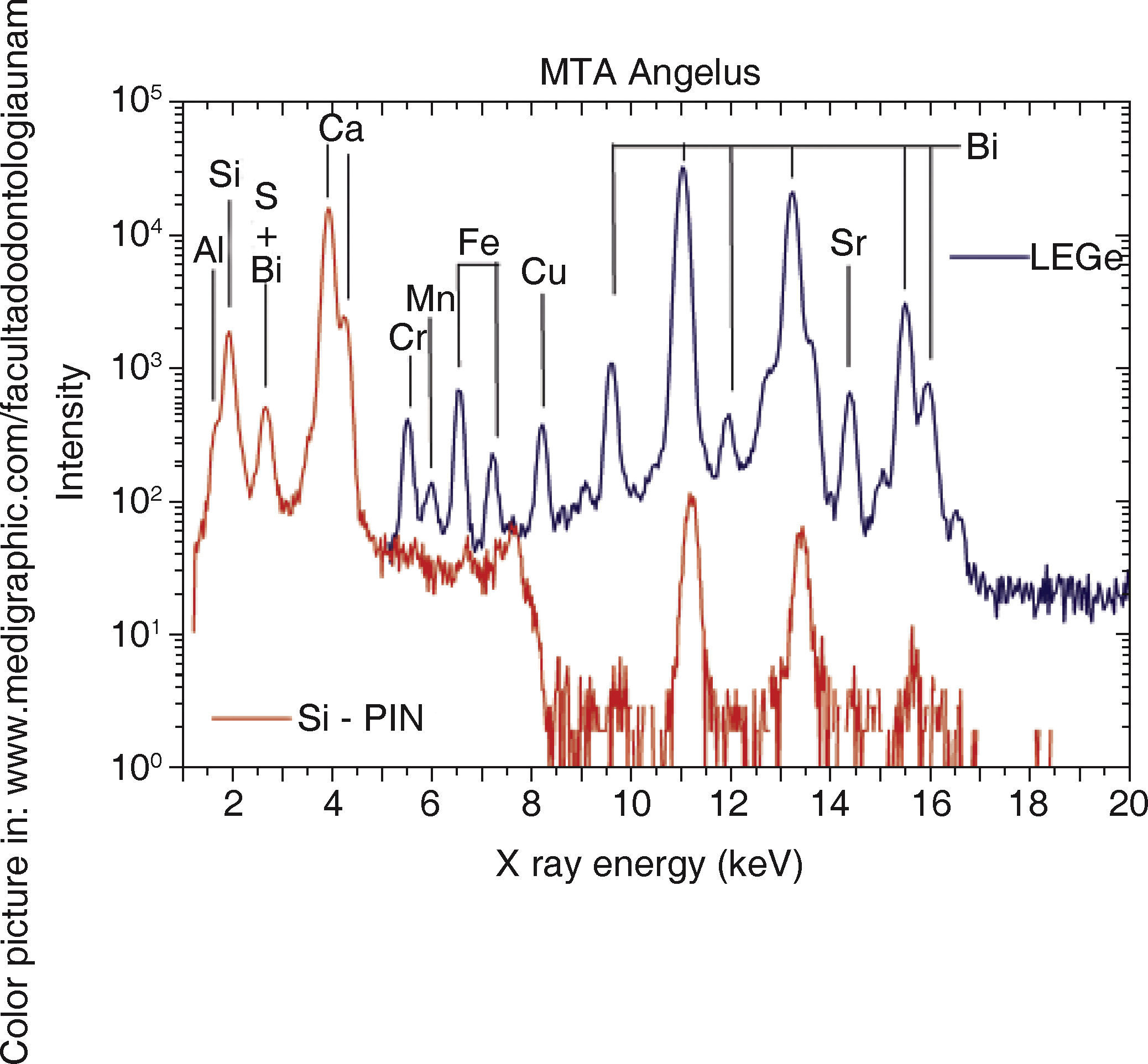
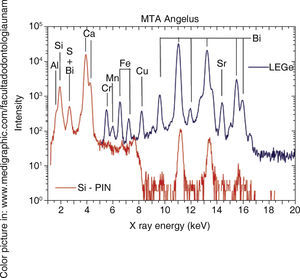 PIXE spectrum of
PIXE spectrum of 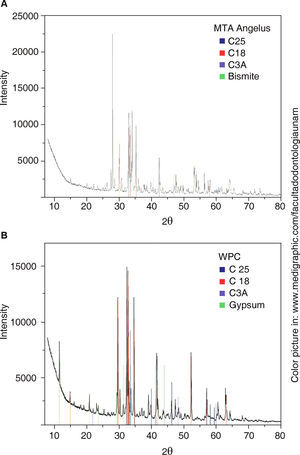 XRD) A)
XRD) A) 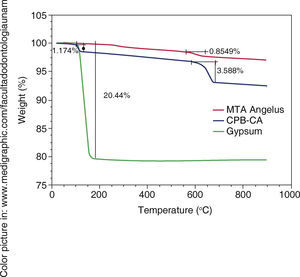 TGA). Green line represents weight loss associated to water in gypsum, a 1.17WT% water loss associated to WPC-CA was observed,
TGA). Green line represents weight loss associated to water in gypsum, a 1.17WT% water loss associated to WPC-CA was observed, 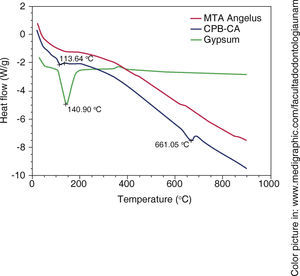 DSC). The green line shows changes in heat flow associated to gypsum's superficial water, it is minimally observed in WPCCA (blue) nevertheless, this change is not observed in
DSC). The green line shows changes in heat flow associated to gypsum's superficial water, it is minimally observed in WPCCA (blue) nevertheless, this change is not observed in