To ascertain inflammatory response through interleukin 1β presence and identify pathogenic microorganisms as possible immunological and microbiological markers in diagnosis and treatment non-surgical periodontal in patients with gingivitis and moderate chronic periodontitis in a sample of Mexican population.
Material and methodsIn the present prospective cohort study, 18 patients with signs of gingivitis and 17 patients with moderate chronic periodontitis were selected. Samples of subgingival biofilm and of crevicular gingival fluid were collected. Interleukin 1β was quantified during the pre-treatment, post-treatment and maintenance phases of the non- surgical periodontal treatment. Continuous variables were analyzed with the Student test, as well as categorical variables which were analyzed with the Turkey-Kramer test. For independent groups the Pearson test was used.
ResultsMicrobiological response variables showed that Porphyromonas gingivalis Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans significantly decreased in subjects with gingivitis, Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans and Actinomyces ssp. decreased in cases. Biochemical response variables showed significant decrease in IL-1β concentration and total count in individuals with moderate chronic periodontitis in treatment maintenance phase. The same result applied to clinical response variables.
ConclusionsThere is a decrease in Interleukine 1β levels with decrease in microflora. Interleukin 1β are sensitive markers for diagnosis of periodontal disease and assessment of its severity.
Conocer la respuesta inflamatoria a través de la presencia de interleucina 1β e identificar microorganismos patógenos como posibles marcadores inmunológicos y microbiológicos en el diagnóstico y tratamiento periodontal no quirúrgico en sujetos con gingivitis y periodontitis crónica moderada en población mexicana.
Material y métodosEn este estudio prospectivo de cohortes, se seleccionaron 18 pacientes con signos clínicos de gingivitis y 17 pacientes con periodontitis crónica moderada, se recolectaron las muestras de biopelícula subgingival y de fluido gingival crevicular. Se cuantificó la interleucina 1β durante las fases pretratamiento, postratamiento y de mantenimiento del tratamiento periodontal no quirúrgico.
ResultadosLas variables de respuesta microbiológica mostraron que Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans disminuyeron significativamente en individuos con gingivitis. Así como Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans y Actinomyces sp. en periodontitis crónica moderada. Las variables de respuesta bioquímica mostraron una disminución significativa en la concentración y cuenta total de interleucina 1β en los individuos con periodontitis crónica moderada en la fase de mantenimiento del tratamiento así como de las variables de respuesta clínica.
ConclusiónHay reducción de los niveles de interleucina 1β con la disminución de la microflora. Los niveles de interleucina 1β son marcadores sensibles para el diagnóstico y severidad de la enfermedad periodontal.
log
μL
mL
pg
%
rpm
sec
Antigen presenting cells.
Nα-Benzol-DL-Arginineβ-Naphtilamide.
Bone morphogenic proteins.
Gingival epitelial cells.
Crevicular gingival fluid.
Gamma interferon.
Interleukin.
Interleukin 1 alpha.
Interleukin 1 beta.
Accessory protein of receptor to IL-1.
Antagonist receptor.
Type I receptor to IL-1.
Type II receptor to interleukin 1.
Nitric oxide oxidase.
B lymphocyte.
T lymphocyte.
Bacterial lipopolysaccharide.
Main histocompatibility complex.
Matrix metalloproteinases.
Natural leukocytes of «natural killers».
Pathogen-associated molecular patterns.
Prostaglandin E2.
Prostaglandin I2.
Polymorphonuclear leukocytes.
Root scaling and planing.
T cell receptors.
Cooperative T lymphocyte.
Cooperative T lymphocyte 1.
Cooperative T lymphocyte 2.
Inhibitor factors of matrix metalloproteinases.
Alpha tumoral necrosis factor.
Beta tumoral necrosis factor.
Colony forming units.
Vascular cell adhesive molecule.
Within the scope of periodontal disease two entities are distinctive, and they possess clearly defined phenotypes: gingivitis (G) and periodontitis (P). These conditions can be clinically observed through chronic inflammatory processes, although in one case (periodontitis) this process evolves and destroys the periodontal attachment apparatus, and in the other case (gingivitis) the inflammatory process is maintained with no evolution towards destruction.1–6 This inflammatory and immune response is determined by the presence of periodontal pathogens which are Gram negative anaerobic bacteria involved in the subgingival biofilm1,7–11 such as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans (Ac), Tannerella forsythia (Tf), Prevotella intermedia (Pi), Fusobacterium nucleatum (Fn), Parvimonas micra (Pm), Campylobacter rectus (Cr) and Actinomyces ssp.11–16 as well by biological modifying factors such as: systemic factors, genetic factors17,18 and behavioral factors such as oral hygiene, smoking habits and stress.19 In periodontal attachment tissue, inflammatory response comes forth with great amounts of neutrophil polymorphonuclear leukocytes (PMNs) and macrophages with phagocytosis and destructive functions in the sites of bacterial interaction with tissue surface, inciting the presence of inflammatory infiltrate, the activation of the immune system, complement cascade and the cytokine production system.10,20,21 Most of the substances released by inflammatory and immune cells concentrate in an exudate, characteristic of inflammatory processes observed in G and moderate chronic periodontitis (MCP). This substance (fluid) is called crevicular gingival fluid (CGF). In this fluid it is possible to identify the following pro-inflammatory cytokines: 1-beta interleukin (IL-1β) as well as alpha tumor necrosis factor (TNF-α). Both cytokines are mediators of the inflammatory process because they modulate the extracellular component of bone and connective tissue. In periodontal disease they show high CGF levels, therefore they can have diagnostic interest in G and MCG, since they can be associated to the active phase of these conditions.22–25
The aim of the present study was to contribute to the knowledge of microbial flora in this sample of Mexican population and assess links between microbial flora and immunological response in G and its progression to MCP. A prospective cohorts study was conducted to endeavor to find differential aspects with respect to etiological factors and host response. For this purpose quantification of IL-1β in the CGF was used as immunological marker and to examine the effect of NSPT at the pre-treatment, post-treatment and maintenance phases.
MATERIAL AND METHODSPatient selection18 patients were selected with clinical and radiographic diagnoses of G (evidence of gingival inflammation, volume increase of the gums, redness and haemorrhage when probing, without loss of epithelial attachment) and 17 patients with MCP (loss of insertion in three or more sites in all quadrants, with pocket depth of 5-7mm in three or more sites, radiographic evidence of bone loss at a half of the root length in three or more sites of all quadrants, bleeding when probing in three or more sites of each quadrant). These patients attended the San Lorenzo and Tepepan Stomatological Clinics, attached to the Metropolitan Autonomous University, campus Xochimilco, in Mexico City. All patients granted informed consent, had not received previous periodontal treatment, and participated of their own volition in the study. Mean time between sampling was 12 months.
Study outlineAfter an initial screening visit for recruitment, during the first eight weeks, baseline measurements were recorded and samples taken. Subsequently, hygiene instruction was undertaken and NSPT (scaling and root planing) (SRP) was performed. Patients were reassessed two months after the SRP procedure. On the following visit, post treatment clinical measurements were recorded, and microbiological and immunological samples were taken from the same sites. Patients were then reassessed six months later, this was considered the maintenance phase visit. At this point, clinical measurements were recorded and microbiological and immunological samples were taken from the same sites.
Clinical measurements and samplingFour sites were selected in each G and MCP patient, whenever possible, one site was selected in each quadrant. The following tests were conducted and recorded: Quigley and Hein plaque index (PI) modified by Turkey. Löe and Silness gingival index (GI), probing of sulcus depth (PSD), probing of pocket depth (PPD) and clinical attachment loss (CAL).26,27 Each tooth was PI determined, GI assessed; pocket depth and attachment loss were measured at each site using the f-6 pgf Hu-Friedy probe. After the clinical measurements were recorded, a supragingival plaque sample was obtained from each site using sterile curettes. Each tooth was isolated with sterile gauze and then air-dried. A sub-gingival plaque sample was obtained with two paper points, introduced for 10 seconds each, following the Mombelli technique.
Each sample was immediately placed in a sterile Eppendorf tube containing reduced transport fluid (RTF) (Herrera GD, Stijine-van NA, Bosch TCJ, Dentokom MEC, Boersme H, Zeiler G, Winkelhoff AJ. [1998]). Microbiological Procedures with Periodontal Anaerobic Bacteriae. Working protocol, Microbiology Laboratory, School of Dentistry, UCM, Spain.28
And was processed within the two hours after recollection. Lastly, GCF was collected using paper strips, in place for 10seconds each.22,29,30 Each sample was immediately placed in a sterile Eppendorf tube at -70° C until analyzed. The volume of the fluid was determined using a Periotron® 8000 and a previously constructed calibration curve.
Microbiological sample processingA vortex-mixed sub-gingival plaque was dispersed for 30seconds. Dilution was performed from the initial sample. Four successive dilutions were carried out at the tenth part with phosphate buffering solution (PBS) at neutral pH. 0.1/ mL of the diluted sample was inoculated over the plaques containing agar-blood (non-selective) media; incubation was performed at 37° C. P. gingivalis, P. intermedia, P micra, F. nucleatum, T. forsythia, C. rectus and Actinomyces sp. were grown on fastidious anaerobe agar, and harvested after seven days. A fifteen-day period was observed for slow growing species soy agar vancomycin bacitracin (TSBV) (selective media). Incubation was carried out at 37° C. A. actinomycetemcomitans was grown in 5% carbon dioxide atmosphere, and harvested after five days (Herrera GD, Stijine-van NA, Bosch TCJ, Dentokom MEC, Boersme H, Zeiler G, Winkelhoff AJ. [1998]). Microbiological Procedures with Periodontal Anaerobic Bacteriae. Working protocol, Microbiology Laboratory, School of Dentistry, UCM, Spain, UCM, Spain.28 Colony morphology was carried out, as well as optical microscopy, Gram stain and BIG, APY, ZYM biochemical tests for bacterial identification were equally performed.30–32
Analysis of GCF samplesGCF samples were eluted into incubation buffer (PBS) at 7.4 pH on a rotary mixer. Paper strips were discarded. 50μL of GCF aliquot was used by IL-1β, analysis was measured using the enzyme-linked immune-sorbent assay (ELISA) by according to the method recommended by the manufacturer. Quadratic regression was calculated to obtain formulae and graphs which translate capacitance units of digital readings of each paper strip, from the Periotron® 8000 measuring device to indicate μL volume. (Norma Oficial Mexicana-Ensayos Clínicos NOM-013-SSA2-1994 y el Comité de Bioética de la UAM Xochimilco.) The guides ethical to the cohort study was the Mexican Official Statement of Clinical Assay and the Bioethic Comite UAM Xochimilco.
STATISTICAL ANALYSIS OF DATATo perform analysis for differences to be found among groups of the G group when compared to the MCP group for continuous variables, the t Student test was calculated. For categorical variables the Tukey-Kramer test was used with a p < 0.0001 between pretreatment, post-treatment and maintenance phase of each studied condition. To analyze differences among groups in independent G groups versus MCP, the Pearson test was applied with a p α 0.05 for all three phases.
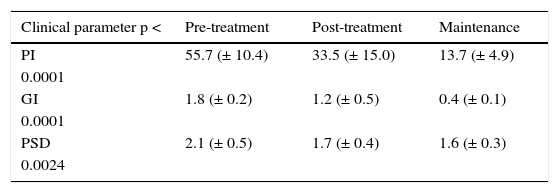
RESULTSVariable clinical responsesThe clinical parameters in the pre-treatment, post-treatment and maintenance phases, in the SRP in G and MCP is shown in tables I and II. NSPT resulted in significant reduction in PSD in the G group and PPD in the MCP group with a p < 0.0001, difference found among the groups was p α 0.03 and CAL in MCP group p < 0.0001. The PI in G and MCP group with p < 0.0001 and inter-group comparison was p α 0.03. Gingival Index in group G and MCP with a p < 0.0001 with inter-group difference of p α 0.03.
Clinical variables in gingivitis.
| Clinical parameter p < | Pre-treatment | Post-treatment | Maintenance |
|---|---|---|---|
| PI | 55.7 (± 10.4) | 33.5 (± 15.0) | 13.7 (± 4.9) |
| 0.0001 | |||
| GI | 1.8 (± 0.2) | 1.2 (± 0.5) | 0.4 (± 0.1) |
| 0.0001 | |||
| PSD | 2.1 (± 0.5) | 1.7 (± 0.4) | 1.6 (± 0.3) |
| 0.0024 |
Clinical variables after non surgical periodontal treatment in G. For clinical variables the following were considered: plaque index (PI), gingival index (GI), probing sulcus depth (PSD) in the three phases of the non surgical treatment in subjects with gingivitis.
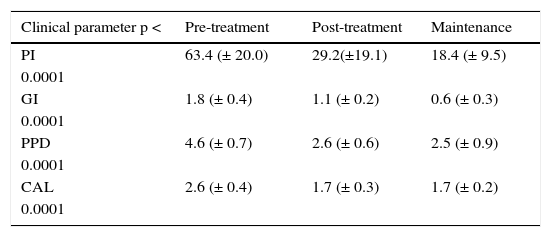
Clinical variables in moderate chronic periodontitis.
| Clinical parameter p < | Pre-treatment | Post-treatment | Maintenance |
|---|---|---|---|
| PI | 63.4 (± 20.0) | 29.2(±19.1) | 18.4 (± 9.5) |
| 0.0001 | |||
| GI | 1.8 (± 0.4) | 1.1 (± 0.2) | 0.6 (± 0.3) |
| 0.0001 | |||
| PPD | 4.6 (± 0.7) | 2.6 (± 0.6) | 2.5 (± 0.9) |
| 0.0001 | |||
| CAL | 2.6 (± 0.4) | 1.7 (± 0.3) | 1.7 (± 0.2) |
| 0.0001 |
Clinical variables after non surgical periodontal treatment in MCP the following were considered as clinical variables: plaque index (PI), gingival index (GI), probing pocket depth (PPD), clinical attachment level (CAL) in the three phases of non surgical treatment in subjects with moderate chronic periodontitis.
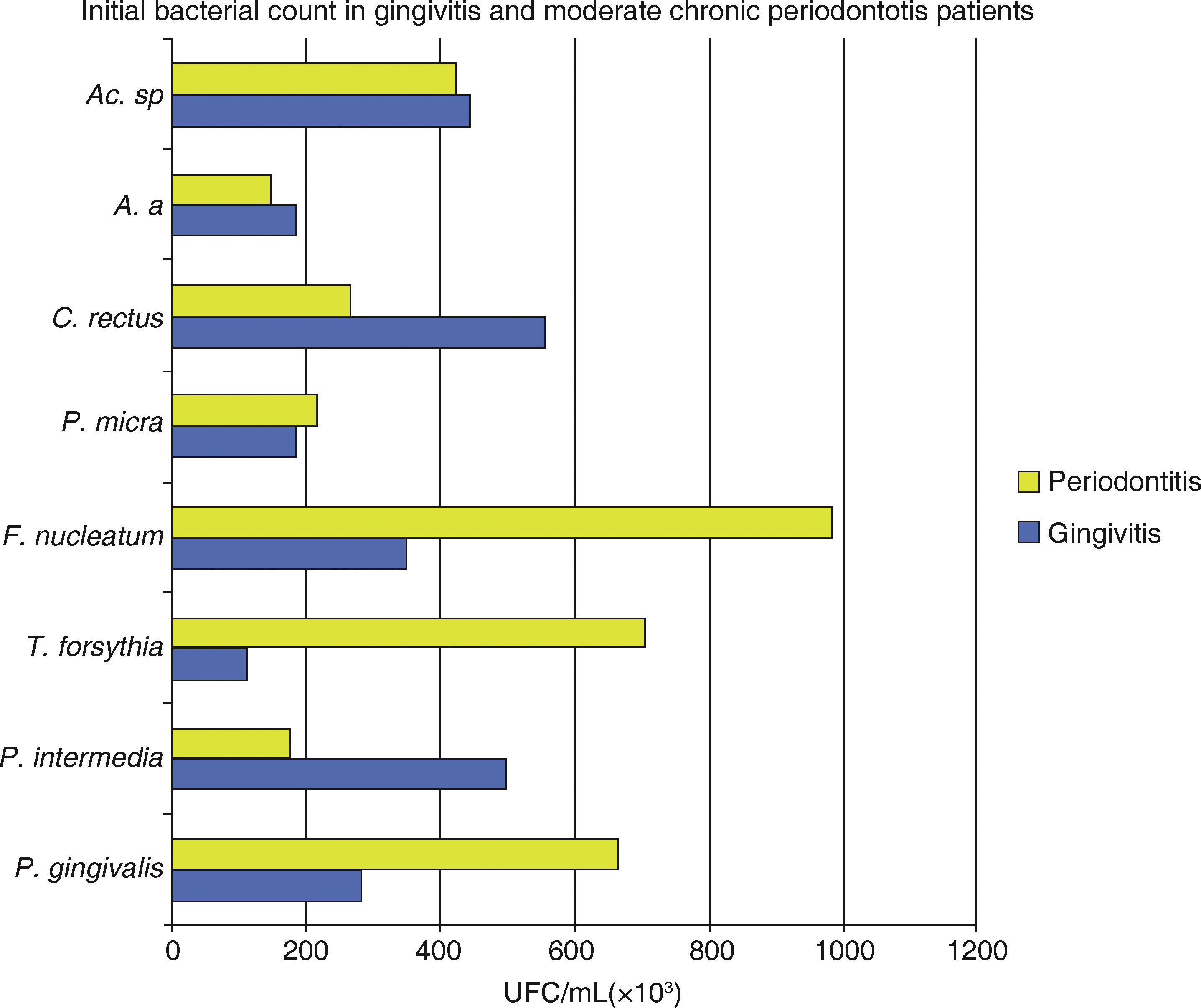
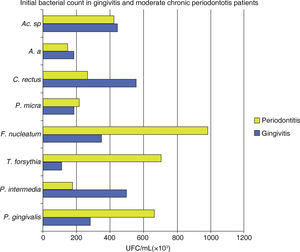
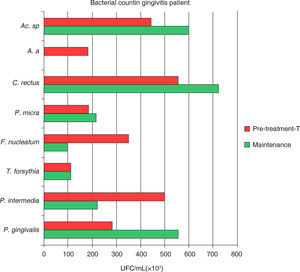
In both groups it was observed that all assessed species presented a decrease in the total bacterial count (UFC/mL × 103). A statistically significant difference was observed between among treatment phases with a p < 0.0001, with inter-group difference of p α 0.02 (Figure 1andTable III).
Mean total bacterial count of UFC/ mL of eight bacterial species in samples taken from 18 gingivitis patients and 17 moderate chronic periodontitis patients. Attention must be brought to the fact that the highest bacterial count in gingivitis was P. intermedia, C. rectus and Actinomyces sp. in periodontitis it was P. gingivalis, T. forsythia and F. nucleatum.
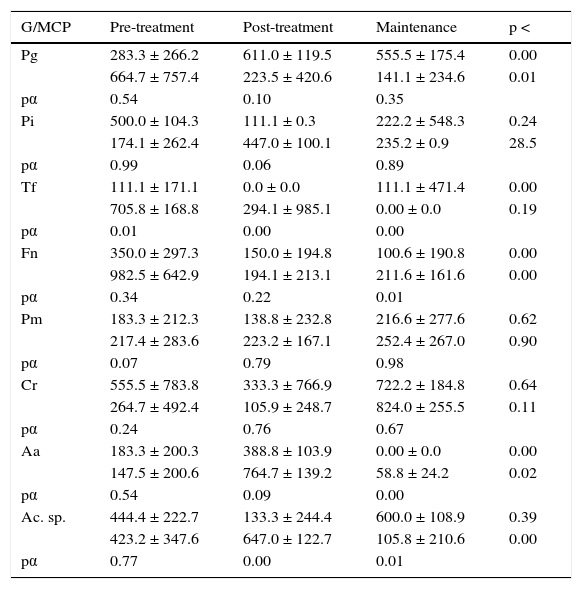
Microbiological data for each species.
| G/MCP | Pre-treatment | Post-treatment | Maintenance | p < |
|---|---|---|---|---|
| Pg | 283.3 ± 266.2 | 611.0 ± 119.5 | 555.5 ± 175.4 | 0.00 |
| 664.7 ± 757.4 | 223.5 ± 420.6 | 141.1 ± 234.6 | 0.01 | |
| pα | 0.54 | 0.10 | 0.35 | |
| Pi | 500.0 ± 104.3 | 111.1 ± 0.3 | 222.2 ± 548.3 | 0.24 |
| 174.1 ± 262.4 | 447.0 ± 100.1 | 235.2 ± 0.9 | 28.5 | |
| pα | 0.99 | 0.06 | 0.89 | |
| Tf | 111.1 ± 171.1 | 0.0 ± 0.0 | 111.1 ± 471.4 | 0.00 |
| 705.8 ± 168.8 | 294.1 ± 985.1 | 0.00 ± 0.0 | 0.19 | |
| pα | 0.01 | 0.00 | 0.00 | |
| Fn | 350.0 ± 297.3 | 150.0 ± 194.8 | 100.6 ± 190.8 | 0.00 |
| 982.5 ± 642.9 | 194.1 ± 213.1 | 211.6 ± 161.6 | 0.00 | |
| pα | 0.34 | 0.22 | 0.01 | |
| Pm | 183.3 ± 212.3 | 138.8 ± 232.8 | 216.6 ± 277.6 | 0.62 |
| 217.4 ± 283.6 | 223.2 ± 167.1 | 252.4 ± 267.0 | 0.90 | |
| pα | 0.07 | 0.79 | 0.98 | |
| Cr | 555.5 ± 783.8 | 333.3 ± 766.9 | 722.2 ± 184.8 | 0.64 |
| 264.7 ± 492.4 | 105.9 ± 248.7 | 824.0 ± 255.5 | 0.11 | |
| pα | 0.24 | 0.76 | 0.67 | |
| Aa | 183.3 ± 200.3 | 388.8 ± 103.9 | 0.00 ± 0.0 | 0.00 |
| 147.5 ± 200.6 | 764.7 ± 139.2 | 58.8 ± 24.2 | 0.02 | |
| pα | 0.54 | 0.09 | 0.00 | |
| Ac. sp. | 444.4 ± 222.7 | 133.3 ± 244.4 | 600.0 ± 108.9 | 0.39 |
| 423.2 ± 347.6 | 647.0 ± 122.7 | 105.8 ± 210.6 | 0.00 | |
| pα | 0.77 | 0.00 | 0.01 |
Microbiological data consist on UFC/mL (× 103) expression for each species obtained during the three phases of the non-surgical periodontal treatment in 18 subjects with gingivitis and 17 subjects with moderate chronic periodontitis, P. gingivalis (Pg), P. intermedia (Pi), T. forsythia (Tf), F. nucleatum (Fn), Parvimonas micra (Pm), A. actinomycetemcomitans (Aa) y Actinomyces sp. (Ac. sp.).
The behaviour of studied species Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Fusobacterium nucleatum (Fn), shows a bacterial count (UFC/mL × 103) greater in subjects in initial phase periodontitis Prevotella intermedia (Pi), and Campylobacter rectus (Cr), presented a greater bacterial count in subjects with gingivitis at the same phase.
Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa) and Actinomyces sp. (A.sp) manifest a statistically significant reductions after treatment, in maintenance phase a lower bacterial count in MCP patients, Prevotella intermedia (Pi), Fusobacterium nucleatum (Fn) and Aggregatibacter actinomycetemcomitans (Aa) manifested a lower count in gingivitis patients, twelve months after treatment, during the maintenance phase.
After NSPT gingivitis patients showed positive Porphyromonas gingivalis without manifestation of significant decrease and nevertheless revealing a 49% increase in the UFC, as shown in figure 1 to 3.
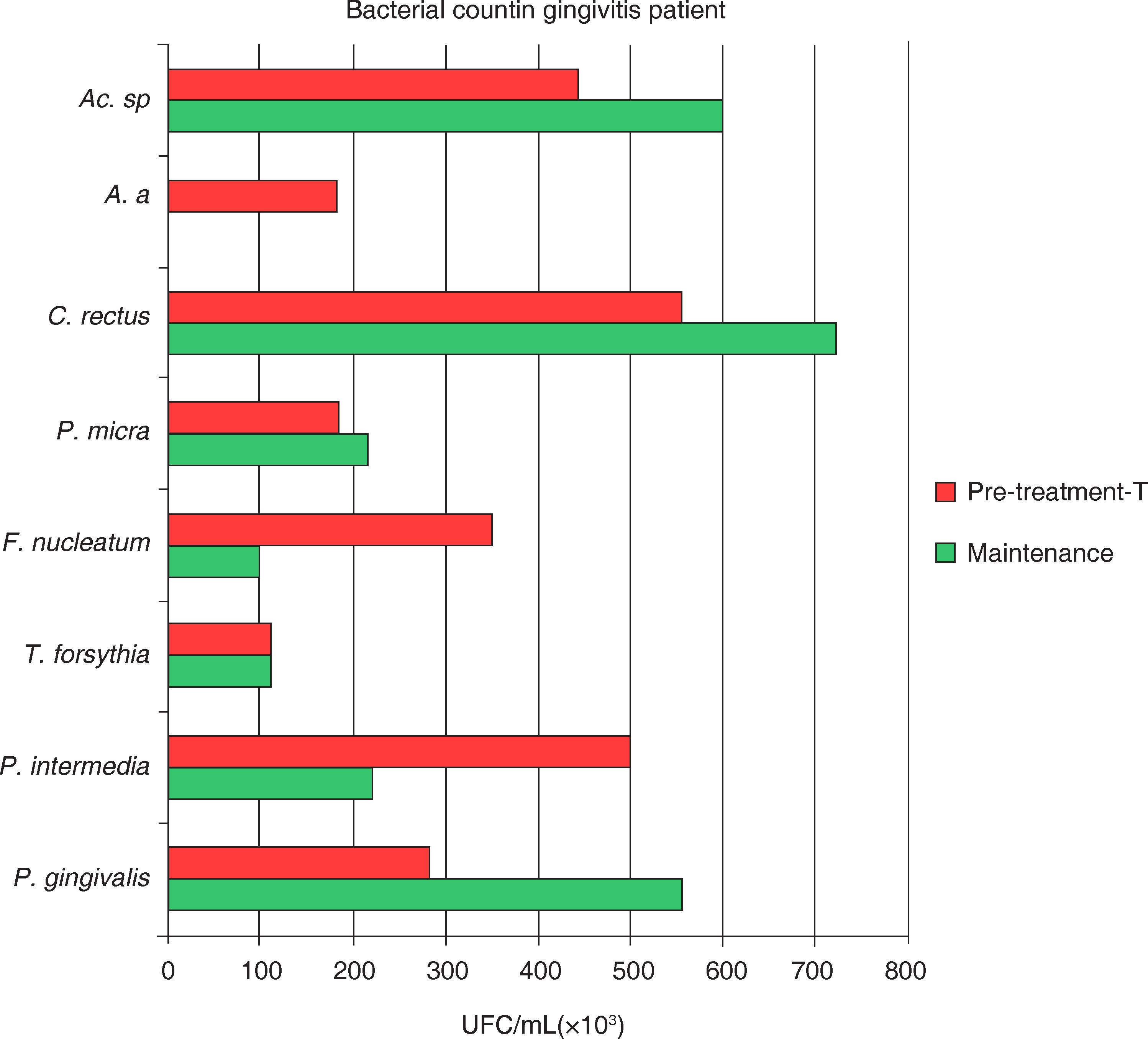
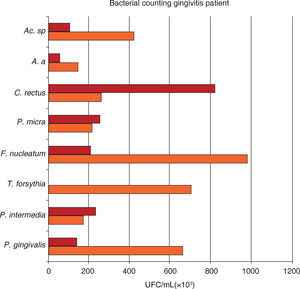
Mean total bacterial count of UFC/mL of eight bacterial species in samples taken from 18 gingivitis subjects. Samples were obtained at initial and maintenance phases of the periodontal treatment. Attention must be brought to the fact that the higher bacterial count in gingivitis corresponded to P. gingivalis, C. rectus, and Actinomyces sp. during the maintenance phase.
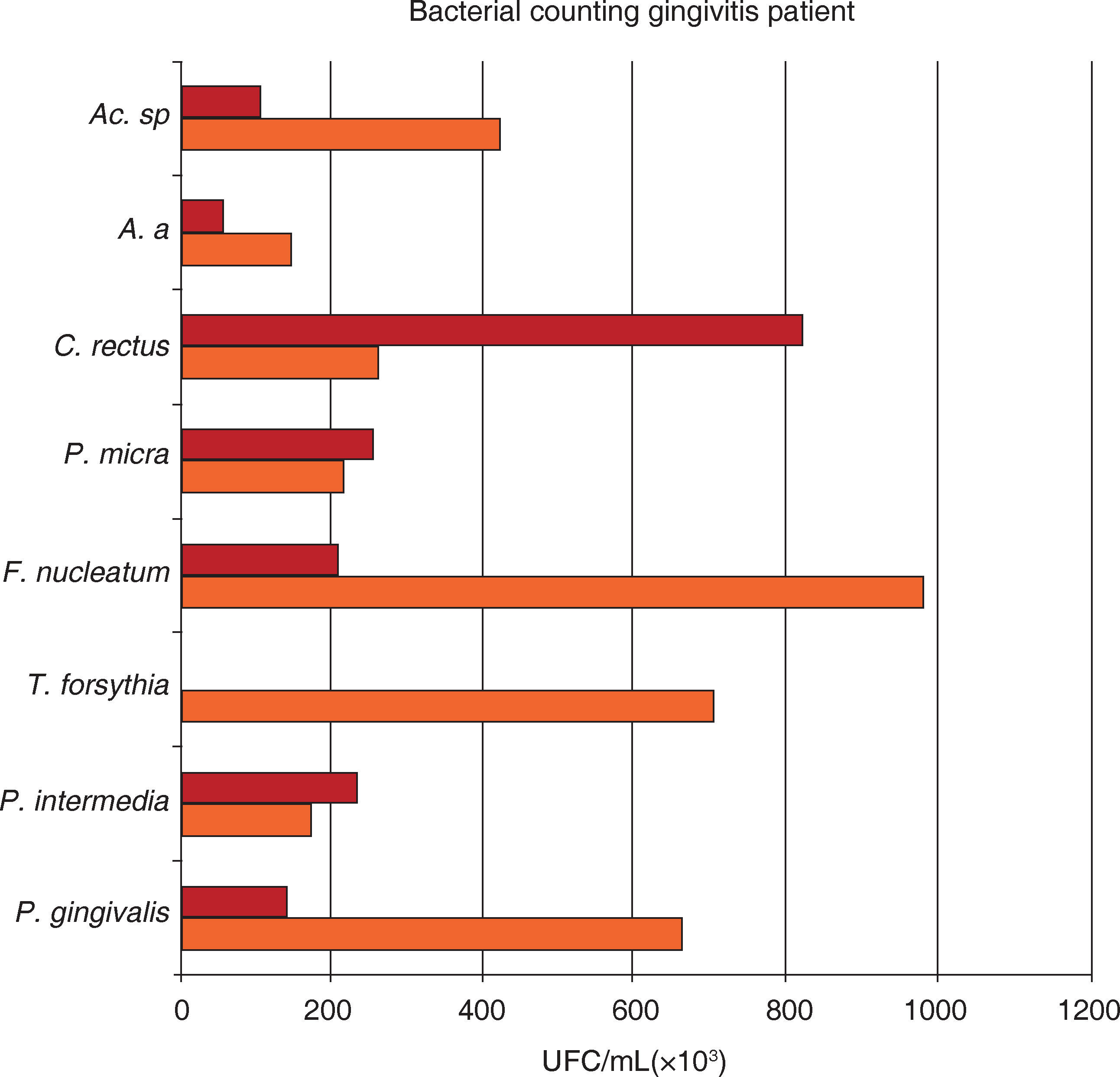
Mean total bacterial count of UFC/mL on eight bacterial samples taken from 17 moderate chronic periodontitis subjects. Samples were obtained at initial and maintenance phase of the periodontal treatment. Attention must be brought to the fact that the higher bacterial count in moderate chronic periodontitis corresponded to P. intermedia and C. rectus during the maintenance phase.
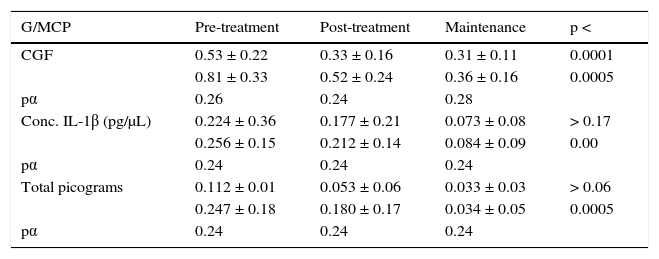
Results of CGF volume in microliters (μL) showed that it was more abundant in subjects with MCP when compared to G patients (Table IV). Values obtained from the pre-treatment phase up to the maintenance phase revealed that CGF volumes experienced a statistically significant reduction with a p < 0.0001 in G and p < 0.0005 in MCP. Table IV shows quantification of concentration in pictograms per microliter (pg/μL) of 1β interleukin where, a greater reduction can be seen between initial and maintenance phase in MCP patient group, with a p = 0.000 statistically meaningful. This situation did not occur in the G patient group who presented a p > 0.17. IL-1β total picogram quantification for each group of disease measured per treatment phase showed that G group patients showed p > 0.06 and MCP group patients p< 0.00 it was statistically significant only for the MCP group.
Crevicular gingival fluid and IL-1β concentration and total picograms.
| G/MCP | Pre-treatment | Post-treatment | Maintenance | p < |
|---|---|---|---|---|
| CGF | 0.53 ± 0.22 | 0.33 ± 0.16 | 0.31 ± 0.11 | 0.0001 |
| 0.81 ± 0.33 | 0.52 ± 0.24 | 0.36 ± 0.16 | 0.0005 | |
| pα | 0.26 | 0.24 | 0.28 | |
| Conc. IL-1β (pg/μL) | 0.224 ± 0.36 | 0.177 ± 0.21 | 0.073 ± 0.08 | > 0.17 |
| 0.256 ± 0.15 | 0.212 ± 0.14 | 0.084 ± 0.09 | 0.00 | |
| pα | 0.24 | 0.24 | 0.24 | |
| Total picograms | 0.112 ± 0.01 | 0.053 ± 0.06 | 0.033 ± 0.03 | > 0.06 |
| 0.247 ± 0.18 | 0.180 ± 0.17 | 0.034 ± 0.05 | 0.0005 | |
| pα | 0.24 | 0.24 | 0.24 |
Crevicular gingival fluid mean volume (CGF) comparison, (pg/μL) concentration and IL-1β total picograms in CGF samples of subjects with G and MCP during the three phases of non surgical procedure. pα = 0.05.
In the present study, disease of moderate chronic periodontitis (MCP) patients was controlled with non surgical periodontal treatment (NSPT). There was an unequivocal relationship of NSPT treated cases with the reduction of all clinical parameters. Probing depth showed average reduction of 2.02mm. This is higher than what is normally reported in scientific literature.33,34 In average, the amount of gained attachment was higher to that presented in previous reports: amount gained was in average 0.9mm. In MCP cases, twelve months after treatment there was a decrease in the amount of periodontal pathogenic bacteria P. gingivalis, T. forsythia, F. nucleatum, A. actinomycetemcomitans, and Actinomyces sp. This is in concordance with other reports.35,36 In this study, NSPT decreased IL-1β concentration in CGF this is in direct relationship with clinical parameters where GI of 1.8 ± 0.5 was reduced to 0.6 ± 0.3 (p < 0.0001). These facts are in agreement with research conducted by Engebretson,23 and Lein-Tuan.37
Etiologic factorsIn the present study, differences found among sub-gingival biofilm samples of Mexican subjects with gingivitis (G) and moderate chronic periodontitis (MCP) confirm, in general terms, previous descriptions manifested in scientific literature. The periodontal pathogenic microbiota showed quantitative differences between both study groups. This is in concordance with studies conducted by Sanz,11 and specifically in Mexican population and this coincides with the frequency of P. gingivalis and F. nucleatum.14 This was not the case for the frequency of the other six studied species. The differing results may reflect the microbiological test used or differences in the groups of examined patients. Non surgical periodontal treatment (NSPT) was effective for reduction of the eight periodontal pathogenic bacteria in MCP, with a statistically significant difference, which is associated to the reduction of clinical parameters when compared with studies conducted by Rawlinson,38 Haffajee,33 Mombelli.39 This reduction was consolidated in the maintenance phase of individuals with MCP; and was due to the control of the subgingival bio-film. In the group of G individuals, after NSPT, P. gingivalis showed an increase in bacterial count in the maintenance phase when compared to the pre-treatment phase. This can be related to the poor response to treatment of patients with sites that were positive to these bacteria, as well as to the specific virulence factors of these periodontal pathogenic bacteria. Nevertheless, we did not find studies in agreement with our findings. Notwithstanding, there were studies where P. gingivalis induce inflammatory mediators in blood.40 In MCP, NSPT was associated to the decrease of IL-1b levels, as well as reduction of P gingivalis, T. forsythia, F. nucleatum, A. actinomycetemcomitans, Actinomyces sp. at twelve months, in the maintenance phase. This is statistically significant when compared with studies conducted by Mogi.41 In these studies, the prevalence of periodontal pathogenic bacteria is associated to the concentration of IL-1β, due to the fact that CGF volume was correlated to the distinctive process of periodontitis.42
Host factorsThis study shows that in the concentration of IL- 1β in GCF pg/μL as well as in total pg in G there is no statistically significant difference among NSPT phases when compared with MCP condition, where statistically significant differences among treatment phases were manifested. For the aforementioned reasons, this research supports the fact that periodontal pathogenic bacteria are ubiquitous in the oral cavity, and in many individual infections and diseases are limited or absent. We can infer that from this fact derives the importance of the host response as susceptibility potential determinant, as previously.43 It can equally be inferred that the balance or imbalance between bio-film and inflammatory process is the determinant factor for the severity of the lesion in MCP lesions. This is due to the fact that enzyme levels, antibodies, complement factors, interleukines, and other antibacterial factors contained in the CGF augment in quantity. This increase is an indication of the severity of periodontal-gingival inflammation. Its sulcus progressing to pocket depth is an unequivocal sign of these conditions.44 It is also in concordance with clinical and experimental evidence which support the idea that MCP is not a conventional bacterial infection, it rather is an inflammatory condition triggered by the hosts immune response towards the poly-microbial infection of the associated bio-film.45 Our findings in this research show that quantity or concentration of IL- 1β in the CGF are useful markers for the association of severity and progression in gingivitis and periodontitis cases. This is in agreement with researchers such as Eley,22 Goutodi,46 Orozco47 Castrillón25 since, determining CGF volume and proinflammatory cytokines is useful to predict and associate progressive attachment loss. This is the case of IL-1β, which is liberated by macrophages, polymorphonuclear leukocytes, lymphocytes and gingival fibroblasts. This molecule is considered as involved with inflammatory processes, matrix destruction and scarring. To the aforementioned, we must add the fact that there is a strong relationship with bone resorption process, which in turn suggests destructive stages of the condition. We can therefore state that IL-1β is the main proinflammatory cytokine produced in the gums and associated with periodontitis.
CONCLUSIONTo conclude, we beg to state that etiological factors showed that in a Mexican population sample the number of bacteria species in the initial phase was greater in MCP than in G cases. In G, there was a reduction in species proportion of P. intermedia, F. nucleatum, A. actinomycetemcomitans . In periodontitis cases there was a reduction of P. gingivalis, F. nucleatum, T. forsythia. This reduction is associated to the decrease of clinical parameters, as well as with IL-1β reduction of concentration and total pg. With respect to host factors, it was confirmed that CGF volume and levels of IL-β are sensitive markers for diagnosis and severity assessment of periodontal disease. This study showed that in NSPT response, maintenance phase is all important to consolidate clinical, microbiological and biochemical improvement of the treatment. It can be inferred that, the non surgical periodontal treatment had more impact in control periodontal disease in MCP.