Chronic stress can influence immune response to vaccines. Healthcare workers are exposed to stressors and biological hazards, the health effects of which may be prevented through vaccination.
ObjectivesThis study aims to evaluate the association between stress in nurses and: (1) insufficient response to influenza vaccine, assessed one month after vaccination (T1); (2) the drop in haemagglutination-inhibition (HAI) antibodies (ab) six months after vaccination (T6).
MethodsA nested case–control study was carried out with 136 healthy hospital nurses. Individual interviews, the General Health Questionnaire (GHQ12) and Maslach Burnout Inventory (MBI-HSS) were applied in order to determine the presence of stress, using the triangulation method at the beginning of the study (T0). Influenza vaccine was administered and titres of HAI above each strain composing influenza vaccine before vaccination (T0), at T1 and T6 were assessed.
ResultsThere was no statistically relevant (5%) relationship between stress and the insufficient immune response to the vaccine at T1. Nevertheless, there was an association between stress and the drop in HAI ab AH1 at T6, when we assessed stress by the triangulation method using an interview (p=0.006), GHQ12 (p=0.045) and combination of criteria (p=0.001), even after multivariate analysis (respectively, p=0.01, p<0.05 and p=0.002). The odds ratios were, respectively, 3.64, 2.73 and 5.22.
ConclusionsThe association we found, between chronic stress and the drop in HAI ab at T6, corroborates the hypothesis that stress can negatively influence immune response. Therefore, it seems reasonable to consider this issue when we implement vaccination programmes for healthcare workers.
O stresse crónico pode influenciar a resposta imunitária à vacinação. Os profissionais de saúde estão expostos a stressores de natureza profissional e ainda a agentes biológicos cujos efeitos poderão ser prevenidos pela vacinação.
ObjetivosEstudar a associação entre a presença de stresse e (1) a “insuficiente” resposta imunitária à vacina contra a gripe, avaliada um mês após a vacinação (T1); (2) a redução dos títulos de anticorpos dirigidos às hemaglutininas (HAI) seis meses após a vacinação (T6).
MétodosRealizou-se um estudo caso-controlo incorporado num estudo de coortes com a participação de 136 enfermeiros hospitalares saudáveis. Realizaram-se entrevistas individuais e aplicaram-se os questionários The General Health Questionnaire (GHQ12) e Maslach Burnout Inventory (MBI-HSS) para determinação da presença de stresse crónico pelo método da triangulação, no início do estudo (T0). Foi administrada a vacina contra a gripe e determinou-se os títulos de HAI dirigidos a cada estirpe componentes da vacina contra a gripe, antes da vacinação(T0), em T1 e em T6.
ResultadosNão se encontrou associação significativa (5%) entre a presença de stress e a “insuficiente” resposta à vacina contra a gripe em T1. Contudo, encontrou-se uma associação entre a presença de stress e a diminuição do título de HAI dirigidos à estirpe A(H1N1) em T6 quando se avaliou a presença de stresse pelo método da triangulação usando a entrevista (p=0,006), o GHQ12 (p=0,045) e a combinação dos três critérios (p=0,001), que se manteve após análise multivariada (respetivamente p=0,01, p<0.05 e p=0.002). Os odds ratio ajustados foram de 3,64, de 2,73 e de 5,22.
ConclusõesA associação encontrada entre a presença de stresse crónico e a redução do título de HAI em T6 vem apoiar a hipótese de que o stresse poderá influenciar negativamente a manutenção dos títulos de anticorpos, mesmo em indivíduos adultos não idosos. Assim, parece razoável considerar este aspeto quando se pretende implementar programas de vacinação dirigidos a profissionais de saúde.
Healthcare workers are exposed to many stressors, some of them related with organisational work conditions and others, more specific to this profession, related with their activity of caring for the ill.1–3
Chronic stress and burnout seem to be very common in nurses.4–6 For example, López-Castillo and colleagues found high levels of emotional disturbance determined by the General Health Questionnaire (GHQ28) in 39% of hospital nurses.7
Amongst the consequences of chronic distress, whether they are related or not with work, are the possible effects on the immune system, including effects on the immune response to vaccination.
Healthcare workers are strongly advised to be vaccinated against influenza in order to protect themselves against the disease, reduce staff absenteeism and minimise the risk of nosocomial transmission to the patients they take care of. Vaccination is a possible model for immune response, testing mostly the humoral immune response. Vaccinated people develop antibodies (ab) that bind and neutralise the virus, in most cases ab against the surface glycoprotein hemagglutinin. Those ab can be used as markers of protection against the disease,8 caused by strains that are similar to the vaccine composition.
According to meta-analysis by Segerstrom and Miller, chronic exposure to stressors such as taking care of spouses with dementia, unemployment and suffering from physical disability is associated with a smaller ab response to influenza vaccine.9 Some reviews also suggest that chronic stress is associated with a smaller ab response to influenza vaccine.10–12
Generally speaking, studies evaluating the association between chronic stress and immune response to influenza vaccine showed relatively consistent results in old people. In those people, chronic exposure to stressors was associated with chronic anxiety and symptoms of depression and a lower response to influenza vaccine, in comparison to a control group.13–17
The use of a standardized dose of antigen which promotes a good immune response in most adults, could make it difficult the detection of the influence of chronic stress in the immune response to vaccination in younger adults, with a robust immune system.
Older people have a weaker immune system, related with age, so this could be an explanation for the greater consistency of results showing a negative association between chronic stress and immune response to vaccines in them. Vedhara and colleagues did not find any association between taking care of spouses with multiple sclerosis and ab response to influenza vaccine in adults under the age of 55.18 However, those adults showed similar stress levels as the control group.
In younger adults, such as university students, some studies19–22 found an association between stress, characterised in different ways, and the immune response to influenza vaccine (assessed by ab titres or by response rate) one month after vaccination. However, other studies did not find that association.23–25
Some of the studies found an association between stress and a drop in ab titres assessed 4–6 months after vaccination,19–23,25 even in the youngest adults. The drop in the ab titres associated with stress was only observed against one strain of vaccine components, suggesting that different exposures or different past vaccinations can be responsible for those results.
In an occupational context of healthcare settings, where healthcare workers are in the labour market (and are therefore not very old), but are simultaneously exposed to chronic stressors and biological risk hazards, it seems important to study the influence of stress on immune response to influenza vaccine.
Therefore, this study analyses the association between stress in nurses and: (1) insufficient response to influenza vaccine, assessed one month after vaccination (T1); (2) the drop in influenza aemagglutination-inhibition (HAI) ab titres six months after vaccination (T6), as compared to one month post vaccination HAI ab titres.
Materials and methodsStudy design and participantsThe study was a nested case–control study, conducted over six months in a university hospital during the 2007/2008 influenza season. Subjects were hospital nurses who were not taking any regular medication, including drugs that could affect immunity (such as cancer therapy drugs or corticosteroids). They did not have any medical condition that could affect the immune response and they also had no major surgery in the preceding three months. They did not have a history of drug consumption or alcohol consumption greater than 10 units per week, nor did they handle citotoxic drugs or work regularly with ionising radiation (n=136). The hospital's Ethics Committee approved the study and all the participants signed their agreement to participating in it.
One-month and six-month drop-out criteria:
- •
clinical diagnosis of medical condition that may affect immune response after the beginning of the study or taking any regular medication that can affect immunity (assessed by interview, at T1 and T6);
- •
workplace changes with regular exposure to ionising radiations or citotoxic drugs handling;
- •
clinical influenza symptoms with virus identification in nasal or oropharyngeal swab, during the six months of the study;
- •
a rise in HAI Ab titre to A(H1N1), A(H3N2) or B strains six months after vaccination, as compared with the titres measured one month after vaccination. Such a rise in HAI Ab titre suggests an exposure to influenza strains during T1 and T6 instead of a delayed response to the vaccine.
Structured individual interviews were conducted at the beginning of the study (T0) in order to:
- •
identify socio-demographic characteristics and possible confoundable variables related with immunity (physical exercise, nutritional parameters, nutritional supplements, hours of sleep per day, smoking habits, shift work) and influenza vaccination history;
- •
identify work-related and non-work-related stressors, using a Likert scale from 1 to 5;
- •
assess perceived stress, using a Likert scale from 1 to 5;
- •
identify stress-related behavioural changes or psychosomatic symptoms.
We also applied the Portuguese versions of the General Health Questionnaire (GHQ12) and Maslach Burnout Inventory (MBI-HSS or MBI) exhaustion scale at the beginning of the study (T0). Alfa Cronbach for those scales was 0.855 and 0.874 respectively.
In order to assess the presence of chronic stress, we applied the triangulation method at T0, as suggested by Cox and colleagues,26 in four different ways:
- •
through interviews: we accepted the presence of chronic stress using interviews if there were identified stressors classified as 4 or 5, plus perceived stress classified as 4 or 5, plus at least one behavioural change or one psychosomatic symptom stress-related;
- •
through GHQ12: we accepted the presence of chronic stress using GHQ12 if there were identified stressors classified as 4 or 5, plus GHQ12 higher than 2, plus at least one behavioural change or one psychosomatic symptom stress-related;
- •
through MBI: we accepted the presence of chronic stress using MBI if there were identified stressors classified as 4 or 5, plus MBI exhaustion scale higher than 24, plus at least one behavioural change or one psychosomatic symptom stress-related;
- •
combination of criteria: we accepted the presence of stress using combination of criteria if there was stress using interview and stress using GHQ12 or if there was stress using interview and stress using MBI exhaustion scale.
Venous blood was drawn at three stages between October 2007 and April 2008: (i) immediately before influenza vaccination (T0); (ii) one month following immunisation (T1); and (iii) six months after T0 (T6).
The samples rested 1h at ambient temperature following centrifugation at 3500rpm for 10min. Sera were stored at −20°C until used. All the samples drawn at T0, T1 and T6 were processed at the same time and under the same conditions.
A commercially available 2007/2008 trivalent influenza vaccine, with the recommended composition for that season in North Hemisphere, was administered intramuscularly, in the deltoid muscle, during October. All the vaccines belonged to the same group (AFLUA290AD).
Haemagglutination inhibition reaction was used to assess specific HAI ab titre against influenza A(H1N1), A(H3N2) and B strains included in the influenza vaccine, in accordance with the World Health Organisation's manual.27 Immediately before the laboratorial procedures, the sera were treated by a Receptor-Destroying Enzyme (RDE) in order to remove unspecific agglutinins and inhibitors.
The reference antigens were diluted to obtain 4 units against haemmaglutinin per 25μl and incubated with the treated sera samples. Erythrocytes were then added to the fluid.
HAI Ab titre corresponded to the inverse of the last dilution of serum that completely inhibited haemagglutination. We used progressive dilutions, starting with 1:10 up to 1:20.480.
The serological parameters obtained were:
- •
HAI Ab titre against influenza A(H1N1), A(H3N2) and B strains included in influenza vaccine, before (T0) and after vaccination (T1 and T6);
- •
rise in HAI Ab titre against influenza A(H1N1), A(H3N2) and B strains included in influenza vaccine, assessed one month after vaccination (compared to HAI Ab titre immediately before vaccination);
- •
drop in HAI Ab titre against influenza A(H1N1), A(H3N2) and B strains included in influenza vaccine, between T1 and T6.
For analysis at T1 and at T6, we defined the following groups:
responders at T1 (one month after vaccination): participants that showed, at T1, at least a fourfold rise in HAI ab titre compared to the titre before vaccination;
non-responders at T1 (one month after vaccination): participants that did not show, at T1, a fourfold rise in HAI ab titre compared to the titre before vaccination;
HAI ab titre drop group at T6 (six months after vaccination): participants with at least a fourfold rise in HAI ab titre at T1, but who showed a drop in HAI ab titre at T6, as compared to HAI ab titre at T1;
no change in HAI ab titre group at T6 (six months after vaccination): participants with at least a fourfold rise in HAI ab titre at T1, but with no change in HAI ab titre at T6, as compared to HAI ab titre at T1.
For dichotomous variables, we used the Qui-square and Fisher exact tests and determined the odds ratio.
For numerical variables, we used the Kolmogorov–Smirnov and Shapiro–Wilk tests to assess normal distribution in non-responders and responders (T1) and in the HAI Ab titre drop group and no change in HAI Ab titre group (T6).
For normal distributions, the T Student test was used to compare means, and the Levene test to assess variance homogeneity. For no normal distributions, we applied the Mann–Whitney test to compare medians.
We also used the multivariate analysis and determined the adjusted odds ratio for the confounding variables.
We considered a statistical significance of 5%. All tests were run in the Statistical Package for Social Sciences – SPSS® software, version 14.0 for Windows.
ResultsWe studied 136 nurses, most of whom were female (83.8%), Caucasian (96.3%) and did not smoke (77.9%). Their average age was 33 and the median age was 29 (22–63 years old). Only one participant was more than sixty years of age.
More than one half of the participants had been given an influenza vaccine shot at least one of the four seasons prior to the beginning of the study (52.9%), mostly in the year immediately before (44.1%). Nurses included in the study worked mostly on a shift work basis (70.6%), had a corporal index mass (kg/m2) between 18.5 and 24.9 (72.1%) and slept at least 7h per day (66.2%). The majority did not take vitamin supplements (86.8%) or fish oil (98.5%). Only 54.4% did regular physical exercise and 46.3% ate yogurt daily.
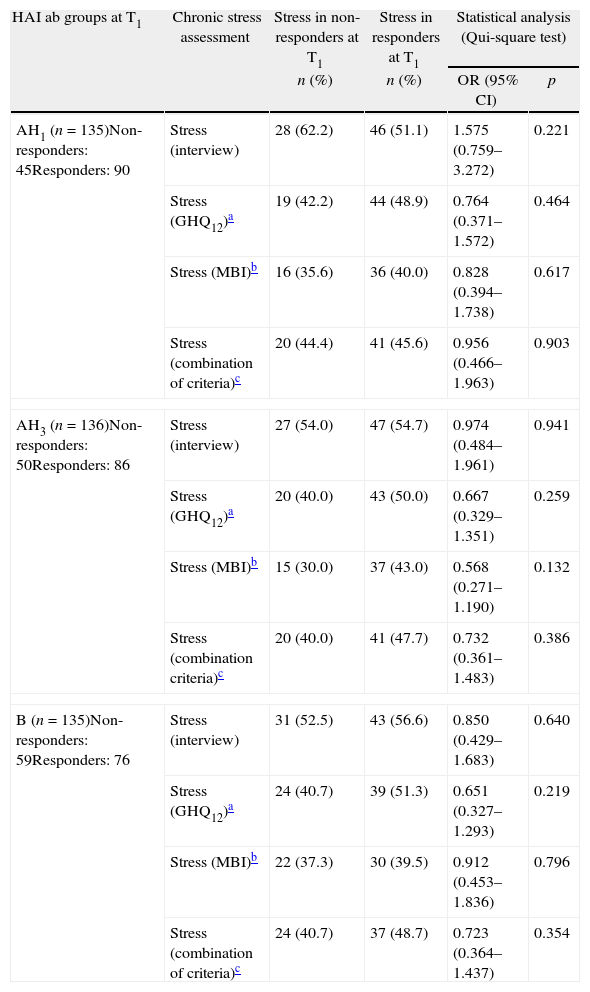
Association between chronic stress and non-responders at T1One month after vaccination (T1), we did not find any association of statistical significance between non-responders for A(H1N1) virus strain included in the influenza vaccine and the presence of chronic stress at T0, assessed in four different ways. Similarly, there was also no association between non-responders for A(H3N2) or non-responders for B strains included in the influenza vaccine and chronic stress at T0. To simplify the table, we named responders or non-responders for A(H1N1) and for A(H3N2) as responders or non-responders AH1 and AH3 respectively (Table 1).
Stress in non-responders and in responders AH1, AH3 and B at T1.
| HAI ab groups at T1 | Chronic stress assessment | Stress in non-responders at T1 | Stress in responders at T1 | Statistical analysis (Qui-square test) | |
| n (%) | n (%) | OR (95% CI) | p | ||
| AH1 (n=135)Non-responders: 45Responders: 90 | Stress (interview) | 28 (62.2) | 46 (51.1) | 1.575 (0.759–3.272) | 0.221 |
| Stress (GHQ12)a | 19 (42.2) | 44 (48.9) | 0.764 (0.371–1.572) | 0.464 | |
| Stress (MBI)b | 16 (35.6) | 36 (40.0) | 0.828 (0.394–1.738) | 0.617 | |
| Stress (combination of criteria)c | 20 (44.4) | 41 (45.6) | 0.956 (0.466–1.963) | 0.903 | |
| AH3 (n=136)Non-responders: 50Responders: 86 | Stress (interview) | 27 (54.0) | 47 (54.7) | 0.974 (0.484–1.961) | 0.941 |
| Stress (GHQ12)a | 20 (40.0) | 43 (50.0) | 0.667 (0.329–1.351) | 0.259 | |
| Stress (MBI)b | 15 (30.0) | 37 (43.0) | 0.568 (0.271–1.190) | 0.132 | |
| Stress (combination criteria)c | 20 (40.0) | 41 (47.7) | 0.732 (0.361–1.483) | 0.386 | |
| B (n=135)Non-responders: 59Responders: 76 | Stress (interview) | 31 (52.5) | 43 (56.6) | 0.850 (0.429–1.683) | 0.640 |
| Stress (GHQ12)a | 24 (40.7) | 39 (51.3) | 0.651 (0.327–1.293) | 0.219 | |
| Stress (MBI)b | 22 (37.3) | 30 (39.5) | 0.912 (0.453–1.836) | 0.796 | |
| Stress (combination of criteria)c | 24 (40.7) | 37 (48.7) | 0.723 (0.364–1.437) | 0.354 | |
OR – odds ratio; 95% CI – 95% confidence interval.
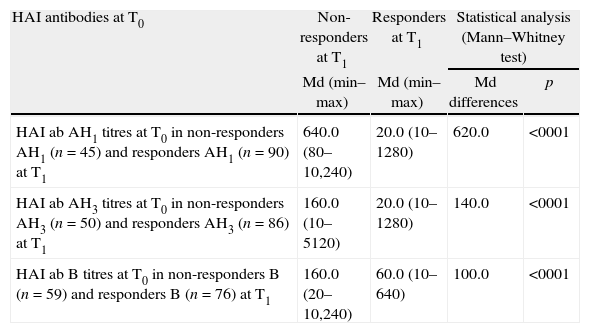
Using the Kolmogorov–Smirnov and Shapiro–Wilk tests, we found that there is no normal distribution in non-responders and responders at T1 for the considered continuous variables (p<0.001). Therefore, we applied the Mann–Whitney test to compare medians between HAI ab titres at T0 in responders and non-responders at T1. We found that non-responders AH1 at T1 had significantly higher HAI ab AH1 titres at T0 than responders AH1 at T1. The same happened with non-responders AH3 at T1 and non-responders B at T1, who had significantly higher HAI AH3 titres at T0 and HAI B titres at T0 than the corresponding responders (Table 2).
HAI antibodies AH1, AH3 and B titres at T0 in non-responders and in responders AH1, AH3 and B at T1.
| HAI antibodies at T0 | Non-responders at T1 | Responders at T1 | Statistical analysis (Mann–Whitney test) | |
| Md (min–max) | Md (min–max) | Md differences | p | |
| HAI ab AH1 titres at T0 in non-responders AH1 (n=45) and responders AH1 (n=90) at T1 | 640.0 (80–10,240) | 20.0 (10–1280) | 620.0 | <0001 |
| HAI ab AH3 titres at T0 in non-responders AH3 (n=50) and responders AH3 (n=86) at T1 | 160.0 (10–5120) | 20.0 (10–1280) | 140.0 | <0001 |
| HAI ab B titres at T0 in non-responders B (n=59) and responders B (n=76) at T1 | 160.0 (20–10,240) | 60.0 (10–640) | 100.0 | <0001 |
Md - median.
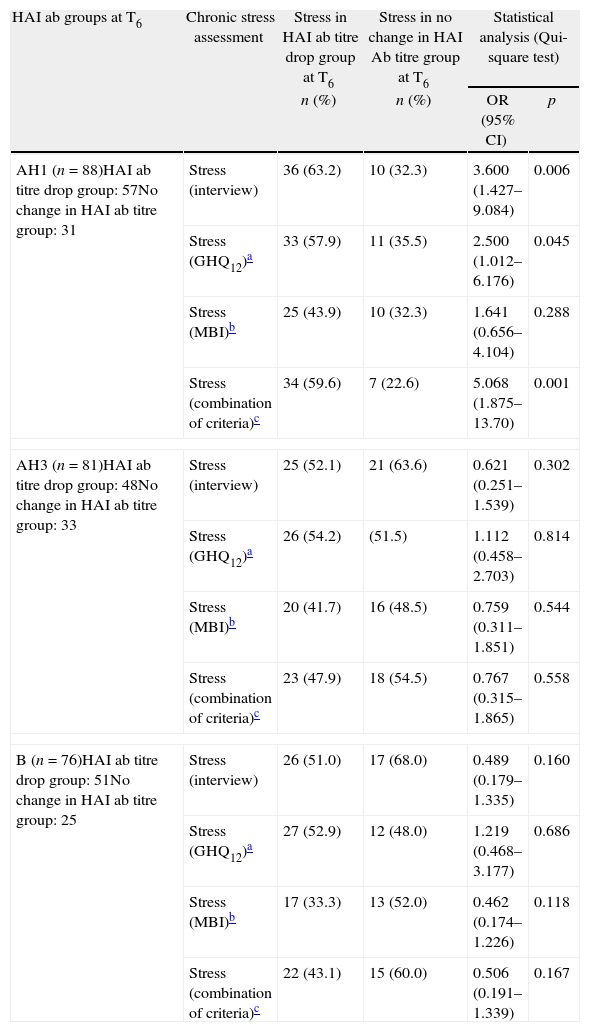
At T6 (six months after vaccination), the presence of stress in the HAI ab AH1 titre drop group was higher than in the no change in HAI ab AH1 titre group, when we assessed stress by all the considered different ways, being statistically significative when we assessed the presence of chronic stress by triangulation method at T0 using interviews, GHQ12 and the combination of the three methods. On the contrary, we did not find any statistically significant association between the others HAI ab titre drop groups at T6 and the presence of chronic stress (Table 3).
Stress in HAI antibodies titre drop group and in no change in HAI Ab titre group AH1, AH3 and B at T6.
| HAI ab groups at T6 | Chronic stress assessment | Stress in HAI ab titre drop group at T6 | Stress in no change in HAI Ab titre group at T6 | Statistical analysis (Qui-square test) | |
| n (%) | n (%) | OR (95% CI) | p | ||
| AH1 (n=88)HAI ab titre drop group: 57No change in HAI ab titre group: 31 | Stress (interview) | 36 (63.2) | 10 (32.3) | 3.600 (1.427–9.084) | 0.006 |
| Stress (GHQ12)a | 33 (57.9) | 11 (35.5) | 2.500 (1.012–6.176) | 0.045 | |
| Stress (MBI)b | 25 (43.9) | 10 (32.3) | 1.641 (0.656–4.104) | 0.288 | |
| Stress (combination of criteria)c | 34 (59.6) | 7 (22.6) | 5.068 (1.875–13.70) | 0.001 | |
| AH3 (n=81)HAI ab titre drop group: 48No change in HAI ab titre group: 33 | Stress (interview) | 25 (52.1) | 21 (63.6) | 0.621 (0.251–1.539) | 0.302 |
| Stress (GHQ12)a | 26 (54.2) | (51.5) | 1.112 (0.458–2.703) | 0.814 | |
| Stress (MBI)b | 20 (41.7) | 16 (48.5) | 0.759 (0.311–1.851) | 0.544 | |
| Stress (combination of criteria)c | 23 (47.9) | 18 (54.5) | 0.767 (0.315–1.865) | 0.558 | |
| B (n=76)HAI ab titre drop group: 51No change in HAI ab titre group: 25 | Stress (interview) | 26 (51.0) | 17 (68.0) | 0.489 (0.179–1.335) | 0.160 |
| Stress (GHQ12)a | 27 (52.9) | 12 (48.0) | 1.219 (0.468–3.177) | 0.686 | |
| Stress (MBI)b | 17 (33.3) | 13 (52.0) | 0.462 (0.174–1.226) | 0.118 | |
| Stress (combination of criteria)c | 22 (43.1) | 15 (60.0) | 0.506 (0.191–1.339) | 0.167 | |
OR – odds ratio; 95% CI – 95% confidence interval.
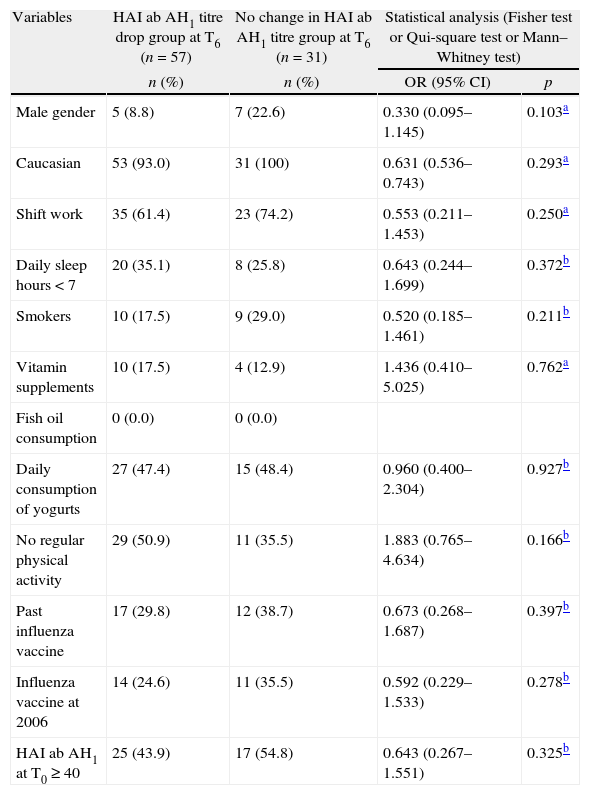
Some conditions that can affect immunity could have been possible confounding factors, when we considered the association between stress and the HAI ab AH1 titre drop group at T6. Using the Kolmogorov–Smirnov and Shapiro–Wilk tests, we found that there is no normal distribution in HAI ab AH1 titre drop group at T6 and in no change in HAI ab AH1 titre at T6 for the considered continuous variables (p<0.001). Therefore, we applied the Mann–Whitney test to compare their medians.
The statistical analyses did not find any significant difference between groups at T6 for the variables (continuous and dichotomous) taken into consideration (Table 4).
Variables distribution (stress not included) in HAI ab AH1 titre drop group at T6 and in no change in HAI ab AH1 titre group AH1 at T6.
| Variables | HAI ab AH1 titre drop group at T6 (n=57) | No change in HAI ab AH1 titre group at T6 (n=31) | Statistical analysis (Fisher test or Qui-square test or Mann–Whitney test) | |
| n (%) | n (%) | OR (95% CI) | p | |
| Male gender | 5 (8.8) | 7 (22.6) | 0.330 (0.095–1.145) | 0.103a |
| Caucasian | 53 (93.0) | 31 (100) | 0.631 (0.536–0.743) | 0.293a |
| Shift work | 35 (61.4) | 23 (74.2) | 0.553 (0.211–1.453) | 0.250a |
| Daily sleep hours<7 | 20 (35.1) | 8 (25.8) | 0.643 (0.244–1.699) | 0.372b |
| Smokers | 10 (17.5) | 9 (29.0) | 0.520 (0.185–1.461) | 0.211b |
| Vitamin supplements | 10 (17.5) | 4 (12.9) | 1.436 (0.410–5.025) | 0.762a |
| Fish oil consumption | 0 (0.0) | 0 (0.0) | ||
| Daily consumption of yogurts | 27 (47.4) | 15 (48.4) | 0.960 (0.400–2.304) | 0.927b |
| No regular physical activity | 29 (50.9) | 11 (35.5) | 1.883 (0.765–4.634) | 0.166b |
| Past influenza vaccine | 17 (29.8) | 12 (38.7) | 0.673 (0.268–1.687) | 0.397b |
| Influenza vaccine at 2006 | 14 (24.6) | 11 (35.5) | 0.592 (0.229–1.533) | 0.278b |
| HAI ab AH1 at T0≥40 | 25 (43.9) | 17 (54.8) | 0.643 (0.267–1.551) | 0.325b |
| Variables | HAI ab AH1 titre drop group at T6 (n=57) | No change in HAI ab AH1 titre group at T6 (n=31) | Statistical analysis (Fisher test or Qui-square test or Mann–Whitney test) | |
| Md (min–max) | Md (min–max) | Md differences | p | |
| Age | 31.0 (23.0–63.0) | 26.0 (22.0–56.0) | 5.0 | 0.072c |
| Body index mass | 22.7 (17.7–37.8) | 22.0 (18.0–37.5) | 0.7 | 0.793c |
| HAI ab AH1 titres at T0 | 20.0 (10–640) | 40.0 (10–1280) | −20.0 | 0.276c |
| HAI ab AH1 titres at T1 | 1280.0 (40–20,480) | 1280.0 (160–20,480) | 0.0 | 0.265c |
Md – median.
Stress – assessed by interview, GHQ12 or using the combination of the three methods – was the exclusive variable associated with HAI ab AH1 titre drop group at T6, but we also took the age variable into consideration in the multivariate analysis. That option was made because age is a strong factor influencing immunity and, in the simple analysis, the median difference between groups would be different if we considered a confidence level of 90% (instead of 95%).
Furthermore, Beyer and colleagues showed that basal HAI ab AH1 titres influence HAI ab AH1 titres one month after vaccination.28 Therefore, we also considered HAI ab AH1 titres at T0 and T1 in the multivariate analysis.
We found that stress, assessed by triangulation method using GHQ12, using interview and using the combination of the three methods, maintained the association with HAI ab AH1 titre drop group at T6. The association between HAI ab AH1 titre drop group at T6 and the others variables did not reveal any statistical significance (Table 5).
Multivaried analysis (multiple logistic regression) for stress (assessed by triangulation method using GHQ12, using interview and using the combination of the three methods) in HAI ab AH1 titre drop group at T6 considering age, HAI ab AH1 titres at T0 and at T1.
| Chronic stress assessment | Considered variables in multivaried analysis | Statistical analysis (multiple logistic regression) | |
| Adjusted OR (95% CI) | p | ||
| GHQ12a | Age | 1.038 (0.989–1.089) | 0.134 |
| Stress | 2.733 (1.039–7.186) | 0.042 | |
| HAI ab AH1 at T0 | 0.998 (0.995–1.000) | 0.100 | |
| HAI ab AH1 at T1 | 1.000 (1.000–1.000) | 0.793 | |
| Interview | Age | 1.043 (0.994–1.094) | 0.083 |
| Stress | 3.643 (1.371–9.684) | 0.010 | |
| HAI ab AH1 at T0 | 0.999 (0.996–1.001) | 0.236 | |
| HAI ab AH1 at T1 | 1.000 (1.000–1.000) | 0.987 | |
| Combination of criteriab | Age | 1.044 (0.994–1.096) | 0.087 |
| Stress | 5.223 (1.828–14.924) | 0.002 | |
| HAI ab AH1 at T0 | 0.999 (0.996–1.001) | 0.255 | |
| HAI ab AH1 at T1 | 1.000 (1.000–1.000) | 0.892 | |
Adjusted OR – adjusted odds ratio; 95% CI – 95% confidence interval.
When we assessed stress by triangulation method using GHQ12, the model was statistically significant (p<0.029) and suitable, given that null hypothesis was not rejected in the Hosmer–Lemeshow test (p<0.106). The model showed a validity rate of 65.9.
When we assessed stress by triangulation method using interview, the model was statistically significant (p<0.009) and suitable, given that null hypothesis was not rejected in the Hosmer–Lemeshow test (p<0.761). The model showed a validity rate of 71.6.
When we assessed stress by triangulation method using the combination of the three methods, the model was statistically significant (p<0.002) and suitable, given that null hypothesis was not rejected in the Hosmer–Lemeshow test (p<0.679). The model showed a validity rate of 73.9.
Discussion and conclusionsWhen human beings are exposed to chronic stressors, they may respond to them with neuroendocrine changes that include the release of neuropeptides, monoamines and hormones. Most of those substances are able to change the immune cells behaviour.29
Psychological chronic stress can change antibody (ab) production and kinetics after vaccination, in particular after the influenza vaccine is given to elderly people who take care of spouses with dementia.9–12
Various studies of elderly people have found an association between exposure to a long-term stressor (such as dementia spouse caregiving) and a small proportion of them who reached at least ab HAI titres that were fourfold what they had before flu vaccination, assessed one month after vaccination.13–15 Bereavement and marriage seem to be associated with antibody response to influenza vaccination in the elderly as well.17
In our study, as in some other studies involving young adults,18,23–25 we did not find any association between the presence of chronic stress in nurses and the proportion of them that reach at least four times the ab HAI titre levels they had before flu vaccination. On the contrary, other studies have found an association among perceived distress,19 life events,20 neuroticism21 and loneliness22 and the immune response to flu vaccination, assessed one month or five weeks after.
It is possible that methodological issues can explain discrepancies in results verified in studies with younger groups, such as: (1) different ways of characterising independent variables; (2) samples with differing demographic characteristics and dimensions; (3) differing histories of flu virus exposure.
With respect to the latter issue, our study found that non-responders had significantly higher ab HAI AH1N1, AH3N2 and B titres at T0 than responder groups. Therefore, as postulated by Beyer and colleagues,28 ab HAI titres at T0, showed to be an important confounding variable when studying the relationship between stress and immune response to flu vaccine one month after vaccination and must be considered.
Nevertheless, we found an association between the presence of chronic stress (measured in three different ways) and a drop in ab HAI (AH1N1) at T6. Other studies also found an association between distress,19,25 life events,20 life events weighed with perceived stress,23 neuroticism,21 or loneliness22 and a drop in ab HAI four to six months after vaccination. Those associations were found for at least one strain composing the flu vaccine.
Our study found a large proportion of nurses with chronic stress in the HAI ab AH1 titre drop group at T6, as compared to the no change in HAI Ab AH1 titre group at T6, when we measured stress by triangulation method, using interview or GHQ12 to assess perceived stress, and using the combination of the three methods (as described in the methods section). We did not find any statistically significant association when we assessed the presence of stress by the triangulation method but using the MBI exhaustion scale to measure perceived stress. A possible explanation is the fact that the MBI exhaustion scale measures specifically work-related stress and the possible immunologic repercussions of chronic stress seem to be independent of the stress source.
As described in other studies,19–23,25 the association between stress and a drop in ab HAI at T6 did not occur for all the vaccine strains components.
Strain novelty can be an important factor in that analysis, as argued by other authors.15,20 Pressman and colleagues, for example, only found an association between stress and a drop in HAI ab, four months after vaccination, for a strain that was not included in previous vaccinations the participants received.25 In our study, the exclusive strain that was not included in flu vaccines in the three preceding years was the A(H1N1) strain.
In Portugal the predominant circulating strains with high flu activity since 1990 have been A(H3N2) and B. From 1990 to the beginning of the study, the A(H1N1) strain was only predominant in 2005, simultaneously with strain B, and 2005 was a year with very low flu activity.30 We also know that A strains undergo more drift mutations than B stains,31 so this can contribute to their being a relative novelty for the participants’ immune system.
Finally, in our study, the A(H1N1) influenza strain proved to be the most immunogenic one, showing a rise in the HAI Ab titre geometric mean of 11.1. A (H3N2) and B strains showed rises of 6.2 and 4.6 times, respectively, between T0 and T1. It is possible that the best immunogenicity observed for the A (H1N1) strain was related with the fact that some participants had had a primary infection with an A (H1N1) strain, so the response to A (H1N1) antigens have been more robust in them.32 That could be a factor that may influence the detection of the association between stress and drops in HAI ab after a period of time.
Given that our sample was not a randomised sample because it depended on nurses voluntarily being vaccinated and participating in the study, we analysed distribution differences for some variables in the HAI ab AH1 titre drop group at T6 and the no change in HAI Ab AH1 titre group at T6 that are not included in drop out criteria. As there are a lot of variables for which we do not yet know if they can influence immunity, we studied those that are most referred to in the relevant literature.33
We did not find any differences in the distribution of the studied variables in the two groups at T6. Nevertheless, we decided to include ab AH1 titres at T0, ab AH1 titres at T1 and age in multiple logistic regression. The reason for including the first two variables was the strong suggestion in literature that they can influence ab titres after vaccination (immune response),28 even though we found no references to the influence they have on a drop in titres six months after flu vaccination. Age is strongly related with immunity33 but we did not find any difference in terms of age between the two groups considered at T6 at the significance level we considered (5%). If we considered a significance level of 10% the result would be different. Hence, we also included age in the multivariate analysis.
After the multivariate analysis, we still found an association with statistical significance between the presence of chronic stress and the HAI ab AH1 titre drop group at T6, when we assessed stress in three different ways, all of them using the triangulation method, as suggested by Cox and colleagues, as a good way of measuring stress.26 Therefore, the relationship that we found between chronic stress and a drop in HAI ab at T6 supports the thesis that stress can negatively influence HAI ab titres some months after flu vaccination even in people in adults under the age of 60. As we could notice, this is the first study assessing the association between chronic stress and immune response to influenza vaccine in healthcare workers, who is an important target group for influenza vaccine. Therefore, in an occupational health environment, it is reasonable to consider the possible interference of chronic stress with ab titres when we implement vaccination programmes to prevent biological occupational risks.
FundingAutoridade para as Condições de Trabalho (ACT) funded the laboratorial evaluation of the study.
Conflicts of interestThe authors have no conflicts of interest to declare.