There is great variability in plasma levels of clozapine. The objective of this study is to know the characteristics of patients treated with clozapine and the relationship between them and the variability of plasma levels.
Material and methodsDescriptive, cross-sectional study of all patients currently treated with clozapine in a Psychiatric Service with a diagnosis of schizophrenic psychosis or schizoaffective disorder. The present study assessed physical situation, psychopathology and functionality of the patients and explored the associations and correlations between clinical variables and plasma levels.
ResultsWe studied 39 patients, predominantly men, with negative and depressive symptoms and cardiovascular risk factors (metabolic syndrome and smoking). Significant variability in dose and even greater in clozapine levels were observed. The levels of clozapine at equal doses/kg of body weight were higher in non-smokers, they had positive correlation with BMI and negative correlation with systolic BP, disruptive behaviours and number of cigarettes consumed.
ConclusionPlasma level monitoring clozapine is an important tool to avoid clozapine plasma levels monitoring and minimise undesirable clinical situations (metabolic syndrome, sedation, negative symptoms and functional impairment). It is also important to control the effects of a smoking habit for optimum drug bioavailability.
Existe mucha variabilidad en las concentraciones plasmáticas de clozapina. El objetivo de este trabajo es conocer las características de pacientes tratados con clozapina y la posible asociación entre estas y las concentraciones plasmáticas.
Material y métodosEstudio descriptivo y transversal de todos los pacientes actualmente tratados con clozapina en un Servicio de Psiquiatría con diagnóstico de psicosis esquizofrénica o trastorno esquizoafectivo. Se valoró la situación física, psicopatología y funcionalidad, y se exploraron las asociaciones y correlaciones entre las variables clínicas y las concentraciones plasmáticas.
ResultadosSe estudiaron 39 pacientes, predominantemente hombres, con sintomatología negativa, síntomas depresivos y factores de riesgo cardiovascular (síndrome metabólico y consumo de tabaco). Se observó variabilidad importante en las dosis y mayor aún en las concentraciones plasmáticas de clozapina. A igualdad de dosis/kg de peso las concentraciones plasmáticas fueron más altas en no fumadores, y presentaron correlación positiva con el IMC y correlación negativa con la PA sistólica, conductas disruptivas y cantidad de cigarrillos consumidos.
ConclusiónLa monitorización de concentraciones plasmáticas de clozapina es un instrumento importante para evitar la variabilidad de dosis y minimizar situaciones clínicas no deseadas (síndrome metabólico, sedación, síntomas negativos y deterioro funcional). Es importante controlar los efectos del consumo de tabaco para la optimización de la biodisponibilidad del fármaco.
Clozapine is an antipsychotic drug with a complex pharmacodynamic profile. It has been shown to be more effective than the other first and second generation antipsychotic drugs in the treatment of resistant schizophrenia.1 It is fundamentally metabolised by the hepatic microsomal enzymes CYP1A2 and CYP34A, and one of its 2 main metabolites, the desmethyl metabolite N-desmethyl-clozapine (norclozapine) is pharmacologically active. Plasmatic concentrations of clozapine are highly variable and depend on absorption, hepatic metabolism and other factors such as nicotinism, age and sex.2 The determination of plasmatic concentrations of clozapine and norclozapine are used to evaluate compliance with the therapy, to optimise treatment and to minimise the risk of adverse effects.3 In spite of the therapeutic importance of the drug and the severity of the patients who receive it, there are few studies of plasmatic concentrations of clozapine. The aims of this work are: to examine the clinical situation of patients treated with clozapine, the doses and plasmatic concentrations of the same and the possible association between plasmatic concentrations and patient characteristics.
Material and methodsThis is a descriptive transversal study of all the patients currently treated with clozapine in a Psychiatry Department with the diagnosis of schizophrenic psychosis or schizoaffective disorder. The diagnosis was made by the psychiatrist in charge of the case, according to CIE-10 criteria. The study was approved by the Local Clinic Research Ethics Committee and it took place according to the norms of good clinical practice. The specific norms governing the use of clozapine were followed in all cases. Additionally, the patients diagnosed schizoaffective disorder who were taking clozapine without indication did so on prescription by the psychiatrist in charge of their case following the consideration that other psychopharmacological resources had been exhausted and that this complied with legal requisites (RD 1015/2009, of 19 June). Prior to undertaking this study no systematic determination of plasmatic concentrations of clozapine had been systematically undertaken.
Sociodemographic and clinical variables were studied, together with anthropometric measurements, life signs, haemogram and metabolic parameters. Plasmatic concentrations of clozapine and norclozapine were determined (this took place while fasting in the morning, without having eaten during the night or taken the breakfast dose of clozapine). Psychopathological examination covered the following areas: psychotic symptoms on the PANNS4 scale; cognition on the SCIP5 scale; functionality on the PSP6 scale and depressive symptoms on the Phq-97 scale.
The categorical variables are described using their frequencies and percentages, while continuous variables are described using their averages and standard deviations (SD). Differences between groups are compared using the chi-squared test or Fisher's exact test when a group smaller than 5 was found. In the case of continuous variables, to compare differences between the averages among groups the Student's t-test was used for two independent samples, or analysis of variance (ANOVA) in the case of 3 or more groups. Pearson's correlation coefficient was used to determine the relationship between 2 quantitative variables. The level of statistical significance used is ≤5%.
Results39 subjects were included in the study. Of the 49 patients diagnosed with schizophrenia or schizoaffective disorder and treated with clozapine in the Department at the time of the study, 10 could not be included due to the following reasons: 5 as they refused to sign the informed consent document, 4 because their psychopathological situation prevented the implementation of procedures, and one because of the presence of a severed physical pathology unconnected with their antipsychotic treatment.
The patients were aged from 20 to 61-years-old, with an average age of 43.8-years-old (SD=8.9). 84.6% were men. Thirty three patients (84.6%) were diagnosed with schizophrenia and 6 (15.4%) with schizoaffective disorder. The average duration of evolution of the disease was 16.8 years (SD=7.2). 59% had been in treatment with clozapine for more than 5 years, and in no case had the dose of the same been changed during the 6 months prior to the study.
Only 15% of the patients were at normal weight (BMI<25) and 19 (48.7%) were obese (BMI≥30). Seventeen patients (43.6%) fulfilled the criteria for metabolic syndrome.8 59% were smokers, with an average consumption of 20.6cigarettes/day (SD=10.1). In terms of their psychopathological situation, predominantly mild to moderate negative symptoms and depressive symptoms were observed in 51% of cases. The sample had a high level of disability, and 43.6% required intensive help in their everyday functioning. In the cognitive evaluation 4 patients (10.3%) showed mild cognitive deterioration, while the others scored within the normal range according to SCIP criteria.
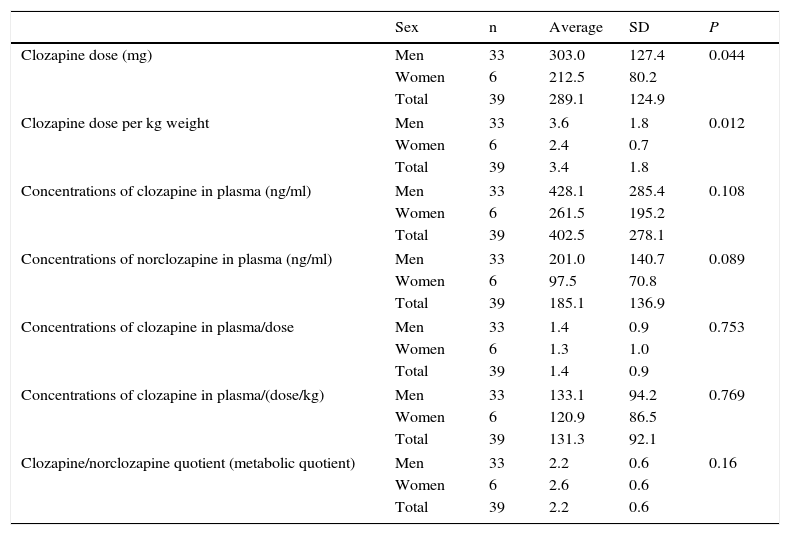
The doses of clozapine prescribed varied from 125 to 600mg/day, while plasmatic concentrations varied from 37 to 1071ng/ml. Table 1 shows the dose of clozapine and plasmatic concentrations of clozapine and norclozapine broken down according to sex. The men received significantly higher doses of clozapine than the women, in terms of absolute dose as well as dose/kg. Plasmatic concentrations of clozapine and norclozapine were also higher in the men, although this difference was not statistically significant. The differences in plasmatic concentrations between the sexes fell when they were adjusted for weight and dose.
Clozapine dose and concentration in plasma of clozapine and norclozapine broken down according to sex.
| Sex | n | Average | SD | P | |
|---|---|---|---|---|---|
| Clozapine dose (mg) | Men | 33 | 303.0 | 127.4 | 0.044 |
| Women | 6 | 212.5 | 80.2 | ||
| Total | 39 | 289.1 | 124.9 | ||
| Clozapine dose per kg weight | Men | 33 | 3.6 | 1.8 | 0.012 |
| Women | 6 | 2.4 | 0.7 | ||
| Total | 39 | 3.4 | 1.8 | ||
| Concentrations of clozapine in plasma (ng/ml) | Men | 33 | 428.1 | 285.4 | 0.108 |
| Women | 6 | 261.5 | 195.2 | ||
| Total | 39 | 402.5 | 278.1 | ||
| Concentrations of norclozapine in plasma (ng/ml) | Men | 33 | 201.0 | 140.7 | 0.089 |
| Women | 6 | 97.5 | 70.8 | ||
| Total | 39 | 185.1 | 136.9 | ||
| Concentrations of clozapine in plasma/dose | Men | 33 | 1.4 | 0.9 | 0.753 |
| Women | 6 | 1.3 | 1.0 | ||
| Total | 39 | 1.4 | 0.9 | ||
| Concentrations of clozapine in plasma/(dose/kg) | Men | 33 | 133.1 | 94.2 | 0.769 |
| Women | 6 | 120.9 | 86.5 | ||
| Total | 39 | 131.3 | 92.1 | ||
| Clozapine/norclozapine quotient (metabolic quotient) | Men | 33 | 2.2 | 0.6 | 0.16 |
| Women | 6 | 2.6 | 0.6 | ||
| Total | 39 | 2.2 | 0.6 | ||
Respecting the clinical and psychopathological variables, no significant associations were found between plasmatic concentrations of clozapine and the clinical variables studied, except in the case of systolic blood pressure and disruptive behaviour. Both of these variables were negatively correlated in a significant way with plasmatic concentrations.
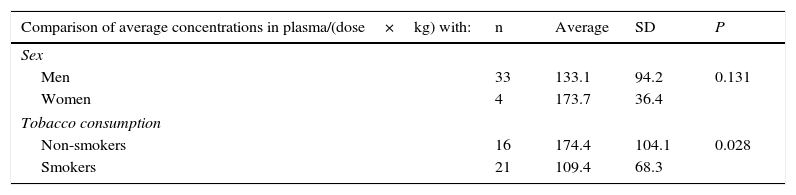
No differences were found in the average values of the coefficient of plasmatic concentrations/(dose/kg) depending on sex, and nor was any correlation found with these for age. It was found that, at an equal level of dose/kg, non-smokers had significantly higher concentrations of plasmatic clozapine than the smokers. It was also found that at equal levels of dose/kg, plasmatic concentrations of clozapine increased with increasing BMI and fell when smokers consumed a higher number of cigarettes (Table 2).
Statistically significant values in comparison of the average concentrations of clozapine in plasma/(dose×kg) according to sex and tobacco consumption, and correlation of the same variable with clinical variables, age, body mass index (BMI) and the number of cigarettes smoked.
| Comparison of average concentrations in plasma/(dose×kg) with: | n | Average | SD | P |
|---|---|---|---|---|
| Sex | ||||
| Men | 33 | 133.1 | 94.2 | 0.131 |
| Women | 4 | 173.7 | 36.4 | |
| Tobacco consumption | ||||
| Non-smokers | 16 | 174.4 | 104.1 | 0.028 |
| Smokers | 21 | 109.4 | 68.3 | |
| Correlation between concentrations in plasma/(dose×kg) with: | Pearson's correlation coefficient | P |
|---|---|---|
| Systolic blood pressure | −0.364 | 0.023 |
| Disruptive behaviour (PSP) | −0.331 | 0.039 |
| Age | −0.08 | 0.961 |
| BMI | 0.362 | 0.024 |
| Number of cigarettes (smokers) | −0.428 | 0.050 |
This work studies the sociodemographic and clinical characteristics of a group of 39 schizophrenia or schizoaffective disorder patients treated with clozapine, seeking possible associations of these variables with dosage and plasmatic concentrations of clozapine. The weak points of this work can be said to be the sample size and the predominance of men in the same.
The characteristics of the sample are those which could be expected based on previous studies of groups of patients with resistant schizophrenia: the majority are men, they have high rates of metabolic syndrome,9 smoking10 and predominantly negative symptoms. A striking finding is the high frequency of depressive symptoms, above all when it is taken into account that 25% of the patients were taking antidepressant medication.
Unless there are justified exceptions, it is considered that concentrations of clozapine in plasma should stand at from 250ng/ml to 350ng/ml.11 Only 7 patients (20%) were within the interval that is considered to be therapeutic, while the majority (45.7%) had concentrations in plasmas higher than 350ng/ml.
The negative influence of tobacco consumption on the bioavailability of clozapine was foreseeable, given that nicotine induces cytochrome P450 (CYP) 1A2 and CYP2B6 activity.12 These enzymes influence clozapine metabolism, so that smoking 7–12 cigarettes per day may be sufficient to cause maximum clozapine induction and mean that the dose of clozapine required to achieve a certain concentration in plasma increases by 50%.13 The opposite situation was the case for BMI, which correlates positively with concentrations of clozapine in plasma.14 If patients are overweight this should not dissuade doctors from considering treatment with clozapine,15 although it must be taken into account when deciding on the dosage.
Previous studies found differences in clozapine metabolism and elimination according to sex16 which would lead to differences in the metabolic quotient (clozapine/norclozapine). This difference does not attain statistical significance in this work, although this datum should be evaluated with care due to the small number of women in the sample.
To conclude, the variability of the dose of clozapine and the physical, psychopathological and functional situation of patients underline the importance of monitoring concentrations in plasma to adjust the dose of clozapine. This has the purpose of avoiding possible adverse effects such as sedation, hypotension or metabolic syndrome, while also minimising undesirable clinical situations (secondary negative symptoms or functional deterioration), as the clinical situation of patients does not seem to be a valid indicator of whether their concentrations in plasma are outside the therapeutic range. It was also found to be important to monitor tobacco consumption and patient weight to optimise the bioavailability of the drug.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have adhered to the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Iglesias García C, Iglesias Alonso A, Bobes J. Variaciones en las concentraciones plasmáticas de clozapina en pacientes con esquizofrenia y trastorno esquizoafectivo. Rev Psiquiatr Salud Ment (Barc). 2017;10:192–196.