Breast carcinoma (BC) is one of the most common cancer-related mortality among women worldwide. S100A9 and S100A8 are calcium-binding proteins involved in BC metastasis. Toll-like receptors (TLRs) are membrane-bound proteins that play a vital role in immune systems and carcinogenesis through inflammatory cytokines.
ObjectivesWe aimed to evaluate of S100A8, S100A9 and TLR5 in breast carcinoma by IHC, their gene expression by PCR and investigate their correlation with clinicopathological characters and hormone status.
Materials and methodsThis study was done in Biochemistry & Molecular Biology and Pathology Departments, Zagazig University, Egypt. Biopsy was taken from 72 patients with invasive breast carcinoma cases admitted to Surgery Department, Zagazig University, between January 2018 and January 2021. 72 breast biopsies were obtained from adjacent normal breast (as a control). IHC, gene expression by q real-time PCR using S100A8, S100A9 and TRL5.
ResultsPositive S100A8, S100A9, TLR5 were 81.9%, 76.4%, 86.1% respectively with statistically significant association between advanced stage, presence of lymph node metastasis, ER.PR status and HER2 negative expression.
ConclusionesBased on our findings, we postulated that S100A8, S100A9, TLR5 play an important role in progression of BC and can be used as novel molecular targets for earlier BC detection and prediction for future therapies in breast carcinoma.
El carcinoma de mama (BC, por sus siglas en inglés) es una de las muertes relacionadas con el cáncer más comunes entre las mujeres en todo el mundo. S100A9 y S100A8 son proteínas de unión al calcio implicadas en la metástasis del BC. Los receptores tipo peaje (TLR, por sus siglas en inglés) son proteínas unidas por membrana que juegan un papel vital en los sistemas inmunitarios y carcinogénesis a través de citocinas inflamatorias.
ObjetivosEvaluar S100A8, S100A9 y TLR5 en el BC por IHC, su expresión génica por PCR e investigar su correlación con los caracteres clínico patológicos y el estado hormonal.
Material y métodosEste estudio se realizó en los Departamentos de Bioquímica y Biología Molecular y Patología de la Universidad de Zagazig, Egipto. Se tomó biopsia de 72 pacientes con carcinoma de mama invasivo ingresados en el Departamento de Cirugía de la Universidad de Zagazig, entre enero de 2018 y enero de 2021. Setenta y dos biopsias de mama se obtuvieron de mama normal adyacente (como control). IHC, expresión génica por q PCR en tiempo real usando S100A8, S100A9 y TRL5.
ResultadosPositivo S100A8, S100A9 y TLR5 fueron del 81,9, 76,4 y 86,1%, respectivamente, con asociación estadísticamente significativa entre el estadio avanzado, la presencia de metástasis en los ganglios linfáticos, el estado de ER.PR−, expresión negativa de HER2.
ConclusionesSobre la base de nuestros hallazgos, postulamos que S100A8, S100A9 y TLR5 juegan un papel importante en la progresión del BC y pueden utilizarse como nuevas dianas moleculares para la detección temprana del BC y la predicción para futuras terapias en el BC.
Breast cancer (BC) is one of the most common malignant tumors causing death among women worldwide.1 In Egypt, breast carcinoma, based on National Cancer Institute registry 2020 represents about 38.8% of all cancers in female.2 According to profiles of gene expression and hormone receptor status, breast cancer is classified into different subtypes with variable prognosis and treatment strategies. Patients with ERþ breast cancer subtype have the best prognosis. In contrast, hormone receptor-negative ER_, PR_ and Her2_ triple-negative (TN) subtype has the worst prognosis.3 Molecular subtype affects the systemic therapy and clinical outcome of BC.4
S100A8 is calcium-binding protein of low molecular weight. S100A8 and S100A9 are heterodimer proteins, involved in many chronic diseases like rheumatic arthritis, inflammatory bowel disease and atherosclerosis.5,6 They are overexpressed in several cancers like lung, colon, gastric and breast cancer and may participate in tumor cell survival and metastasis.7 In BC, S100A8/A9 enhance metastasis by binding with receptor advanced glycation end-product (RAGE).8
Toll-like receptors (TLRs) are membrane-bound proteins that are expressed in some immunocytes and play a vital role in immunity through inflammatory cytokines.9
Numerous members of the TLR family are detected in different carcinomas. TLR3 overexpression in neuroblastoma10 while TLR4 is reduced in squamous cell carcinoma of the skin.11 TLR5, unlike other TLRs, is not expressed in macrophages and conventional dendritic cells.12 TLR5 is highly expressed in many tumors, as non-small cell lung13 and breast carcinomas.14 It was reported that cancer cell proliferation and tumor growth are inhibited by TLR5 signaling.15
Aim of the study was to evaluate of S100A8, S100A9 and TLR5 in breast carcinoma by IHC, their gene expression by PCR and investigate their correlation with clinicopathological characters and hormone status.
Patients and methodsThis study was done at Biochemistry and Molecular Biology Department, Pathology and general Surgery Departments, Zagazig University, Egypt. The study was done between January 2018 and January 2021 after written informed consent from our Patients. Biopsy was taken from 72 patients with invasive breast carcinoma, then modified radical mastectomy at general Surgery department was done. In addition, normal breast biopsies were obtained from adjacent normal breast tissue (served as controls). None of the patients had received preoperative treatment.
All cases of invasive breast carcinoma were histologically diagnosed as infiltrating duct carcinoma (NST).
The study was done according to The Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans and was approved by the research ethical committee (IRB) of Faculty of Medicine, Zagazig University.
Gene expression by q real-time PCRTotal RNA from breast tissue of each group was extracted using (RNeasy Mini Kit, Qiagen) following the manufacturer's protocol. The purity and integrity of total RNA were monitored by absorbance of ultraviolet spectrophotometer at 260/280nm. For the synthesis of complementary DNA (cDNA), the extracted RNA was reverse transcribed by QIAGEN One step RT-PCR Kit, as recommended by the manufacturer.
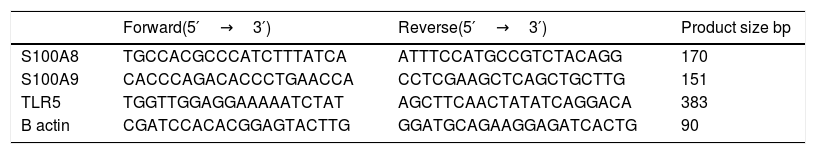
Expression levels of mRNA were determined using Real time PCR (Strata-geneMx3005P-qPCR System). The sequences for primers are listed in (Table 1). The PCR was performed in 25UL containing 12.5 UL SYBR Green PCR Master Mix (Qiagen), 10 p mol of each primer (Invitrogen) and 5 UL cDNA. For S100A8, S100A9 and β actin the PCR cycling conditions were as follows: 95°C for 10min, followed by 40 cycles consisting of 95°C for 15s, 60°C for 1min and 72°C for 50s. For TLR5 the thermal cycle profile for PCR was as follows: 94°C for 5min, 40 cycles of PCR (94°C for 30s; 55°C for 30s; 72°C for 30s). The expression of cellular house-keeping gene β-actin as a control to normalize values. Relative gene expression in terms of fold change was calculated using 2-ΔΔCt method.
primer sequences of S100A9, S100A8, TLR5 and B actin in cancer breast.
| Forward(5′→3′) | Reverse(5′→3′) | Product size bp | |
|---|---|---|---|
| S100A8 | TGCCACGCCCATCTTTATCA | ATTTCCATGCCGTCTACAGG | 170 |
| S100A9 | CACCCAGACACCCTGAACCA | CCTCGAAGCTCAGCTGCTTG | 151 |
| TLR5 | TGGTTGGAGGAAAAATCTAT | AGCTTCAACTATATCAGGACA | 383 |
| B actin | CGATCCACACGGAGTACTTG | GGATGCAGAAGGAGATCACTG | 90 |
Immunohistochemical staining was carried out using the EnVision (USA) method. Paraffin-embedded blocks have been cut into 4μm thick sections. Tissue sections were deparaffinized in xylene and rehydrated in graded alcohol. To block endogenous peroxidase activity the sections were placed in 0.5% hydrogen peroxide in methanol for 10min. Sections were incubated with mouse monoclonal antibody to S100A8 (primary antibody), SC 23657 clone, dilution 1/100, Santa Cruiz international cooperation; rabbit polyclonal antibody to S100A9 GTX129575clone, dilution 1/100, GeneTex International Corporation and rabbit polyclonal antibody clone GTX102590. GeneTex International Corporation, dilution 1/100 anti TRL5 antibody. Secondary antibodies were added to sections for 30min, DAB was used as a chromogen and slides were stained by hematoxylin as counterstain. The slides were dehydrated by deionized water and finally slides were mounted by a cover slip using DPX.
Stained sections were photographed with a digital camera where the original magnification was mentioned underneath each photograph.
Interpretation and evaluation of immunohistochemical stainingRegards both S100A8 and 9 immunoreactivity; they are expressed in cytoplasm or nucleus in tumor cells and may stain stromal cells, positive tumor cells were calculated and scored as follows: 0=0%; 1=1–20%; 2=21–50%; and 3=≥50%.16 Concerning TRL5, cytoplasmic immunostaining, percent of positive cells was scored 0, none; 1, <10%; 2, 10–50%; and 3, >50%. The intensity may be mild, moderate and sever. Staining index equal percent of positive cells score X staining intensity if more than4 describe high expression but if less than 4 this mean low expression.14
Statistical analysisData analysis was performed using the software SPSS (Statistical Package for the Social Sciences) version 20. Quantitative variables using their means and standard deviations. Categorical variables using their absolute frequencies and were compared using chi square test and Fisher exact test when appropriate. To compare quantitative data between two groups, independent sample t test was used to compare means when data was normally distributed. Pearson correlation coefficients were used to assess strength and direction of association between two continuous parametric variables. The level statistical significance was set if P<0.05. P≤0.001 was considered as statistically highly significant.
ResultsPatient characteristicsThis study included 72 patients with breast carcinoma; 58.3% of them aged >50. 68.1% and 45.8% of cases had grade III and stage III respectively. Lymph node metastasis was evident in 90.3%. 41 of cases had tumor size from 2:5cm. About 54%, 65% and 72% had positive ER/PR receptor, negative HER2 and high Ki 67 respectively. Lymphovascular and perineural invasion were detected in 63.9% and 27.8% respectively.
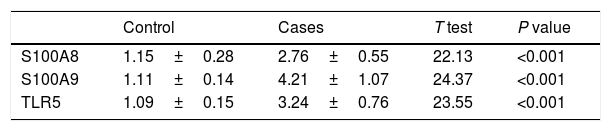
mRNA expression resultsIn breast carcinoma tissues, the mean values of mRNA fold expressions of S100A8, A9 and TRL5 compared to control normal adjacent breast tissues were 2.76±0.55, 4.21±1.07 and 3.24±0.76 respectively (Table 2). There was significant upregulation in S100A8, S100A9 and TLR5 mRNA expression in the breast carcinoma samples in comparison to control samples P value<0.001.
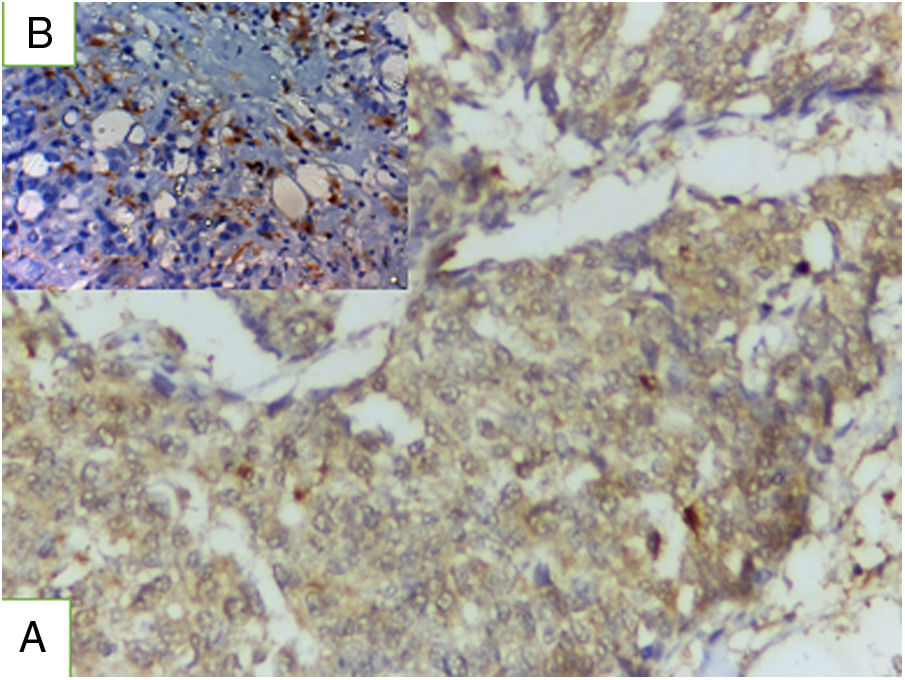
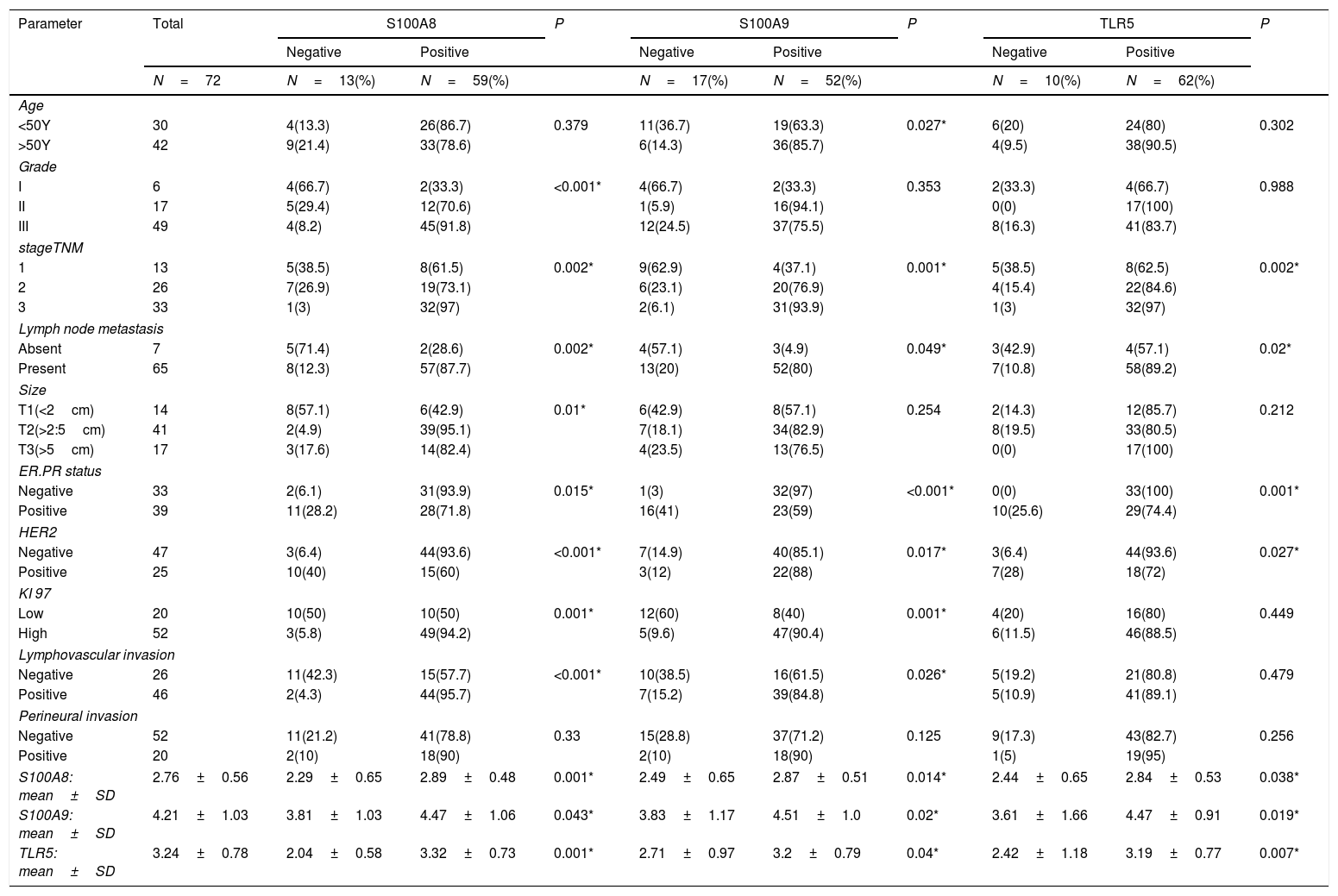
Results of immunohistochemical markers (Table 3)S100A8 immunohistochemical expressionPositive S100A8 was present in 81.9% of the studied cases both in tumor and stromal cells (Fig. 1). High S100A8 expression was present in high grade, advanced stage, large tumor size, ER/PR- status, negative HER2, high Ki-67 expression, presence of lymph node metastasis and lymphovascular invasion (P value was <0.001, 0.002, 0.01. 0.015, 0.01 and <0.001 respectively). No significant association was found between S100A8 expression and either age or perineural invasion (P value 0.379 and 0.33 respectively).
Relation between clinicopathological features and immunohistochemical expression of S100A8, S100A9 and TLR5 among those with breast cancer.
| Parameter | Total | S100A8 | P | S100A9 | P | TLR5 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |||||
| N=72 | N=13(%) | N=59(%) | N=17(%) | N=52(%) | N=10(%) | N=62(%) | ||||
| Age | ||||||||||
| <50Y | 30 | 4(13.3) | 26(86.7) | 0.379 | 11(36.7) | 19(63.3) | 0.027* | 6(20) | 24(80) | 0.302 |
| >50Y | 42 | 9(21.4) | 33(78.6) | 6(14.3) | 36(85.7) | 4(9.5) | 38(90.5) | |||
| Grade | ||||||||||
| I | 6 | 4(66.7) | 2(33.3) | <0.001* | 4(66.7) | 2(33.3) | 0.353 | 2(33.3) | 4(66.7) | 0.988 |
| II | 17 | 5(29.4) | 12(70.6) | 1(5.9) | 16(94.1) | 0(0) | 17(100) | |||
| III | 49 | 4(8.2) | 45(91.8) | 12(24.5) | 37(75.5) | 8(16.3) | 41(83.7) | |||
| stageTNM | ||||||||||
| 1 | 13 | 5(38.5) | 8(61.5) | 0.002* | 9(62.9) | 4(37.1) | 0.001* | 5(38.5) | 8(62.5) | 0.002* |
| 2 | 26 | 7(26.9) | 19(73.1) | 6(23.1) | 20(76.9) | 4(15.4) | 22(84.6) | |||
| 3 | 33 | 1(3) | 32(97) | 2(6.1) | 31(93.9) | 1(3) | 32(97) | |||
| Lymph node metastasis | ||||||||||
| Absent | 7 | 5(71.4) | 2(28.6) | 0.002* | 4(57.1) | 3(4.9) | 0.049* | 3(42.9) | 4(57.1) | 0.02* |
| Present | 65 | 8(12.3) | 57(87.7) | 13(20) | 52(80) | 7(10.8) | 58(89.2) | |||
| Size | ||||||||||
| T1(<2cm) | 14 | 8(57.1) | 6(42.9) | 0.01* | 6(42.9) | 8(57.1) | 0.254 | 2(14.3) | 12(85.7) | 0.212 |
| T2(>2:5cm) | 41 | 2(4.9) | 39(95.1) | 7(18.1) | 34(82.9) | 8(19.5) | 33(80.5) | |||
| T3(>5cm) | 17 | 3(17.6) | 14(82.4) | 4(23.5) | 13(76.5) | 0(0) | 17(100) | |||
| ER.PR status | ||||||||||
| Negative | 33 | 2(6.1) | 31(93.9) | 0.015* | 1(3) | 32(97) | <0.001* | 0(0) | 33(100) | 0.001* |
| Positive | 39 | 11(28.2) | 28(71.8) | 16(41) | 23(59) | 10(25.6) | 29(74.4) | |||
| HER2 | ||||||||||
| Negative | 47 | 3(6.4) | 44(93.6) | <0.001* | 7(14.9) | 40(85.1) | 0.017* | 3(6.4) | 44(93.6) | 0.027* |
| Positive | 25 | 10(40) | 15(60) | 3(12) | 22(88) | 7(28) | 18(72) | |||
| KI 97 | ||||||||||
| Low | 20 | 10(50) | 10(50) | 0.001* | 12(60) | 8(40) | 0.001* | 4(20) | 16(80) | 0.449 |
| High | 52 | 3(5.8) | 49(94.2) | 5(9.6) | 47(90.4) | 6(11.5) | 46(88.5) | |||
| Lymphovascular invasion | ||||||||||
| Negative | 26 | 11(42.3) | 15(57.7) | <0.001* | 10(38.5) | 16(61.5) | 0.026* | 5(19.2) | 21(80.8) | 0.479 |
| Positive | 46 | 2(4.3) | 44(95.7) | 7(15.2) | 39(84.8) | 5(10.9) | 41(89.1) | |||
| Perineural invasion | ||||||||||
| Negative | 52 | 11(21.2) | 41(78.8) | 0.33 | 15(28.8) | 37(71.2) | 0.125 | 9(17.3) | 43(82.7) | 0.256 |
| Positive | 20 | 2(10) | 18(90) | 2(10) | 18(90) | 1(5) | 19(95) | |||
| S100A8: mean±SD | 2.76±0.56 | 2.29±0.65 | 2.89±0.48 | 0.001* | 2.49±0.65 | 2.87±0.51 | 0.014* | 2.44±0.65 | 2.84±0.53 | 0.038* |
| S100A9: mean±SD | 4.21±1.03 | 3.81±1.03 | 4.47±1.06 | 0.043* | 3.83±1.17 | 4.51±1.0 | 0.02* | 3.61±1.66 | 4.47±0.91 | 0.019* |
| TLR5: mean±SD | 3.24±0.78 | 2.04±0.58 | 3.32±0.73 | 0.001* | 2.71±0.97 | 3.2±0.79 | 0.04* | 2.42±1.18 | 3.19±0.77 | 0.007* |
P for Chi square test.
Positive S100A9 was evident in 76.4% of the studied cases both in tumor and stromal cells. S100A9 expression was significantly associated with older age, stage, presence of lymph node metastasis, ER.PR- status, negative HER2 and high Ki 67 expression and lymphovascular invasion (P value was 0.027, 0.001, 0.049, <0.001, 0.017, 0.001 and 0.026. No significant correlation was observed between S100A9 expression and either grade, size, or perineural invasion.
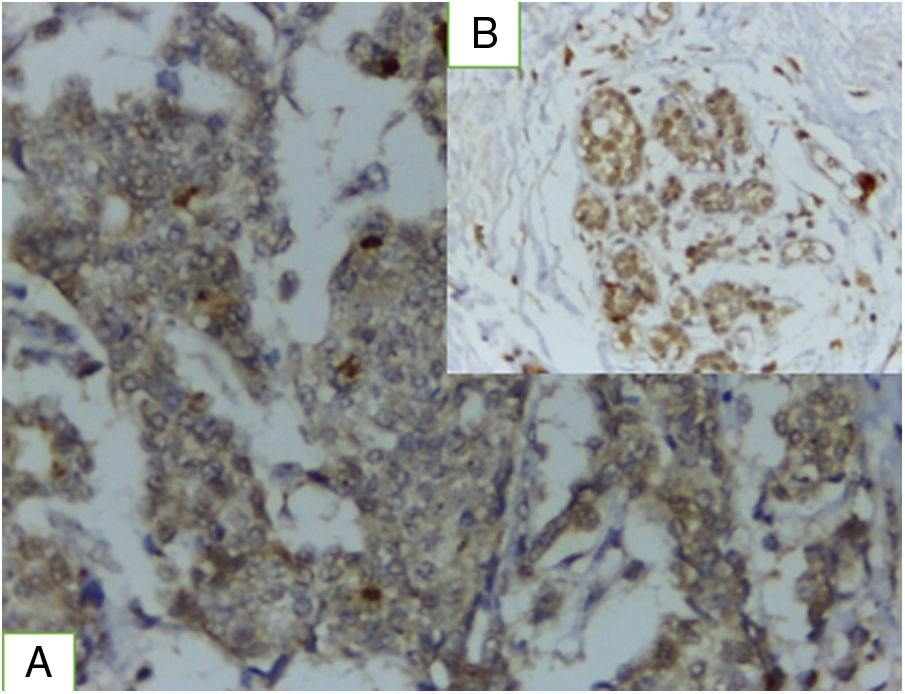
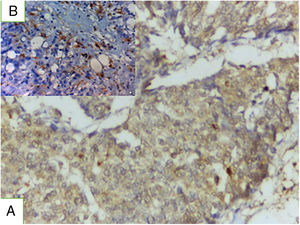
TRL5 immunohistochemical expressionTRL5 was found in 86.1% of cases, it is present in tumor cells and in breast normal adjacent tissue (Fig. 2). There was statistically significant association between TLR expression and advanced stage, presence of lymph node metastasis, ER.PR status-, HER2 negative expression (P value was 0.002, 0.02 0.001 and 0.027). A statistically non-significant association was found between TLR gene expression and either age, grade, Ki 67 index, tumor size, or lymphovascular invasion.
A highly statistically significant positive correlation between each two markers (P<0.001) data not tabulated.
Relation between immunohistochemical staining and gene expressionThere is a significant association between protein expression of S100A8, S100A9 and TRL5 with their gene expression.
DiscussionBreast cancer in Egypt is major health problem with high mortality rate, its incidence increasing and unfortunately most cases presented by advanced stage. In this study we aimed to evaluate of S100A8, S100A9 and TLR5 in breast carcinoma by IHC and their gene expression by PCR.
We found significant upregulation in S100A8, S100A9 and TLR5 mRNA expression in the breast cancer samples in comparison to control samples P value<0.001, this suggest that their expressions are altered in breast cancer.
The current study showed that cytoplasmic and nuclear expression of S100A8 and S100A9 present in 81.9% and 76.4% respectively of the studied cases.
Nuclear expression of S100A8/A9 promotes oncogenic transcription and leads to enhanced breast transformation as confirmed by Song and Struhl16 who reported in their study that S100A8/A9 overexpression is important for breast cellular transformation both act as a classic cytokine that can stimulate signaling pathway and as a transcriptional nuclear coactivator.
S100A8/A9 enhance metastasis by binding with receptor advanced glycation end-product (RAGE) and Toll like receptor through activation of MAPK pathways.8
We found that S100A8 and S100A9 expression both in the tumor and stromal cells, parallel to Tidehag et al.17 who reported that S1008/9 was expressed in stromal cells in prostate cancer.
Bao et al.18 also reported that S100A8 and S100A9 expression was not only present in BC cells, but also on stromal and inflammatory cells.
The current study showed that S100A8 correlated with larger tumor size, high Ki-67 index, nodal stage, ER PgR_and HER2 negative tumors. These results are in concordance with Bergenfelz et al.19 who reported that S100A9 expression in ER_PgR_ breast carcinoma correlated with poor pathological features.
Bao et al.18 also reported strong expression of S100A8/9 in BC that was associated with poor pathological parameters.
Several other studies confirmed our results, Lim et al.,20 confirmed the role of S100A8/A9 in tumor invasion and progression.
Miller et al.21 demonstrated that increased S100A8 protein expression was an independent prognostic indicator of poor outcome in ER-positive tumors. Drews-Elger et al.22 demonstrated that expression of S100A8 cells was associated with basal-like breast cancer.
Wang et al.23 confirmed that increased expression of S100A8 in tumor area than para carcinoma tissue. They reported that mRNA level of S100A8 was increased in relapsed breast cancer patients and correlated with poor prognosis. They also found that S100A8 expression increased in triple negative breast cancer.
Concerning TRL5, the current study, showed that TRL5 expression was detected in 86.1% of the studied cases with statistically significant association between its expression and advanced stage, presence of lymph node metastasis, ER.PR status-, HER2 negative expression (P value was 0.002, 0.02 0.001 and 0.027). Our results are consistent with Shuang et al.14 who indicated that TLR5 is often overexpressed and upregulated in breast carcinoma. They found that TLR5 overexpression correlated significantly with lymph node metastasis and tumor grade.
However, contrary results were found in another study by Shi et al.24 who reported that TLR5 down-regulation increased proliferation, invasion and metastasis of tumor cells in breast carcinoma. The difference may be related to complexity in the process of translation from mRNA to protein in tumorigenesis.
Besides its expression in breast carcinoma, TLR5 is upregulated in many other types of human carcinomas.25,26 TLR5 plays an important role in these carcinomas as TLR5 signaling regulates systemic tumor-promoting inflammation and contributes to distal malignant progression.27
The effects and functions of TLR5 seem to be dependent on cancer type, location and cell line. For example, in gastric carcinoma, stimulation with flagellin increased proliferation by TLR5.28 In contrary to gastric cancer, flagellin activation by TLR5 resulted in tumor suppressive effects in some of the breast carcinomas as reported by Cai et al.29 This is explained by bacterial abundance in the gastrointestinal tract and its less prevalence in the mammary glands.25
Besides immunohistochemical results, we found correlation between S100A8, S100A9 and TLR5 mRNA expression levels in breast carcinoma group that is consistent with similar study.30
ConclusionBased on our findings, we postulated that S100A8, S100A9, TLR5 play an important role in development and progression of breast carcinoma and can be used as novel molecular targets for earlier detection and prediction for further therapies of breast carcinoma.
FundingThis work has not received any funding.
Confidentiality of dataThe authors declare that they have followed the protocols of their center on the publication of patient data.
Conflict of interestThe authors declare no conflict of interest regarding this work.