Clinical trials have shown that Nab-paclitaxel is more effective than paclitaxel in the treatment of metastatic breast cancer (MBC). Although the incidence of cancer increases with age, elderly patients are under-represented in clinical trials and tend to receive suboptimal treatment to avoid toxicity.
The main aim of our study was to compare progression-free survival (PFS) among patients older and younger than 65 years treated with Nab-paclitaxel in real-world clinical practice. A secondary aim was to assess overall survival (OS) as well as the response rate and toxicity.
Methods and patientsThis single-centre study retrospectively analyzed a cohort of 60 patients with MBC treated with Nab-paclitaxel monotherapy in Hospital Clínico Lozano Blesa de Zaragoza.
ResultsThe median PFS was 5.92 months (3.62–11.2) in patients<65 years and was 5.06 months (4.31–12.4) in those ≥65 years (p=0.868). The median OS was 26.9 months (18.6–30.7) in patients < 65 years and was 20.3 months (11.4–33.6) in those ≥65 years (p=0.138).
ConclusionsAlthough patients in the cohort studied had a median age of 60.45 years, which is higher than the median age in most clinical trials, PFS and OS in conditions of real-world clinical practice were similar to previously published data.
The results of the use of Nab-paclitaxel in patients older than 65 years are similar to those in younger patients, with no additional toxicity problems. The results of our study agree with those of other notable studies.
En los ensayos clínicos se ha demostrado que el Nab-paclitaxel es más eficaz que el paclitaxel en el tratamiento del cáncer de mama metastásico (CMM). Aunque la incidencia del cáncer aumenta con la edad, los pacientes ancianos están infrarrepresentados en los ensayos clínicos, y tienden a recibir un tratamiento subóptimo para evitar toxicidades.
El objetivo principal de nuestro estudio es comparar la supervivencia libre de progresión (SLP) entre las mayores y menores de 65 años tratadas con Nab-paclitaxel en la práctica clínica real. El objetivo secundario es evaluar la supervivencia global (SG), así como la tasa de respuestas y la toxicidad.
Métodos y pacientesSe ha analizado de forma unicéntrica y retrospectiva, una cohorte de 60 pacientes con CMM tratadas con Nab-paclitaxel en monoterapia en el Hospital Clínico Lozano Blesa de Zaragoza.
ResultadosMediana SLP<65 años: 5,92 meses (3,62-11,2) y ≥65 años, mediana: 5,06 meses (4,31-12,4) (p=0,868). Mediana de SG en <65 años: 26,9 meses (18,6-30,7) y ≥65 años: 20,3 meses (11,4-33,6) (p=0,138).
ConclusionesAunque las pacientes de la cohorte estudiada tienen una mediana de edad de 60,45 años, que es superior a la mediana de edad de la mayoría de los ensayos clínicos, la SLP y la SG en condiciones de práctica clínica real son similares a los datos publicados anteriormente. En cuanto al uso de Nab-paclitaxel en pacientes de edad avanzada, se obtienen resultados similares a las de aquellas pacientes más jóvenes, sin observarse problemas de seguridad adicionales. Además, con los datos disponibles, los resultados son congruentes con los presentados en otros estudios de impacto.
Breast cancer is the most common malignant tumour among women and the leading cause of cancer's death in Europe.1 Its incidence also increases with age, with approximately half of breast cancers taking place in women over 65 years old.2
Despite advances in the treatment of metastatic breast cancer (MBC), the prognosis for this disease remains poor, with a 5-year survival rate of approximately 23–26%.3 In recent years a decreasing trend in mortality has been observed with a 1–2% annual decrease,4 and an increase in overall survival (OS) between 18 and 28 months.5 However, therapeutic strategies continue to have a palliative purpose and are aimed to symptom control, improvement and maintenance of quality of life and prolongation of survival, with a balance between efficacy and toxicity.6
Although the incidence of cancer increases with age, older patients are under-represented in clinical trials, therefore only a few published data on the efficacy of treatments in this age group are available.7,8
The choice of treatment in these patients is complex.9 The progressive worsening of the general condition and the increase in comorbidities make them more vulnerable to certain treatments, such as chemotherapy.10,11 As a result, they often receive treatments that are less effective than younger patients, and take the risk of under-treating them in order to avoid toxicities. However, several studies show that the efficacy of treatments is the same, regardless of age.12,13
In MBC, taxanes are treatments of choice in the first line and this is reflected in the main clinical guidelines. Nab-paclitaxel is a taxane in which albumin acts as a transporter avoiding the use of solvents.14–16 In several phase II and III trials, Nab-paclitaxel has proved to be more effective than paclitaxel and docetaxel every three weeks (not in paclitaxel weekly regimen), in the first and second lines of MBC treatment in terms of response rate (ORR) and progression free survival (PFS).17 In addition, in the subgroup analysis it was observed that efficacy and safety were the same regardless of age.18
So far, most published data on the efficacy of Nab-paclitaxel have been obtained through clinical trials. However, the strict selection criteria for this type of study often result in populations that may not reflect the profile of patients treated in routine clinical practice. In this sense, there is a growing interest in evaluate the effectiveness and safety of chemotherapy treatments under real clinical practice conditions.19–21
In response to this need, this study aims to evaluate the effectiveness of Nab-paclitaxel treatment in patients with MBC in the real clinical practice, analysing the influence of age on treatment effectiveness.
Material and methodsStudy designThis is an observational, unicentric, retrospective study, based on a cohort of 60 patients diagnosed with MBC, who are in follow-up, or were until their death, by the Medical Oncology Service of the Hospital Clínico Universitario Lozano Blesa of Zaragoza, from January 2011 to January 2019. The inclusion's criteria were patients diagnosed with Her2-negative MBC and treated with Nab-paclitaxel in monotherapy.
The demographic characteristics of the patients, the stage of the disease at diagnosis, the expression of hormone receptors, the number of previous treatments received, the disease's location, the duration of treatment with Nab-paclitaxel, the treatment pattern used, toxicities and the reasons for end of treatment were collected.
The dose regimen used are divided in weekly (125mg/m2, the first three cycles, and then 100mg/m2) or every three weeks (260mg/m2).
The main objective was to compare the median PFS in the groups of patients older and younger than 65 years. The secondary objective was to assess the OS in both age groups, as well as to analyze response to treatment and toxicities.
Statistical analysisThe statistical analysis has been carried out with the statistical package R. The median with confidence intervals (CI) estimated using booststrap resampling with n=104 has been used as measure of location and the standard deviation (SD) as measure of dispersion. Relative frequencies as percentages have been used in the case of qualitative variables. To test independence in 2×2 contingency tables, the Fisher test (two-sided) is used with mid-up adjustment as recommended by Agresti22 and usual Fisher's Exact test for 2×K contingency tables with K>2. For the quantitative variables the Mann–Whitney U test was used for location comparison after rejecting the normal distribution with the Shapiro–Wilk test.
For the estimation of the survival curves (OS and PFS) the Kaplan–Meier estimator was used, as well as Greenwood's formula for its variances. The Log-rank test has been used for testing equality between survival curves.
Descriptive analysisA descriptive analysis of the collected variables was carried out. Categorical variables are presented by the relative frequency corresponding to each category. For continuous variables, estimators of central tendency (median) and dispersion (SD) will be presented.
Inferential analysisFor the main variable (median of PFS) and the secondary variable (median of OS) the grouping of older and younger than 65 years has been carried out to compare them by the log-rank test.
ResultsDescriptive analysisThe median age of our sample is 60.45 years (±11.61). Of the 60 patients in our sample, there were 22 patients over 65 years (36.6%) and 38 patients under 65 years (63.4%). In the group of under-65s the median is 56 years (±7.77). Those over 65 have a median of 73 years (±5.2).
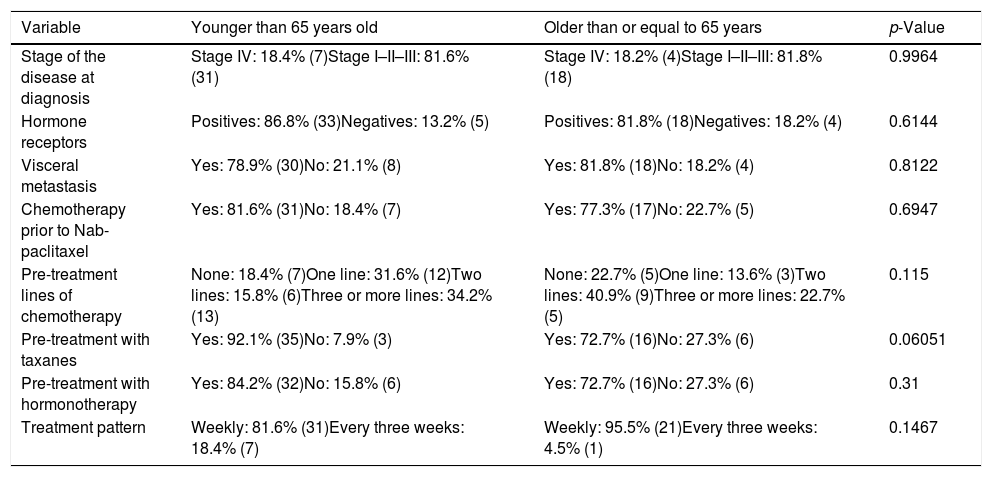
Table 1 shows the demographic characteristics of the patients in our sample, as well as the homogeneity of both groups.
Demographic characteristics of the patients with their p-value showing the homogeneity of both groups.
| Variable | Younger than 65 years old | Older than or equal to 65 years | p-Value |
|---|---|---|---|
| Stage of the disease at diagnosis | Stage IV: 18.4% (7)Stage I–II–III: 81.6% (31) | Stage IV: 18.2% (4)Stage I–II–III: 81.8% (18) | 0.9964 |
| Hormone receptors | Positives: 86.8% (33)Negatives: 13.2% (5) | Positives: 81.8% (18)Negatives: 18.2% (4) | 0.6144 |
| Visceral metastasis | Yes: 78.9% (30)No: 21.1% (8) | Yes: 81.8% (18)No: 18.2% (4) | 0.8122 |
| Chemotherapy prior to Nab-paclitaxel | Yes: 81.6% (31)No: 18.4% (7) | Yes: 77.3% (17)No: 22.7% (5) | 0.6947 |
| Pre-treatment lines of chemotherapy | None: 18.4% (7)One line: 31.6% (12)Two lines: 15.8% (6)Three or more lines: 34.2% (13) | None: 22.7% (5)One line: 13.6% (3)Two lines: 40.9% (9)Three or more lines: 22.7% (5) | 0.115 |
| Pre-treatment with taxanes | Yes: 92.1% (35)No: 7.9% (3) | Yes: 72.7% (16)No: 27.3% (6) | 0.06051 |
| Pre-treatment with hormonotherapy | Yes: 84.2% (32)No: 15.8% (6) | Yes: 72.7% (16)No: 27.3% (6) | 0.31 |
| Treatment pattern | Weekly: 81.6% (31)Every three weeks: 18.4% (7) | Weekly: 95.5% (21)Every three weeks: 4.5% (1) | 0.1467 |
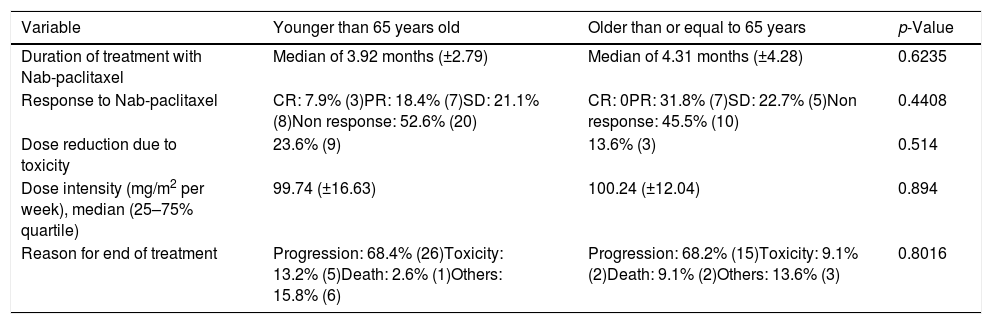
In Table 2 we can observe the descriptive analysis of the variables related to the treatment with Nab-paclitaxel being the differences found between both groups non-significant.
Descriptive analysis of the variables related to the treatment with Nab-paclitaxel with their p-value. CR: complete response, PR: partial response, SD: Stable disease.
| Variable | Younger than 65 years old | Older than or equal to 65 years | p-Value |
|---|---|---|---|
| Duration of treatment with Nab-paclitaxel | Median of 3.92 months (±2.79) | Median of 4.31 months (±4.28) | 0.6235 |
| Response to Nab-paclitaxel | CR: 7.9% (3)PR: 18.4% (7)SD: 21.1% (8)Non response: 52.6% (20) | CR: 0PR: 31.8% (7)SD: 22.7% (5)Non response: 45.5% (10) | 0.4408 |
| Dose reduction due to toxicity | 23.6% (9) | 13.6% (3) | 0.514 |
| Dose intensity (mg/m2 per week), median (25–75% quartile) | 99.74 (±16.63) | 100.24 (±12.04) | 0.894 |
| Reason for end of treatment | Progression: 68.4% (26)Toxicity: 13.2% (5)Death: 2.6% (1)Others: 15.8% (6) | Progression: 68.2% (15)Toxicity: 9.1% (2)Death: 9.1% (2)Others: 13.6% (3) | 0.8016 |
Table 3 summarize toxicities during Nab-paclitaxel in our cohort.
Descriptive analysis of the main toxicities related to the treatment with Nab-paclitaxel.
| Toxicities | Younger than 65 years old | Older than or equal to 65 years | p value |
|---|---|---|---|
| Anaemia (Hb<8g/dL) | 23.68% (9) | 27.27% (6) | 0.766 |
| Neutropenia (<1.500/ml) | 21.05% (8) | 18.18% (4) | 0.835 |
| Diarrhoea | 10.53% (4) | 18.18% (4) | 0.448 |
| Nausea and vomiting | 21.05% (8) | 27.27% (6) | 0.752 |
| Sensorial neuropathy | 42.11% (16) | 36.36% (8) | 0.789 |
| Asthenia | 28.95% (11) | 40.91% (9) | 0.401 |
The OS of our cohort has a median of 24.1 months (±15.27226) (95% CI: 18.6–27 months). The median follow-up sample was 26.15 months.
Analysing PFS (Fig. 1), the median in months in patients younger than 65 years was 5.92 months (95% CI 3.62–11.2) and in patients older than or equal to 65 years, median was 5.06 months (95% CI 4.31–12.4) (p:0.868, log Rank test).
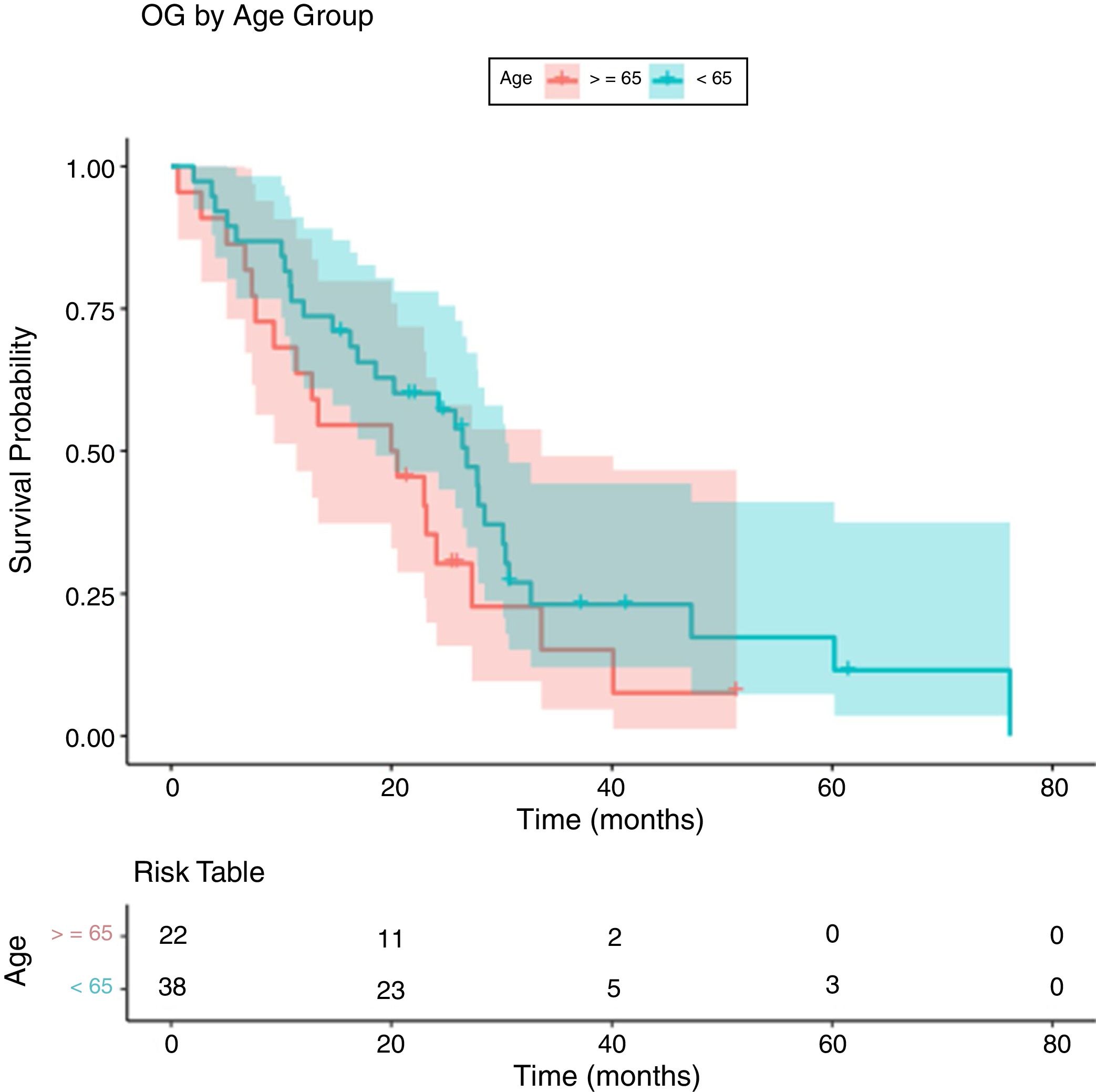
Regarding OS in patients younger and older than 65 years (Fig. 2), the median OS in those younger than 65 years is 26.9 months (95% CI 18.6–30.7) and in the group equal or older than 65 years the median is 20.3 months (95% CI 11.4–33.6) (p:0.138, log-rank test).
DiscussionComparing patients studied in clinical trials with those treated in routine clinical practice is very difficult, mainly because the study population often differs in its characteristics.
Our cohort of 60 patients, for example, has a median age of 60.45 years old compared with 53 years23 or 54 years24 for some of the clinical trials.
In addition, clinical trials usually have strict exclusion criteria which determine that the drugs cannot be studied in patients with comorbidities, that are more in line with those that we will later find in our usual clinical practice.
In the phase III clinical trial that led to the approval of treatment with Nab-paclitaxel in MBC, for example, the following exclusion criteria were used: patients with a life expectancy of less than 12 weeks, who have previously received paclitaxel or docetaxel (taxanes), who have brain metastasis, who have received hormonal therapy, immunotherapy or chemotherapy in the four weeks prior to the start of the drug, among others.25
As for the variables studied descriptively compared to clinical trials, the samples are quite similar. For example, in our cohort around 80% of the patients presented visceral metastases at the beginning of treatment with Nab-paclitaxel and 20% non-visceral metastases. In Gradishar et al. the proportion was 84% and 16% respectively.24
As noted above, older patients tend to be under-treated compared to younger patients. In our study there was a lower percentage of patients older than 65 years (36.6%) compared to those younger than 65 years (63.4%).
Analysing the description of our sample, there was no difference between the stage at which they are diagnosed, the expression of hormonal receptors or the presence of visceral metastases, in relation to age (Table 1).
However, we can observe that patients older than 65 years have been previously treated with taxanes or hormone therapy in a lower percentage than those younger patients (Table 1). Despite these differences (not statistically significant) the response to chemotherapy with Nab-paclitaxel is similar in both groups with a p-value of 0.4408 (Table 2).
One of the causes that led to undertreatment in elderly patients is trying to avoid toxicities.26 In our sample, there are no significant differences in toxicities between the two groups (Table 3), although there is slight difference with a higher percentage of anaemia, diarrhoea, nausea, vomiting and asthenia in patients older than 65 years. However, if we analyze the toxicities that required the suspension of the drug (Table 2), only have happened in the 11.67% of the patients (9.09% in ≥65 years versus 13.15% in <65 years). Both percentages are quite similar and without statistically significant differences. In addition, if we take into account dose reductions due to toxicities, these have occurred in 23.6% in ≤65 years old versus 13.6% in ≥65 years old (Table 2). Therefore, in our sample, as observed in the clinical trials mentioned above, there is no additional safety problem due to the use of Nab-paclitaxel in elderly patients.
Like the response to treatment, PFS did not give a statistically significant result, with no differences between the two groups (median of 5.92 months in younger than 65 years and 5.06 months in older than 65 years with a p: 0.868) (Fig. 1). This supports what was found in other studies, that there are no differences in the efficacy of treatment in elderly patients.12,13
Regarding OS, there were 47 deaths (of 60 patients in our cohort) during a median follow-up of 26.15 months, concentrated in relatively short survival times with respect to the variable range.
The median in months of OS is 24.1 (95% CI 18.6–27). This data does not differ practically from the 103 weeks (23.7 months) of phase III.25
In terms of comparing OS according to the age (Fig. 2), the log-rank test did not give a statistically significant result (p=0.138) (median of 26.9 months in younger than 65 years and 20.3 months in older than 65 years). Nevertheless, a dominance of the curve of the group of under-65s over that of over-65s was observed, although with insufficient differences to reject the equality between curves. This may be due to the fact that patients over 65 years tend to live less than younger patients because of a biological issue.
As recommended by several studies, a comprehensive geriatric evaluation should be carried out in elderly patients in order to improve in understanding the comorbidities of each patient, trying to predict the risk and prognosis of each of them and helping to choose the best individualized treatment.27–30
With regard to the limitations of our study, it should be noted that we are dealing with a unicentric and retrospective study with a small sample size.
ConclusionsAlthough the patients in the cohort studied have a median age of 60.45 years, which is higher than the median age of most clinical trials, OS and PFS under conditions of usual clinical practice, are similar to previously published data.
As for the use of Nab-paclitaxel in elderly patients, similar results as young patients are observed, without posing any additional safety problems. Moreover, with the available data, the results are consistent with those presented in other impact studies.
Compliance with ethical standardsThis study has been approved by the local ethics committee and it has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All the alive patients gave their informed consent to be part of the study and in the death ones, according to the resolution of the ethical committee, it was not achieve.
FundingThis work has not received any funding.
Conflict of interestThe authors have no relevant conflicts of interest to declare.






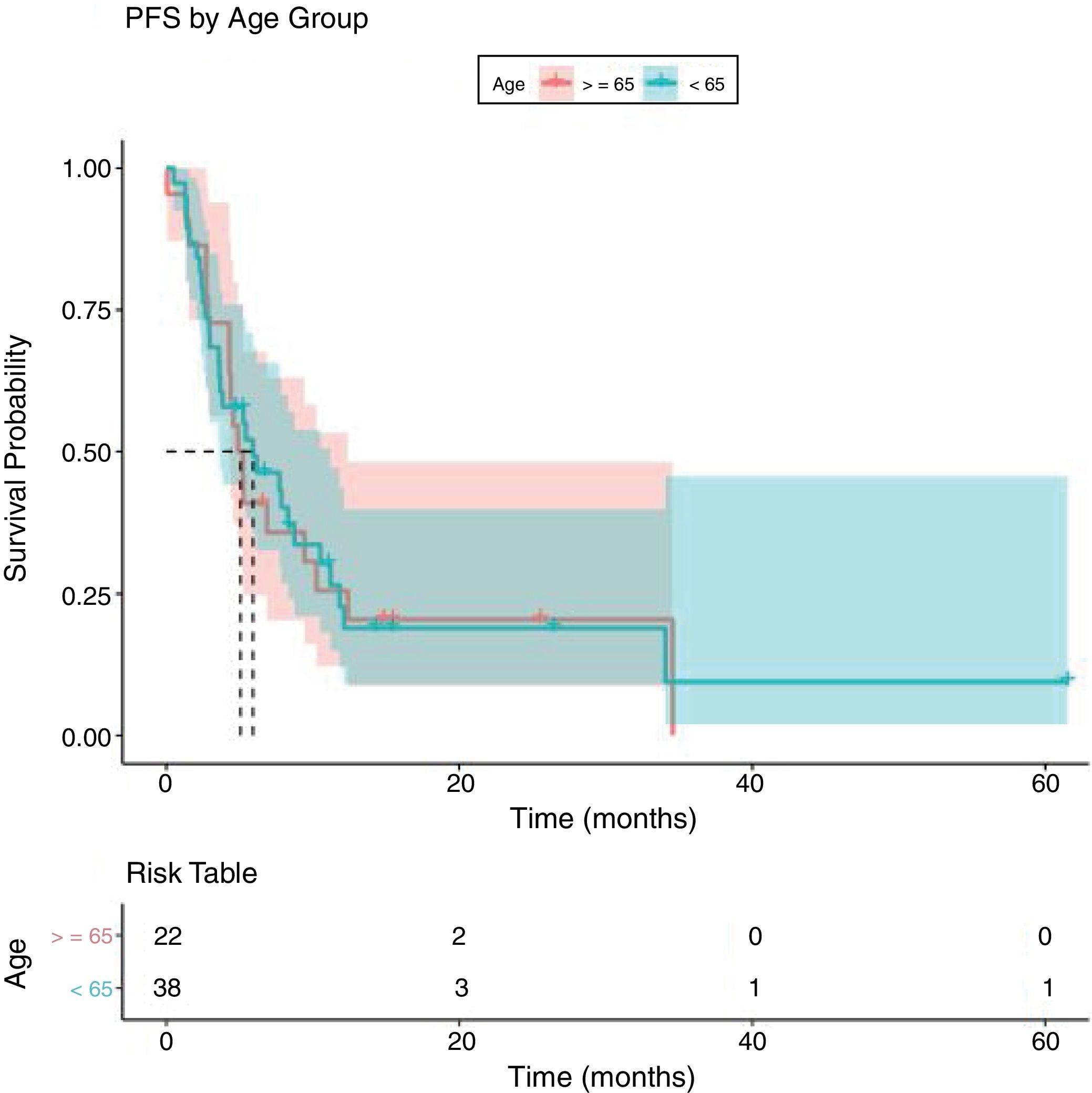
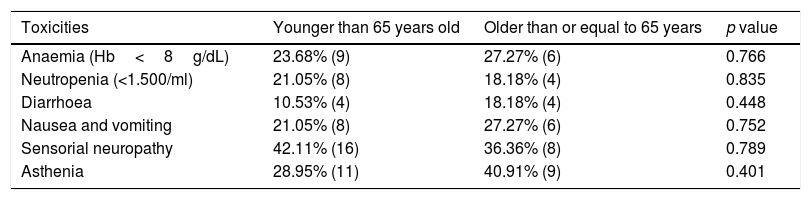
![Progression free survival curve by Kaplan–Meier [median OS<65 years old: 5.92 months (95% IC: 3.62–11.2) and for ≥65 years old 5.06 months (95%IC: 4.31–12.4)]. Progression free survival curve by Kaplan–Meier [median OS<65 years old: 5.92 months (95% IC: 3.62–11.2) and for ≥65 years old 5.06 months (95%IC: 4.31–12.4)].](https://static.elsevier.es/multimedia/02141582/0000003400000002/v1_202103240827/S0214158220301018/v1_202103240827/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Overall survival curve by Kaplan–Meier [median OS<65 years old: 26.9 months (95% IC: 18.6–30.7) and for ≥65 years old 20.3 months (95%IC: 11.4–33.6)]. Overall survival curve by Kaplan–Meier [median OS<65 years old: 26.9 months (95% IC: 18.6–30.7) and for ≥65 years old 20.3 months (95%IC: 11.4–33.6)].](https://static.elsevier.es/multimedia/02141582/0000003400000002/v1_202103240827/S0214158220301018/v1_202103240827/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

