Rosai-Dorfman disease represents a very rare idiopathic disorder mainly characterized by sinus histiocytosis and massive lymphadenopathy. In even rarer occasions (<43%), extranodal involvement has been reported, with one of the least common presentations being isolated breast affection (<1%). We report the case of a 64-year-old woman who debuted with a suspicious breast lump and underwent a prolonged study protocol before a proper histopathological diagnosis could be obtained. Surgical management was effective, and follow-up shows no signs of recurrence. In the context of ruling out breast malignancy, even rare alternatives should be considered when a diagnosis eludes us.
La enfermedad de Rosai-Dorfman representa un trastorno idiopático infrecuente caracterizado por histiocitosis sinusal y linfadenopatía masiva. Aún más rara es la manifestación extranodal de esta patología (<43%), particularmente cuando afecta la mama de forma aislada (<1%). Presentamos el caso de una mujer de 64 años quien debuta con una masa sospechosa en su mama y quien requirió un protocolo de estudio prolongado antes de obtener un diagnóstico histopatológico acertado. El manejo quirúrgico fue efectivo y al seguimiento no ha presentado datos de recurrencia. En el contexto de descartar malignidad en las mamas, inclusive las alternativas infrecuentes deben considerarse cuando un diagnóstico nos elude.
Sinus histiocytosis with massive lymphadenopathy or Rosai-Dorfman disease (RDD) is a very rare pathology which typically presents as a benign massive, bilateral lymphadenopathy in the head and neck region of males between their second and third decade of life.1 While extranodal disease has been described in up to 43% of reported cases, sole breast affection is exquisitely rare with less than 50 cases cited in English literature.2 This kind of presentation can often mimic breast malignancy both clinically and radiologically, presenting a diagnostic challenge that may warrant further studies and treatment despite its typically benign course.3 We present the case of a 64-year-old female patient presenting with breast Rosai-Dorfman disease debuting as a suspicious breast lump.
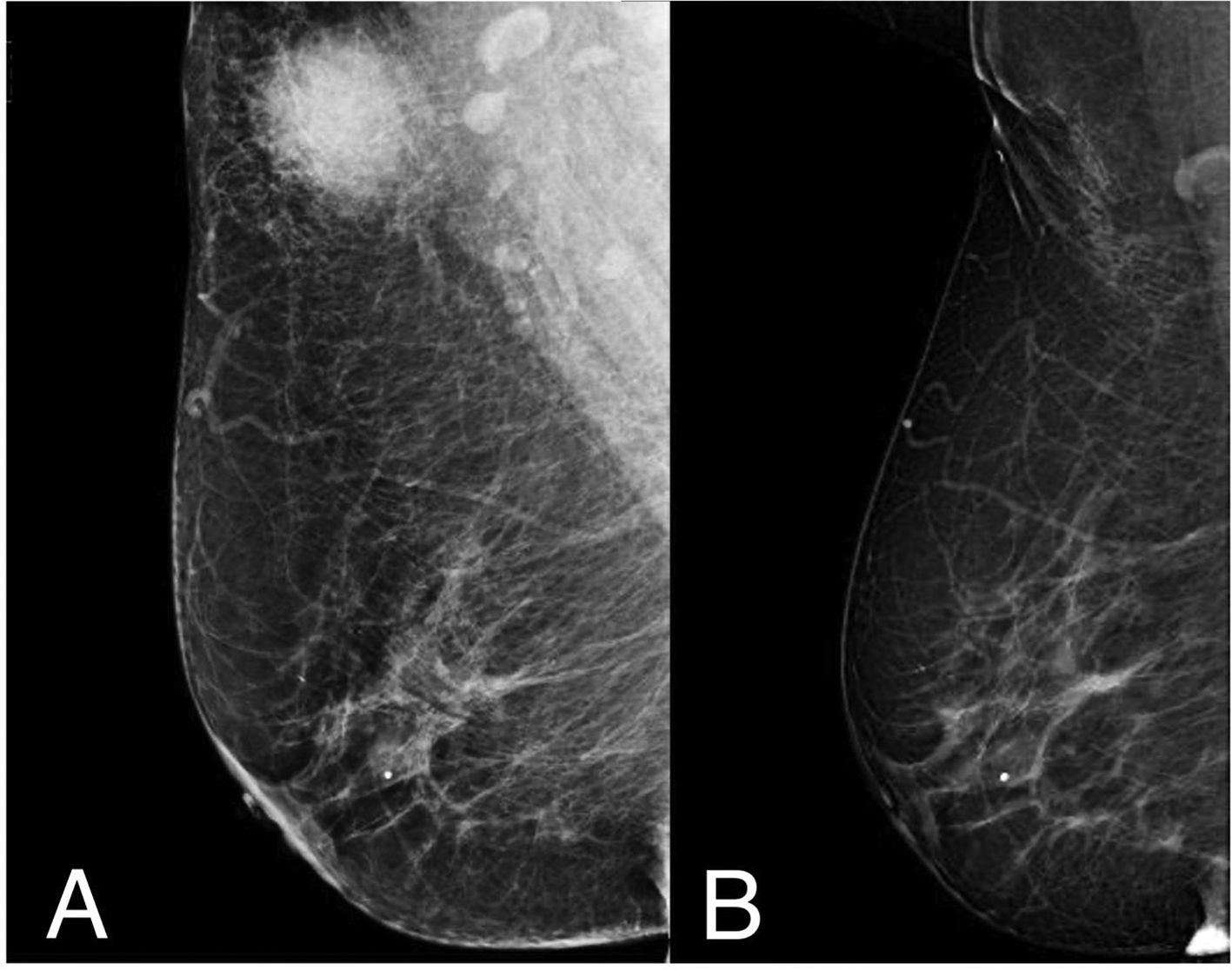
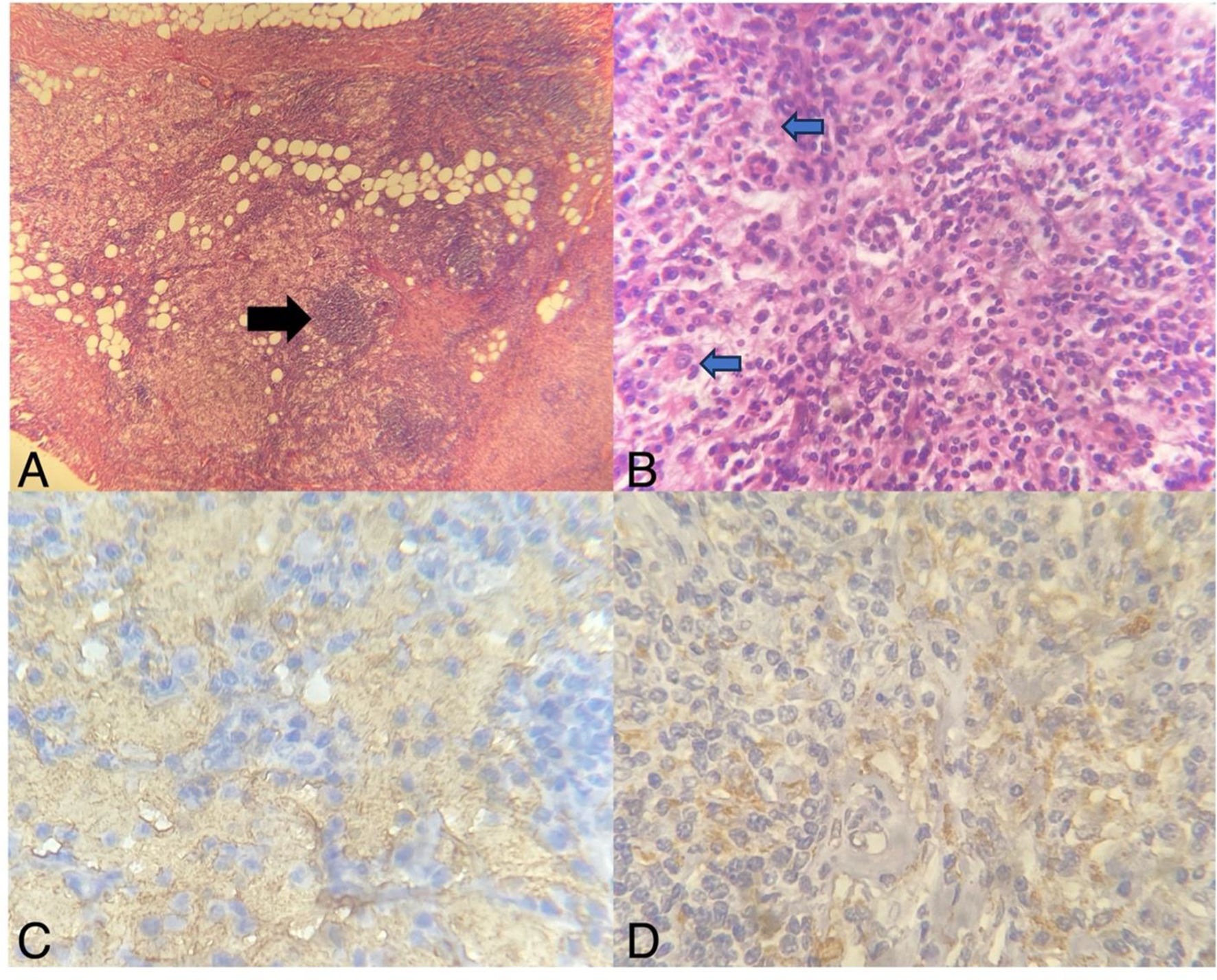
Case presentationA 64-year-old female patient without relevant past medical history is referred to our institution in 2019 after discovering a solitary breast nodule. Her case began in August 2018 when she discovered a tender spot in her right breast during routine self-examination. The lump continued to increase in size during the following months, causing the patient to schedule an appointment in an external clinic. During the initial examination, the presence of a firm, mobile nodule was confirmed. The patient denied breast pain, skin changes, nipple discharge, or systemic manifestations. A mammogram and breast ultrasound were ordered; with both studies reporting a BIRADS 4 image in the upper outer quadrant of the right breast of approximately 22 × 17 × 22 mm in size (Fig. 1A). Due to the characteristics of the mass and the patient’s age group, a breast malignancy was suspected. The patient underwent 2 separated core-needle biopsies. The first biopsy reported signs of inflammatory tissue and lymphocytic proliferation while the second reported inflammatory tissue with a predominance of macrophages. Due to diagnostic uncertainty, the patient was sent to our institution for further studying. Upon arrival, her previous studies were reevaluated by our team. A new physical examination of the lesion yielded findings similar to those previously reported by the referring physician. A magnetic resonance was ordered, revealing a homogeneous T1 hypointense/T2 hyperintense image in the tail of Spence without evidence of additional findings. The patient underwent an excisional biopsy which reported a proliferative lesion with clear cells that stained positively for S100 and CD68, IgG4 staining was not performed. An initial diagnosis of a granular cell tumor (GCT) was made, nonetheless, no other typical morphological signs of GCT were found and the diagnosis was challenged, granting further examination of the specimen. In a second histopathological analysis, evidence of emperipolesis was found, discarding the previous diagnosis, and confirming the disease as an extranodal breast presentation of RDD (Fig. 2). The patient showed optimal post-surgical recovery and continues to be kept in periodical checkup with a yearly mammogram and monthly self-examination. At 3.5 years of follow-up, she shows no signs of either local or systemic recurrence (Fig. 1B).
Breast scan before and after surgical management. (A) Patient’s breast scan after being referred to our institution. A dense, round, heterogeneous mass with irregular margins can be observed in the upper outer quadrant of the right breast which corresponded with the palpable lesion presented by the patient. A BIRADS-4 score was once again assigned to the lesion, warranting further investigation. (B) Follow-up study in the same breast 3.5 years after surgical excision of the lesion with minimal scar tissue and contour deformity in the corresponding site. A small calcification within the glandular tissue is present in both studies without suspicion of malignancy, reported as BIRADS 2.
Histopathological analysis of surgical specimen. (A) Breast parenchyma exhibiting a fatty tissue, peripheral hyalinizing fibrotic bands, lymphoplasmacytic infiltration, and nodules of histiocyte proliferation (black arrow); HE-10X. (B) Characteristic lymphoplasmacytic infiltrate accompanied by moderate sclerosis within breast parenchyma, as well as RDD cells (blue arrow with black outline); HE-100X. (C) Positive S100 staining in immunohistochemistry; 400X. (D) Positive CD68 staining; 400X.
RDD, also known as “Rosai-Dorfman-Destombes disease”, was first described in 1959 by Pierre-Paul Louis Lucien Destombes as a lipid storage disorder after inflammation.4 It wasn’t until 1969 that pathologists Juan Rosai and Ronald Dorfman associated the clinical symptoms of RDD with the pathological features of histiocytosis and lymphoproliferation.5 Initially termed “sinus histiocytosis with massive lymphadenopathy” due to its most typical presentation, this disease is a rare proliferative and inflammatory idiopathic disorder classified as a type of non-Langerhans cell histiocytosis.1,3 Although the exact etiopathogenesis remains unknown, more and more reports suggest and association with certain viral agents such as human herpes virus, Epstein-Barr virus, and parvovirus B19.3,6,7
The main population affected by this disease consists of male patients in their second or third decade of life who present with massive, bilateral lymphadenopathy in the head and neck region, as well as fever, malaise, and increased inflammation markers (particularly lymphocytes and erythrocyte sedimentation rate) on laboratory studies.8 The course of this disease tends to be benign, with spontaneous remission being reported in up to 20% of patients, relapsing and remitting disease in 70% of cases, and a small percentage of cases (<10%) progressing to a systemic affection.4 This more aggressive course has been linked to mutually exclusive recurrent somatic KRAS and MAP2K1 mutations, which may be present in up to a third of RDD specimens.7,8 Poor prognosis has been associated with certain risk factors such as older age, concurrent immunologic dysfunction, and organ involvement of the kidneys, lung, or liver.7,9 Death as a direct result of RDD is thought to vary between 7% and 12% of cases in large series.3
Extranodal affection in RDD has been described concomitantly in 25%–40% of cases, with exlusively extranodal disease appearing in approximately 23% of reports.9 The most common sites for extranodal RDD are the skin, upper respiratory tract, orbits, and bone.1 Sole breast affection is extremely rare within the spectrum of this pathology, with some reviews citing a prevalence of less than 1% of cases and less than 50 reports described in medical literature.2–4,7–13
Most cases of breast RDD present as an asymptomatic solitary lump of variable size (ranging from 0.5 to 6.6 cm) with very few reports of progression to systemic disease requiring aggressive chemotherapeutic management.3,11,14 The main population afflicted by breast RDD consists of women over 50-years-old, with some authors attributing this to an increased accessibility to mammograms and public health campaigns screening for breast malignancy.7,9 Nonetheless cases have been reported within an age range of 15–84 years, with at least 4 of these involving males (less than 15% of reported literature, with an approximate F:M ratio of 10:1).4,7,10,14 Radiologically, this disease most commonly presents as a poorly defined rounded mass in mammograms and as hypoechoic mass in ultrasound, being frequently reported in both as BIRADS 4 or 5.7 This findings, in a patient with a gender and age group such as ours, tend to raise suspicion towards the presence of breast malignancy.11 The mainstay of confirmatory diagnosis is histopathological analysis, nonetheless, a high index of suspicion is necessary as common staining methods and routinary examinations may fail to find conclusive signs of the disease due to insufficient tissue samples, an absence of characteristic findings, or a lack of access to inmunostaining.8 Fine needle aspiration has been diagnostic in only a small cluster of the reported cases and while core biopsy has yielded higher results in comparison, excisional biopsy remains the preferred method for definitive diagnosis.13,15 Proliferative histiocytes with emperipolesis, while not exclusive of RDD, are considered to be pathognomonic.16 Emperipolesis is defined as the presence of an intact cell within the cytoplasm of another cell, but unlike phagocytosis, these remain viable and can exit at any time without causing structural or functional compromise in either cell.8 Although emperipolesis has been reported to be highly prevalent, in a majority of cases, it is also highly inconspicuous, with a review of 22 cases reporting a lack of prominent emperipolesis in 72% of patients, this being the case with the initial examination of our patient’s tissue samples.13 The most prevalent histological findings were reported to be a dense lymphoplasmacytic infiltrate and prominent sclerosis, which can suggest a variety of other pathologies, but should also start to raise suspicion of breast RDD.13 Other important characteristics that may aid in differential diagnosis are the round nuclei, distinctly different from the elongated and grooved nuclei in Langerhans cells, and the immunohistochemical profile of this histiocytes which tend to stain positively with S100 (typically negative in normal histiocytes, also positive in Langerhans cells) and CD68, while remaining negative to CD1a.10,16 Newer studies have reported a high sensitivity with the monocyte-macrophage marker OCT2 and cyclin D1.14 Amongst the differential diagnoses most frequently considered, lymphoma with plasmacytic differentiation, IgG4-related sclerosing mastitis, granulomatous disease, and inflammatory myofibroblastic tumor are the most commonly mentioned in literature.13,14 Within this group of pathologic entities, some of the main aspects to be considered are the absence of positive inmunostains for B-cells (when suspecting lymphoma due to the lymphoplasmocytic infiltrate), low yield or absence of IgG4 positive staining (when suspecting IgG4-related sclerosing mastitis, usually with a IgG4/IgG ratio <30% in most reported cases and requiring >40% for a positive diagnostic criteria), negative ALK immunohistochemical staining (when suspecting inflammatory myofibroblastic tumor), as well as negative Grocott methenamine silver and acid-fast bacillus stains (when suspecting infectious granulomatous mastitis).13,14 Due to the high index of diagnostic uncertainty present even with proper work-up and biopsies, as well as the concern of a malignant breast tumor, most cases undergo surgical excision (up to 80%) with a proper RDD diagnosis being made post-operatively.3,7 Despite most cases having a benign prognosis, it’s always important to maintain a proper follow-up of the patient due to the possibility of recurrence at a local or systemic level, with some cases having reported local or systemic recurrence up to 6 years after the initial management.11–13 Reports on prognosis are scarce and currently, there are no existing protocols or guidelines for the management and vigilance of these patients, with some institutions relying on annual radiological studies.3,8,13 Such is the case of our center where this patient’s follow-up is conducted through a yearly mammogram and office visit. According to the bibliography reviewed for this article, an acceptable algorithm for management may look very similar to those currently established for any suspicious breast nodule, with a core needle biopsy as the initial measure, followed by an excisional biopsy both as the standard surgical treatment as well as a when faced with diagnostic uncertainty in hopes of avoiding overtreatment since the prognosis is generally benign. While most of the reported cases showed an adequate response to these measures, and as such, it is the authors current recommendation, published information on long-term follow-up is limited.
ConclusionRosai-Dorfman disease is a very infrequent pathology, one with an exquisitely rare disposition to present as a solitary breast nodule. Such presentation steps outside the borders of the population, we normally associate with RDD and seems to favor both a different gender and age group. In our patient’s demography, any solid lesion in the breast must be properly studied to discard the possibility of malignancy. While this presentation of RDD is extremely infrequent and scarcely reported, it is useful to keep this diagnosis in mind for cases where the proper diagnosis of a breast lesion seems to elude us.
FundingThis research did not receive any specific grant from funding agencies and all expenses were covered by the authors.
Ethical disclosuresThe authors declare that they have followed their center's protocols on the handling and publication of patient data.
Patient consentThe authors declare that they obtained written patient consent for publication of this article.
Authors contributionsAll authors were involved in the conception, data collection, and drafting of the article.