Phyllodes tumour corresponds to 1% of breast primary tumours. Its primary treatment is presently surgical and, for several reasons, it usually requires breast reconstruction. First of all, an important breast volume is often removed, not only because it can easily achieve great dimensions, thanks to it fast growing potential, but also because wide excision is recommended to avoid its frequent but unpredictable local recurrence (meaning that ideal tumour resection margins are unknown). Second, it frequently affects young woman with more aesthetic concerns.
We present a case of phyllodes tumour recurrence where oncoplastic surgery was necessary at both episodes and discuss the literature controversies on this subject, mainly related to treatment and prognosis prediction factors.
El tumor phyllodes representa el 1% de los tumores primarios de mama. Actualmente, su tratamiento primario es la cirugía y, por muchas razones, requiere normalmente reconstrucción de mama. Primeramente, porque a menudo es extirpado un importante volumen de mama, no sólo porque el tumor puede adquirir grandes dimensiones gracias a su alto potencial de crecimiento, sino también porque es recomendado una amplia excisión para evitar su frecuente pero impredecible recidiva local (los márgenes quirúrgicos ideales no son conocidos). Segundo, porque afecta frecuentemente a mujeres jóvenes con más preocupaciones estéticas.
Presentamos un caso de recidiva de tumor phyllodes donde la cirugía oncoplástica fue necesaria en ambos episodios y discutimos las controversias en la literatura sobre este tema, principalmente dirigidos al tratamiento y a los factores pronósticos.
Phyllodes tumour (PT) is a fibroepithelial tumour which accounts for 1% of breast primary tumours.1 The term phyllodes (which means “leaflike”) refers to its histologic appearance in which a double layer epithelial component is surrounded by its hypercellular stromal/mesenchymal component. The WHO has defined several histologic features that help differentiate PT from fibroadenomas and classify them into benign, borderline or malignant (degree of stromal cellularity, atypia and overgrowth, mitotic count, tumour borders behaviour and the presence of malignant heterologous components).1 However, PTs intratumoural heterogeneity, and the continuous spectrum between benign and malignant categories, makes their grading accuracy and reproducibility less achievable.2 Molecular markers like MED12 (Mediator of RNA polymerase II transcription) mutations or DNA ploidy have been studied to help define more precisely the tumour clinical behaviour. MED12 mutations is frequently associated with a longer disease-free survival while its absence is associated with a higher recurrence rate.3 Aneuploidy (versus diploidy) is also associated with an increased recurrence rate.4 PTs usually have clearly defined margins and can be surgically “enucleated”, however they are well known for their recurrence potential – consensus is lacking to define which optimal margins protect against recurrence.2
Mainly the malignant PT can metastasize hematogenously (like sarcomas). Metastasis are usually of the stromal component, with loss of the epithelial-stromal interdependence.2
Because PT arise in women between the age of 40 and 50 years and because of their usual rapidly growing capability (therefore presenting with large volumes, 5–10cm), they represent an oncoplastic challenge.5 We report a recurring PT and the offered breast reconstruction treatments in each situation.
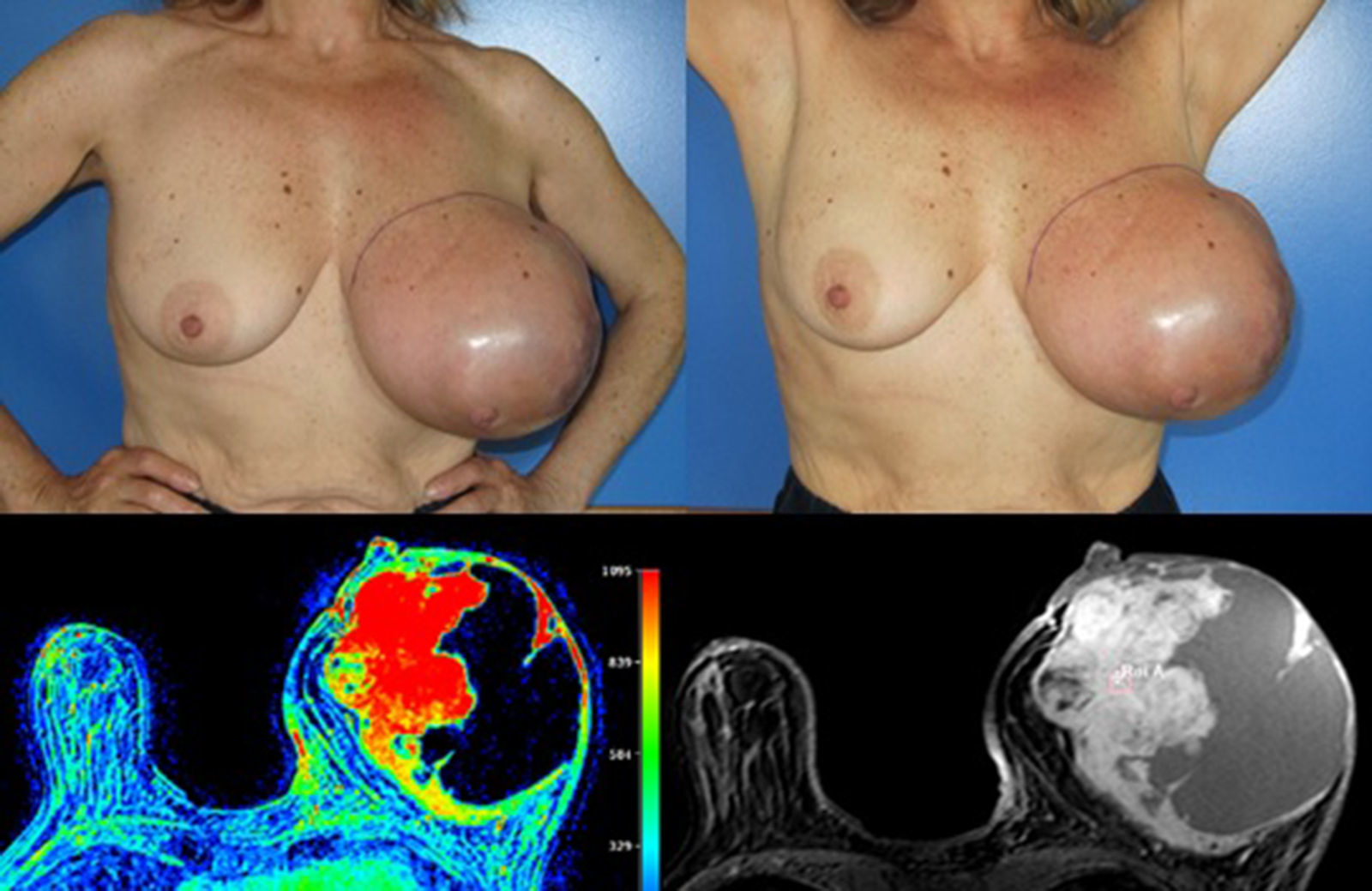
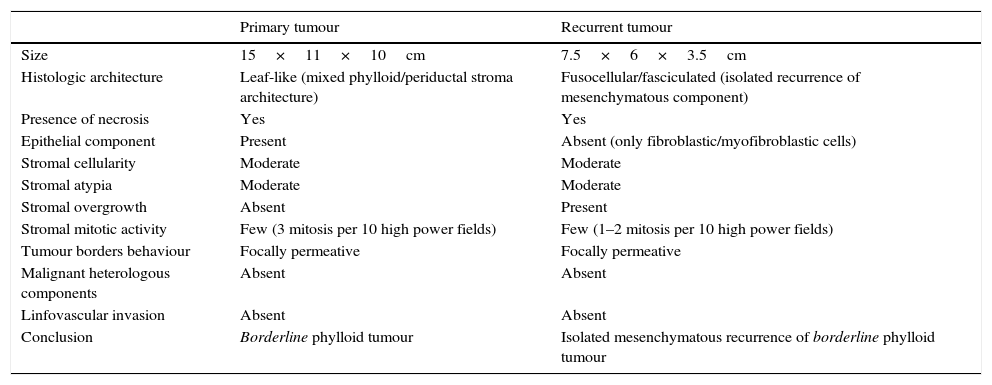
Case reportA 58 years old woman, heavy smoker, otherwise healthy, with no relevant personal or familiar history, presented with a painful, rapidly growing, breast mass with inflammatory characteristics (but without linfedema). To palpation it was a multinodular mass with firm consistency and no palpable lymph nodes. For years she had noticed a stable small lump in the same location, however, after reassured by her doctor, she had never had, so far, a breast imagiologic exam (Fig. 1).
A core needle biopsy revealed a fibroepithelial tumour with hypercellular stroma, no mitosis, no pleomorphism or heterologous components. These findings were suggestive of a benign PT but total excision was required for a conclusive diagnostic.
The magnetic resonance imaging (MRI) showed a 13×11cm ovoid shaped polylobulated mass in the left breast, that had an heterogeneous appearance with 50% of the mass occupied by cystic areas and the rest by solid areas with a rapid contrast enhancement pattern (type 2 curve). The pectoralis major muscle did not appear to contact with the breast tumour. The regional lymph nodes and the right breast where uncharacteristic. The left breast was classified as BIRADS 4b (Breast Imaging-Reporting and Data System Classification) and the right breast as BiRads 2 (Fig. 1).
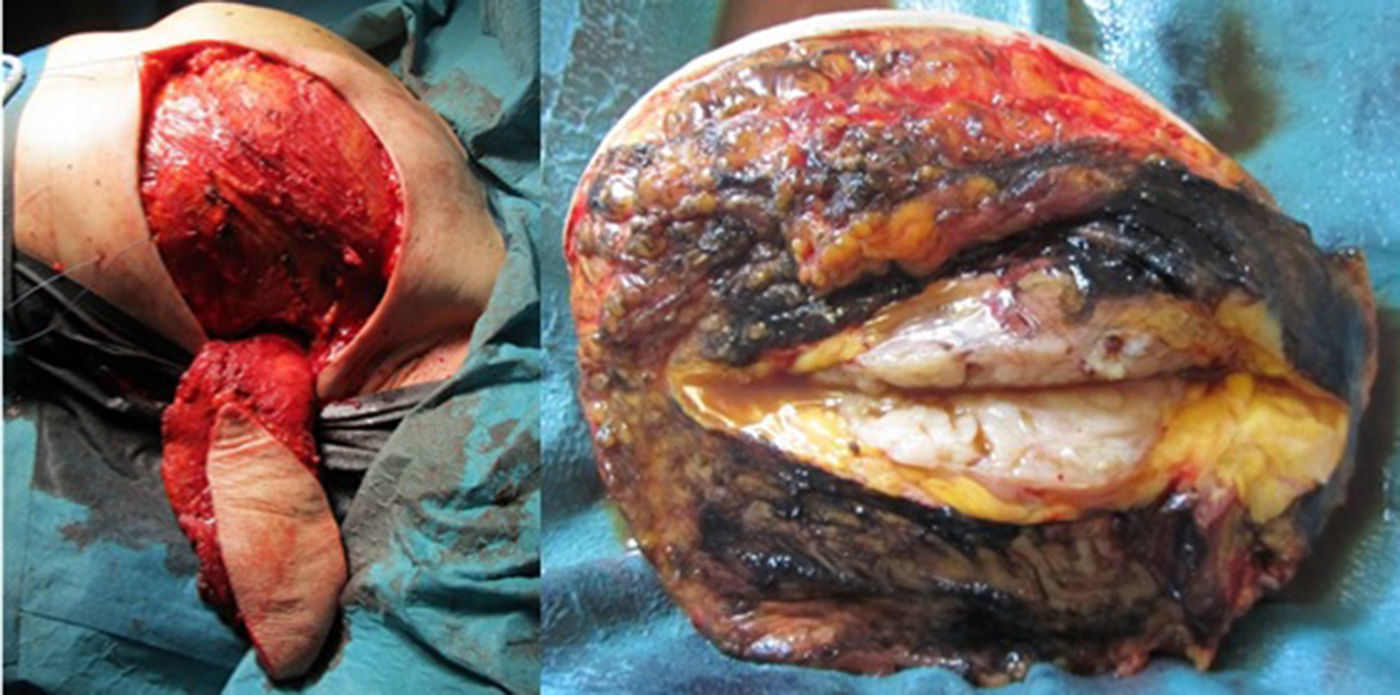
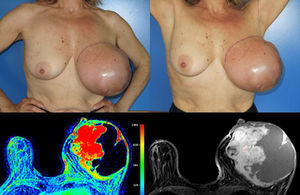
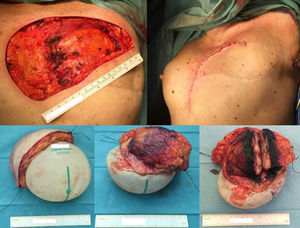
Considering the tumour dimension, a radical surgery, rather than a conservative approach, was considered the appropriate surgical option for this case. A simple mastectomy with maximum breast tissue extirpation was provided, wielding wide macroscopic margins (confirmed by intraoperatory pathological evaluation). Due to the important cutaneous deficit, immediate reconstruction with a flap was necessary. Among the flap possibilities, latissimus dorsi muscle was the best suitable flap due to smoking habits. A symmetrization mammoplasty was done in the right breast (Fig. 2).
The histologic result was a 15×11×10cm borderline PT with the histologic features detailed in Table 1. The right breast fragments had normal histology.
Histopathology of primary and recurrent tumour.
| Primary tumour | Recurrent tumour | |
|---|---|---|
| Size | 15×11×10cm | 7.5×6×3.5cm |
| Histologic architecture | Leaf-like (mixed phylloid/periductal stroma architecture) | Fusocellular/fasciculated (isolated recurrence of mesenchymatous component) |
| Presence of necrosis | Yes | Yes |
| Epithelial component | Present | Absent (only fibroblastic/myofibroblastic cells) |
| Stromal cellularity | Moderate | Moderate |
| Stromal atypia | Moderate | Moderate |
| Stromal overgrowth | Absent | Present |
| Stromal mitotic activity | Few (3 mitosis per 10 high power fields) | Few (1–2 mitosis per 10 high power fields) |
| Tumour borders behaviour | Focally permeative | Focally permeative |
| Malignant heterologous components | Absent | Absent |
| Linfovascular invasion | Absent | Absent |
| Conclusion | Borderline phylloid tumour | Isolated mesenchymatous recurrence of borderline phylloid tumour |
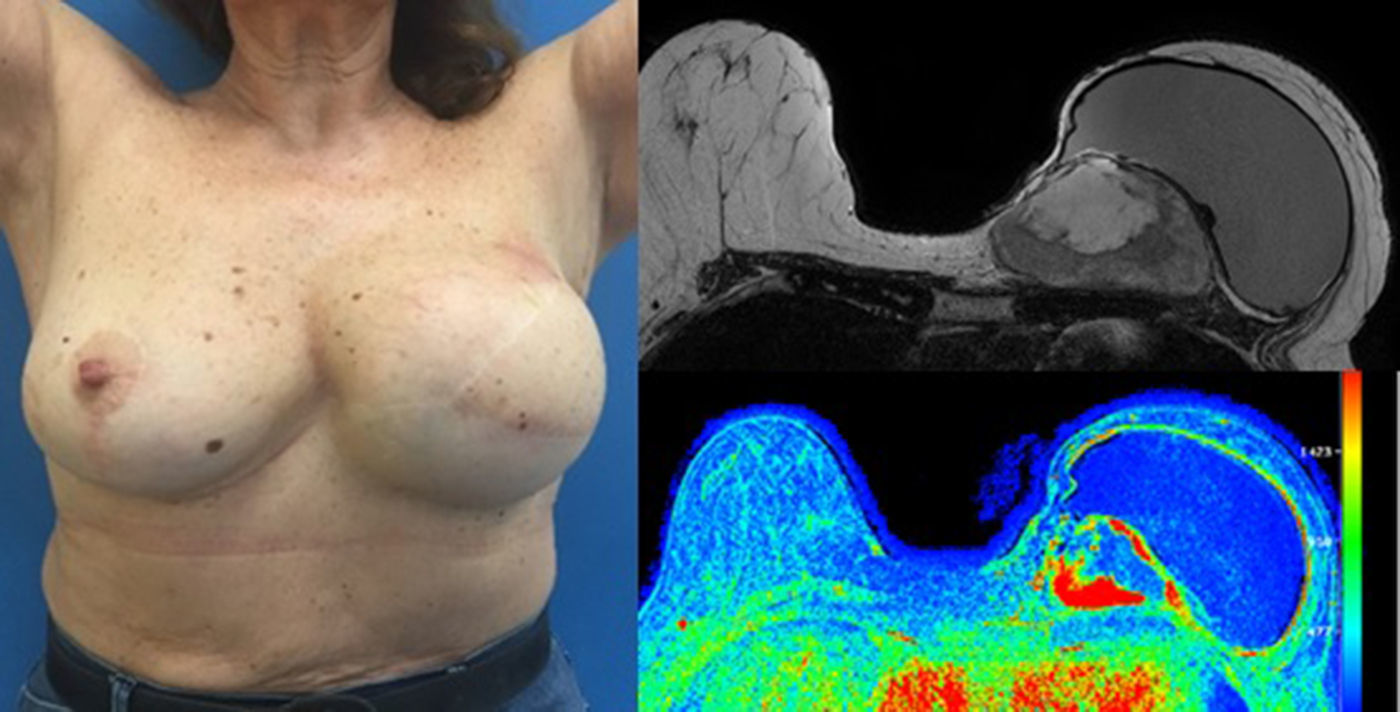
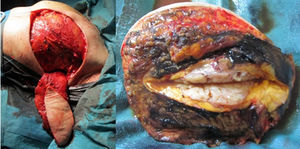
The patient follow-up was done clinically and with an annual mammogram. Four years and 7 months after the first surgery, a rapidly growing mass was clinically evident near the medial end of the surgical incision and the histologic confirmation of the recurrence revealed a borderline PT (with 8 mitosis per 10 high power fields). MRI showed a 8×7×5cm tumour and the rapid contrast enhancement with wash-out (type 3 curve), lymph nodes were unremarkable (Fig. 3).
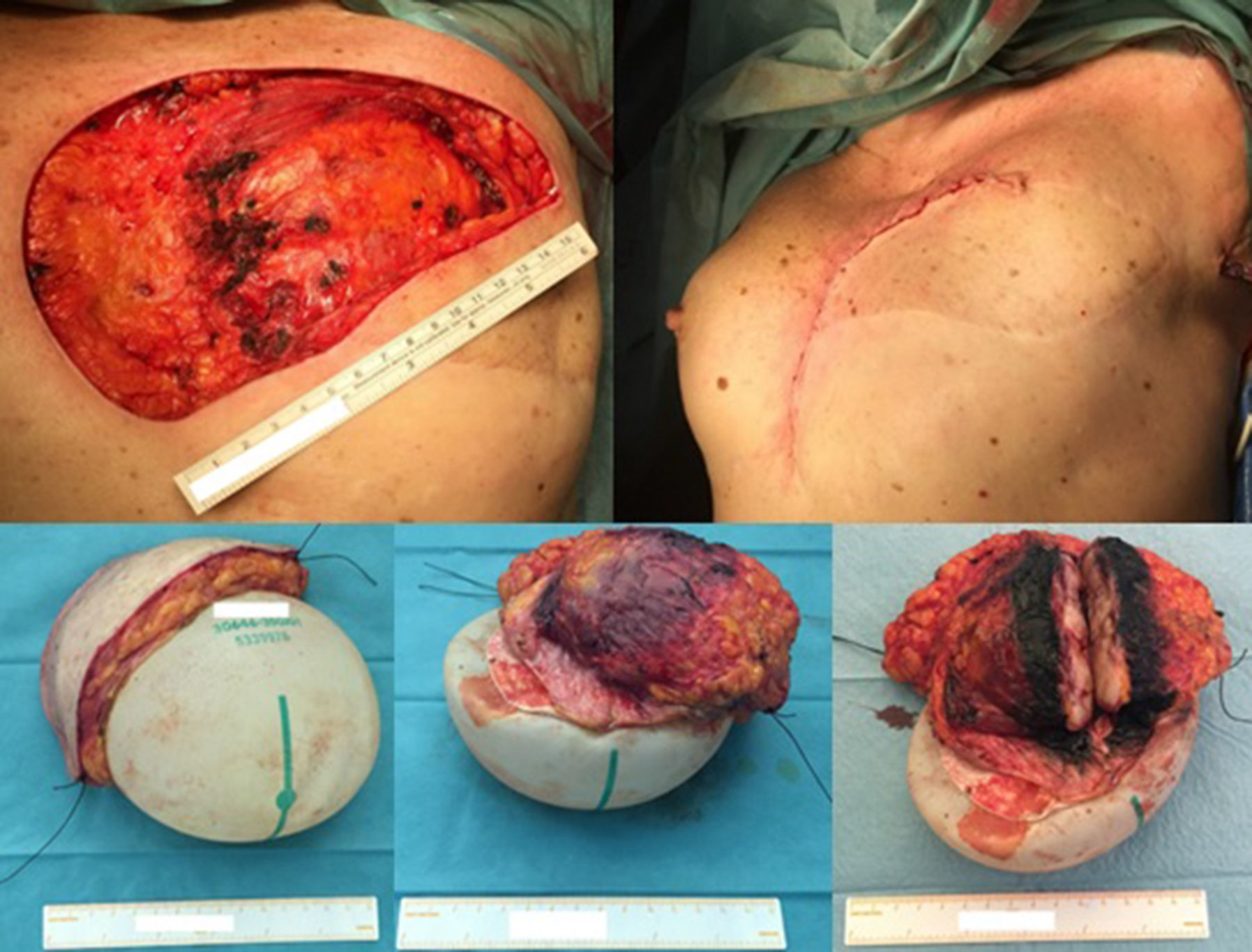
Excision of the tumour, together with the breast implant, part of the pectoralis major muscle (6×3×2.5cm) and partial resection of the breast implant capsule was feasible in one single block. A local tissue flap advancement (skin and remaining latissimus dorsi muscle) was enough to ensure closure with an expander placed. This option conciliated a tumour excision with safety margins, and a definitive reconstruction, avoiding the sacrifice of another flap (Fig. 4).
The wide excision of the local recurrence was accomplished with the partial resection of the pectoralis major and the lastissimus dorsi muscle (together with the breast implant and a segment of its capsule), in one unique piece. Closure was achieved by a local tissue advancement flap – initially corresponding to the tissue covering the lower and lateral part of the breast implant.
The histologic analysis revealed total absence of epithelial cells with an isolated mesenchymal component recurrence. This stromal overgrowth was the only feature suggestive of malignant phylloid tumour according to the WHO's international histologic classification. All the other histologic characteristic were typical of borderline (moderate stromal cellularity and atypia) or benign (less than 5 mitosis per 10 high power fields) phylloid tumour. If malignant heterologous components were present it would be enough to qualify a malignant phylloid tumour. In this case an isolated mesenchymatous recurrence of a borderline phylloid tumour was diagnosed.
DNA ploidy analysis with flow cytometry showed diploidy (this was not analyzed in the primary tumour).
The patient has presently a 2 months follow-up period and continues in the expansion process.
DiscussionA clinically benign lump, even if stable for years before, should always call the attention if it becomes hypersensitive or tender after menopause, grows quickly or becomes painful no matter what age. Once detected, an initial ultrasound or ultrasound plus mammography (according to the patient age or breast density) to assess its initial imagiologic characteristics should be done. In doubt, a core biopsy is usually conclusive. Assisted vacuum biopsy or surgical excision is both valid options if the lesion is symptomatic or if a complete histologic evaluation is required.
So far there is no precise consensus determining which suspected or histologic confirmed fibroepithelial lesions should be totally removed. However, even if imagiologically benign, a biopsy must always be done before surgery (this will also help planning enucleation for fibroadenomas versus wide excision for PTs). Complete excision of all fibroepithelial lesions probably is an overtreatment and is not recommended.
In case of PT, although the primary treatment is surgical, optimal margins are not defined. The French College of Gynecologists and Obstetricians has recently recommended, with low quality of evidence (grade C recommendation), clear margins for grade 1 PT (benign) and 10mm margins for grade 2 PT (borderline).6 For local recurrence or malignant PT, total mastectomy is the preferred approach.5
It is important to inform the patient with PT about the recurrence and metastatic risk according to the histologic features of the tumour (even if, in some cases, the histological grade alone does not correctly predicts the biological aggressiveness of the tumour and the clinical prognosis).7,8
After many classifications have been proposed, the 2003 WHO Phylloid tumour classification has been the most widely accepted and commonly used. Considering the poor relation between histologic grade and prognosis, several authors have tried to identify genetic or molecular markers (MED12 mutations,3 DNA ploidy,4 collagen type III α1,9 others).10,11 This has been a hard task due to long time latency, low number of cases, and intratumoural heterogeneity.11 Nowadays this markers are only investigational, and, due to little prognostic value, none has been recommended for routine use. Other possible prognostic factors have been published like older age, bigger size, presence of necrosis, affected margins and adjuvant treatments.7,12 Precocious recurrence or grade progression with recurrence are also of undetermined informative value. Research continues with the pursuit of identifying more objective and useful variables, needed to identify high risk patients who would benefit from wider margins or adjuvant therapies.
Breast reconstruction with other myocutaneous flaps like deep/superficial inferior epigastric perforator, transverse rectus abdominus, superior/inferior gluteal artery flap are also an option.13 However, they require a higher expertise level (involving microsurgery), a longer operative time and hospital stay.
Adjuvant treatment with chemotherapy,8,14,15 radiotherapy8,14 or hormonal therapy presently have no clear evidence of benefit and remain controversial. Given the rarity of PT, randomized controlled trials are unexpected. Nevertheless, for malignant tumours, adjuvant radiotherapy registered an increase but its use was established based on an individual basis (affected margins, increased tumour volumes, recurrence, others)16 as it has not shown disease free or overall survival benefit (only less local recurrence rate).17
In conclusion, PT represents an oncoplastic challenge. Its natural history is not well known which challenges recurrence prediction or its risk factors identification. Further knowledge about this tumour may have surgical and other therapeutic implications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors declare no conflicts of interest.