Histological lesions of risk of breast cancer are proliferative epithelial lesions associated with a higher incidence of breast cancer. The magnitude of the risk is related to the presence of epithelial atypia. Lesions with atypical hyperplasia have a relative risk of cancer 4 times higher than the control population. Proliferative epithelial lesions present diverse histological characteristics and must be appropriately classified. They include usual ductal hyperplasia, radial scar and complex sclerosing lesions, sclerosing adenosis, apocrine adenosis, microglandular adenosis, papillary lesions, atypical ductal hyperplasia, flat epithelial atypia, lobular neoplasia. They propose the differential diagnosis with ductal carcinoma in situ and invasive carcinoma. When risk lesions type B3 are diagnosed in needle-core and vacuum-assisted biopsies, the underestimation rates for invasive carcinoma should be below 5% and below 10% for ductal carcinoma in situ. In this review, we describe the clinical and histological aspects of the different entities and discuss the possible management options depending on the diagnostic category and the lesion size. Correct management requires close collaboration between surgeons, radiologists, and pathologists.
Las lesiones histológicas de riesgo de cáncer de mama son lesiones epiteliales proliferativas asociadas a una mayor incidencia de cáncer de mama. La magnitud del riesgo está relacionada con la presencia de atipia epitelial. Las lesiones con hiperplasia atípica tienen un riesgo relativo de cáncer cuatro veces mayor que la población control. Las lesiones epiteliales proliferativas presentan características histológicas diversas y deben ser clasificadas adecuadamente. Incluyen la hiperplasia ductal usual, la cicatriz radial y lesiones esclerosantes complejas, la adenosis esclerosante, adenosis apocrina, adenosis microglandular, lesiones papilares, hiperplasia ductal atípica, la atipia epitelial plana y la neoplasia lobulillar. Plantean el diagnóstico diferencial con el carcinoma ductal in situ y con el carcinoma infiltrante. Cuando se diagnostican en biopsias por punción y en biopsias asistidas por vacío lesiones de riesgo tipo B3, la tasa infraestimación de carcinoma infiltrante y carcinoma ductal in situ debe ser inferior a 5% y 10%, respectivamente. En esta revisión describimos aspectos clínicos e histológicos de las distintas entidades y discutimos las posibles opciones de manejo, en función de la categoría diagnóstica y del tamaño de la lesión. El manejo correcto de estas lesiones requiere una estrecha colaboración entre cirujanos, radiólogos y patólogos.
Histological lesions at risk for breast cancer are epithelial proliferative lesions that are associated with a higher incidence of invasive breast carcinoma. The magnitude of the risk is related to the existence of epithelial atypia.
Epidemiological studies indicate the importance of histological classifications (Table 1).1–3 Benign proliferative lesions with atypia have a higher relative risk (RR) of breast cancer than the control population.
Classification of lesions at risk for breast cancer.
| Proliferative lesions without atypia. Low risk. RR 1.27–1.90 |
| Ductal hyperplasia without atypia, usual or florid type |
| Sclerosing and apocrine adenosis |
| Lesions with columnar cells |
| Radial scar and complex sclerosing lesions |
| Papillomas |
| Proliferative lesions with atypia. High risk. RR 3.7–5.9 |
| Atypical ductal hyperplasia |
| Flat epithelial atypia |
| Atypical lobular hyperplasia |
In patients with carcinoma in situ, ductal, or lobular, the risk affects primarily the quadrant of the breast where the initial lesion is diagnosed.4,5 These lesions are considered non-obligatory precursors of invasive breast carcinoma.
Lobular carcinoma in situ (LCIS) behaves like risk lesions and as non-obligatory precursors of breast cancer since their presence indicates a greater bilateral risk of developing invasive carcinoma. Still, the risk is significantly higher for the quadrant where LCIS is first diagnosed.5–7
With the implementation of core needle biopsies (CNB) and vacuum-assisted biopsies (VAB) in patients that present lesions detected by imaging, the question of the appropriate management arises. International societies have recently published consensus and guidelines for diagnosing and treating breast lesions of uncertain malignant potential.8–11
This study aims to review the diagnostic criteria of benign proliferative lesions of the breast, the associated risk of carcinoma, and management based on the diagnosis with minimally invasive biopsies (CNB or VAB) and vacuum-assisted excision (VAE), which aims to remove a similar amount of tissue as diagnostic surgical excision.11
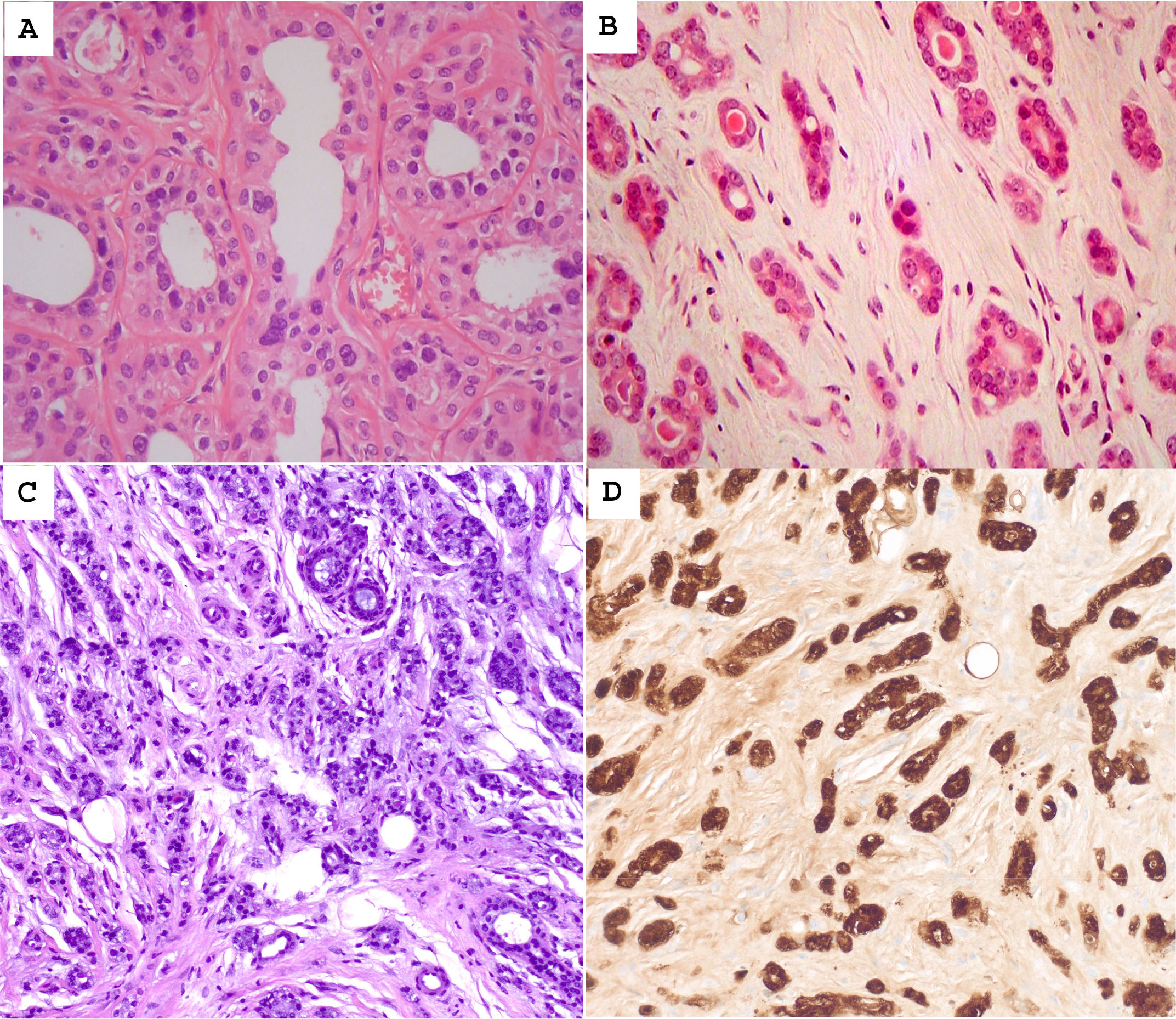
Usual ductal hyperplasia (UDH)UDH presents a heterogeneous intraductal proliferation of cells without nuclear atypia (Fig. 1A).12
(A) Usual ductal hyperplasia showing intraluminal cellular proliferation. The cells are arranged in streams, and they tend to overlap. They delineate irregular-shaped spaces. (B) Radial scar showing a central area of sclerosis and a lobulocentric architecture. (C) Complex sclerosing lesion with irregular acinar proliferation, surrounded by collagenous stroma. (D) Sclerosing adenosis is characterized by the proliferation of epithelial and myoepithelial cells embedded in a collagenous stroma.
It raises the differential diagnosis with atypical ductal hyperplasia (ADH) and low or intermediate-grade ductal carcinoma in situ (DCIS).
The immunohistochemical (IHC) study with high molecular weight cytokeratins, reactive with basal/myoepithelial cells (CK 5/6, CK 14), is helpful since cell proliferation is patchily positive in UDH and negative in ADH and DCIS.12–14 Likewise, stains with estrogen and progesterone receptors are positive in isolated cells in UDH but uniformly positive in ADH/DCIS.12,13
The etiopathogenesis of UDH needs to be better understood. The studies by Boecker et al. propose that cell proliferation is formed by progenitor cells of the ductal epithelium and myoepithelium.14
UDH confers a low RR of infiltrating breast cancer (RR. 1.5–2); therefore, when it is detected in CNB or VAB, a surgical biopsy is not indicated.8
Radial scar (<1 cm) and complex sclerosing lesion (>1 cm)They are asymptomatic and rarely palpable lesions, being able to simulate the image and histology of an invasive carcinoma (Fig. 1B and C). They are detected in 0.5% of CNB, 7% of benign surgical biopsies, and 26% of resections for carcinoma.15–18 Its RR is low (1.5–1.8).18
The radial scar and complex sclerosing lesion may be associated with epithelial proliferation without atypia, ADH, and carcinoma in situ, ductal, or lobular. In surgical resections, carcinoma can be detected in 10% of cases (0%–25%). Lesions that present atypia must be surgically removed. However, open excision (OE) may not be mandatory in lesions without atypia, in which the radiological image agrees with the diagnosis by CNB or VAB, if the radiological target lesion has largely been removed by VAB or VAE.7,8,11
Sclerosing adenosis, atypical apocrine adenosis, and microglandular adenosisSclerosing adenosis (SA) is a benign lesion showing the proliferation of small acini with abundant myoepithelial cells and fibrous stroma (Fig. 1D). It is associated with a low RR (2.2), like other proliferative lesions without atypia.19
SA with apocrine change (apocrine adenosis) may present atypia (Fig. 2A).20
(A) Atypical apocrine adenosis showing acini lined by cells with pink granular cytoplasms. The nuclei are hyperchromatic and moderately pleomorphic. (B) Microglandular adenosis showing rounded acini containing eosinophilic secretions. (C) Atypical microglandular adenosis shows rounded acini with intraluminal secretions. Some acini are irregular and fused. (D) S100 immunostaining shows diffuse positivity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Atypical apocrine adenosis (AAA) preferentially affects postmenopausal women.21 The differential diagnosis of AAA should be established with apocrine DCIS.
Microglandular adenosis (MGA) is an extremely rare proliferative lesion characterized by rounded glandular structures lined by cells containing eosinophilic secretions (Fig. 2B).22,23 It presents an IHC profile characterized by the absence of the myoepithelial layer, positivity with S100, and triple-negative immunophenotype (Fig. 2D). Laminin and collagen IV stains demonstrate the presence of basal lamina. Atypical MGA shows a more complex architecture with cytological atypia (Fig. 2C). Twenty-five per cent of atypical MGA are associated with carcinoma. It is related to triple-negative breast cancer.24,25 The lesion must be surgically removed when atypical MGA is diagnosed in CNB/VAB.
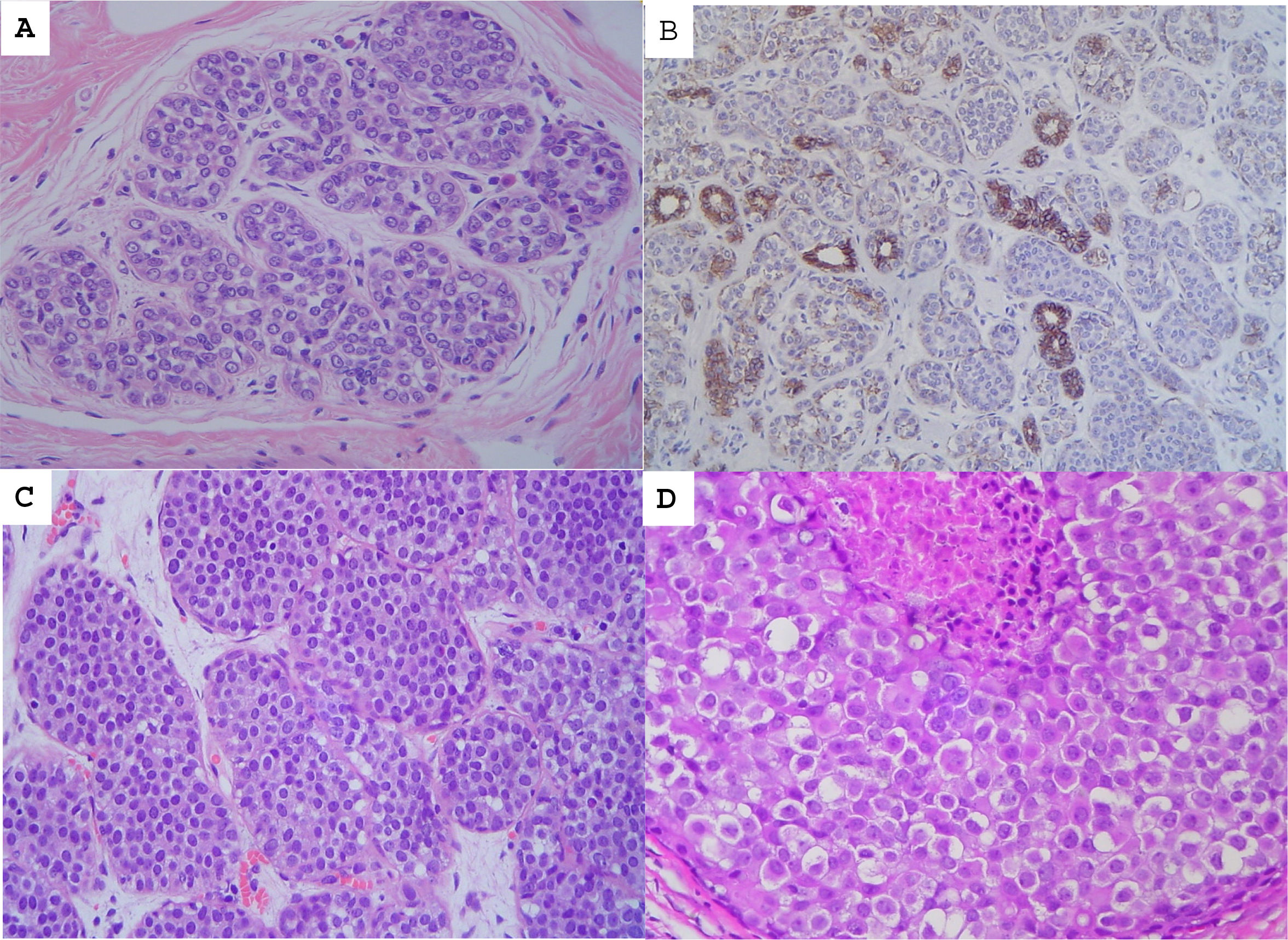
Non-invasive papillary lesionsPapillary lesions can be central or peripheral, solitary, or multiple (Fig. 3). The lesions may be asymptomatic or palpable and produce bloody secretion. They are histologically characterized by intraductal epithelial proliferation associated with fibrovascular cores. They can be benign, atypical, or harbor non-invasive carcinomas.26,27,28 A double epithelial and myoepithelial layer lines benign papillomas (Fig. 3B). Papilloma may present areas of ADH or foci of low-grade DCIS. Their size differentiates these; less than 3 mm for ADH and greater than 3 mm for DCIS.
The IHC study is essential in diagnosing benign intraductal papilloma.27 Myoepithelial stains show positivity in the papillae and ductal layer (Fig. 3C).
The RR of carcinoma associated with central and peripheral papilloma is 2 and 3, respectively. The RR associated with papilloma with atypia is 7.5.28–31. Papillomas without atypia, diagnosed by CNB, present a higher risk lesion at surgery in less than 10% of cases. Therefore, the surgical indication must be individualized based on the patient's characteristics, image, and lesion size. After a diagnosis by CNB/VAB, it is considered acceptable to carry out radiological follow-up if the target image has been removed. However, papillary lesions with ADH present a higher risk lesion with a high frequency (>50%), for which OE is indicated.8,9,11
Atypical ductal hyperplasiaADH is diagnosed histologically based on cytological, architectural, and quantitative criteria (Fig. 4A and B).32–35 The cytology and architecture of ADH are like those of low-grade DCIS. The difference between ADH and low-grade DCIS is quantitative. Atypical proliferative lesions that affect up to 2 lobular units or measure up to 2 mm are diagnosed as ADH, and larger lesions are diagnosed as low-grade DCIS. The WHO recommends a conservative diagnosis, favoring a diagnosis of ADH in small lesions, especially when diagnosed in CNB.7 These quantitative criteria are orientative and arbitrary.
(A) Atypical ductal hyperplasia shows epithelial cell proliferation, with low-grade nuclear atypia, forming micropapillae and cellular bridges. (B) CK14 immunostaining is limited to the myoepithelial layer and a few foci of usual ductal hyperplasia. (C) Flat epithelial atypia showing distended acini with intraluminal calcifications. (D) The acinus is lined by epithelial cells with overlapping, rounded nuclei.
ADH presents genetic alterations like those described in low-grade DCIS, low-grade invasive ductal carcinoma, and lobular neoplasia (LN). These include losses of 16q and 17p and gains of 1q.12
ADH represents 5%–10% of benign breast biopsies; the RR of breast cancer for this population is 3–5 times; the absolute risk of breast cancer is 15% (3.7%–30%). The risk affects both breasts. The mean latency time after the diagnosis of ADH in a surgical biopsy until the development of breast carcinoma is 8.3 years.34
The histological diagnosis of ADH must be established by pathologists who are experts in breast diseases.35
Some cases present histological features that fall between ADH and low-grade DCIS; these cases are designated ADH bordering on DCIS (ADH-BD).36–38
OE is recommended after a diagnosis of ADH on CNB/VAB.9,11
In small lesions, less than 15 mm, VAE could be considered.11
Columnar cell lesions and flat epithelial atypiaLesions with columnar cells frequently contain secretions and microcalcifications (Fig. 4C).39 A subgroup of these lesions, flat epithelial atypia (FEA), shows low-grade nuclear atypia but no significant intraluminal proliferation characteristics in ADH (Fig. 4D).
FEA is detected in up to 10% of CNB and 2.4% of surgical biopsies. The RR of carcinoma is low (2.04). It is frequently associated with ADH (46%). The detection of DCIS in surgical biopsy is 9%.40
After a diagnosis of FEA on CNB, resection with VAB is recommended, and radiological follow-up if 90% or more of the lesion has been removed.9,11
Lobular neoplasiaThe term LN encompasses atypical lobular hyperplasia (ALH) and LCIS.41
LCIS is not a direct precursor of invasive breast cancer but instead behaves as a risk factor.
LN is diagnosed with greater prevalence in premenopausal women. It is frequently a multifocal and multicentric (50%) and bilateral (30%) disease. LN does not present characteristic images. It can be associated with other lesions (SA, radial scar, and columnar cell lesions). Microcalcifications occur in 23%–60% of cases.41,42
Classic LCIS presents a uniform population of cells with rounded and eccentric nuclei that occupy more than 50% of the lumen of the mammary acini, causing them to distend (Fig. 5A). Lesions of smaller volumes are classified as ALH.
(A) Lobular carcinoma in situ, classical type, showing distended acini completely occupied by poorly cohesive, rounded cells. (B) E-cadherin immunostaining with lack of expression in the lobular carcinoma in situ. (C) Lobular carcinoma in situ, florid type, with markedly distended acini and low-grade cellular atypia. (D) Lobular carcinoma in situ, pleomorphic type. The cells are markedly atypical, showing eccentrically located nuclei. Focal necrosis is shown.
The IHC study of LCIS shows a loss of E-cadherin, with the absence or a marked decrease in cytoplasmic membrane staining (Fig. 5B); up to 10% of the cases may show aberrant expression of E-cadherin. Beta-catenin and p120 can also be helpful in defining the immunophenotype in selected cases.
Molecular studies of LN most frequently show a loss of 16q and a gain of 1q. The CDH1 gene mutation, located at 16q 22.1, is related to the loss of expression of E-cadherin, a suppressor gene associated with cell adhesion.5,41
Patients with LN have a higher risk of developing invasive breast cancer, lobular or non-special (ductal) type. The RR is 4–5 times in HLA and 8–10 in LCIS. Invasive carcinoma develops with a low prevalence over a long period (15–30 years). The breast cancer mortality rate is low after the diagnosis of LCIS (1.1% after 12 years). The risk of invasive cancer affects both breasts but is higher for the ipsilateral breast. The absolute risk at 20 years is approximately 20%.5,43
After a diagnosis of CNB/VAB, observation is recommended in most patients rather than surgery.9,11 However, if there is radiological–pathological discordance, OE or VAE is advised.
Histological variants of LCIS require special mention: florid LCIS (FLCIS) and pleomorphic LCIS (PLCIS).5,6,41–46
FCLIS presents low nuclear atypia but causes significant acini distention and may show necrosis (Fig. 5C).
PLCIS is characterized by marked nuclear atypia. They frequently present necrosis and resemble DCIS with comedonecrosis (Fig. 5D). With some frequency, it can be HER2 positive.41,43–46
LCIS variants (FLCIS and PLCIS) usually present target lesions, generally microcalcifications, on imaging. After diagnosis by CNB, surgical excision is recommended due to the frequent presence of a higher-grade lesion in 33%–38% of cases.44
In the case of PLCIS, surgical management like that of DCIS is recommended, and the margins should be free of tumor.41,44,45 Radiotherapy is advised after conservative surgery in PLCIS due to its similarity to high-grade DCIS.44–46
General principles for the management of proliferative lesions at risk for breast cancer (Tables 2 and 3)8–11The pathologist plays a fundamental role in the histological diagnosis of breast lesions and participates in management decisions with radiologists and surgeons. For this, the pathologist must have complete information on the clinical symptoms and the findings of the imaging techniques.
Diagnostic classification system for core needle biopsies.
| B1: Normal breast tissue | Indicate the presence of breast epithelium and microcalcifications |
| B2: Benign lesion | Include fibroadenoma, fibrocystic changes, sclerosing adenosis, ductal ectasia, and others. |
| B3: Lesion of uncertain malignant potential | Benign lesions that may be heterogeneous or associated with a higher risk of malignant lesions. Include ADH, FEA, LN, papillary lesions, radial scar, mucocele-like lesions, and rare lesions. |
| B4: Lesion suspected of malignancy. | Does not allow a definitive diagnosis due to a lack of material or associated artifacts |
| B5: Malignant lesion | If possible, specify the grade and histological type |
| B5a. DCIS, malignant papillary lesion, pleomorphic lobular carcinoma situ | |
| B5b. Invasive carcinoma and other malignant tumors | |
| B5c. Carcinoma cannot be specified if it is in situ or infiltrative. |
Management of risk breast lesions diagnosed by CNB/VAB.
| Type of lesion | Recommendation | Comment |
|---|---|---|
| Atypical ductal hyperplasia | Open resection | Consider VAE in small lesions |
| Flat epithelial atypia and columnar cell lesions | Resection with VAB and radiological follow-up if 90% of the target lesion has been removed | |
| Radial scar/complex sclerosing lesion | Resection with VAB and radiological follow-up | VAE if needed |
| Papillary lesions | After CNB diagnosis of lesions without atypia, complete resection of the target lesion with VAB | Open excision/VAE of lesions with atypia and large papillary lesions |
| LCIS and atypical lobular hyperplasia | After diagnosis with CNB or VAB, radiological follow-up | Open excision/VAE of lesions with radiological-pathological discordance Resection of pleomorphic and florid LCIS |
Minimally invasive biopsies (CNB and VAB) are the procedure of choice for the diagnosis of palpable and non-palpable breast lesions. For the histological classification, it may be helpful to use the diagnostic categories proposed by the Royal College of Pathologists of the United Kingdom (Table 2).47,48
OE is generally recommended for ADH lesions because the underestimation rates should be below 5% for invasive carcinoma and below 10% for DCIS. However, VAE is being implemented for small lesions.
Radiological follow-up is recommended after removal with VAB or VAE in B3 lesions without atypia.
FundingNo funding has been used for this review.
Ethical committee approvalThe characteristics of the review exempt it from ethics committee approval.