The increased survival achieved with surgery and cancer treatments for breast cancer has led to a growing concern about fertility in young women, adversely affecting quality of life outcomes. The potential loss of fertility not only has a psychological but can also affect patients' treatment adherence or decisions, such as selection of treatments associated with a lower risk of infertility despite inferior outcomes. Our objective is to provide an overview of current knowledge about how systemic cancer treatments could impact the future fertility of breast cancer survivors The main hypothesis of how chemotherapy produce damage to the ovaries is a direct damage to the DNA of the oocytes, resulting in the activation of apoptosis and/or autophagy-related pathways. Alkylating agents as cyclophosphamide are the chemotherapeutic agents that are associated with the highest risk of gonadotoxicity; however, breast cancer patients are usually given combinations of chemotherapy drugs. Conversely, endocrine therapy, such as tamoxifen, does not appear to have a permanent effect on fertility but long-term use could lower oocyte quality due to patient aging. There is still not enough evidence about the effect on ovarian reserve for many biological therapies and for new drugs being used in the non-metastatic setting, and further research in this area is needed. Members of multidisciplinary teams in breast cancer should understand the impact on fertility of cancer treatments and the importance of an early referral of premenopausal patients to specialists in reproductive medicine to discuss all possible fertility preservation strategies.
La mayor sobrevida alcanzada con la cirugía y los tratamientos del cáncer de mama ha generado una creciente preocupación por la afectación de la fertilidad en mujeres jóvenes, lo cual puede impactar negativamente en su calidad de vida. La posible afectación de la fertilidad no solo tiene repercusión psicológica, sino que también puede afectar la adherencia al tratamiento o su selección, pudiendo incluso las pacientes optar por tratamientos asociados con un menor riesgo de infertilidad aún con resultados inferiores. Nuestro objetivo es brindar una visión general del conocimiento actual sobre cómo los tratamientos sistémicos oncológicos pueden impactar en la fertilidad futura de pacientes sobrevivientes al cáncer de mama. La principal hipótesis de cómo la quimioterapia produce daño en los ovarios es el daño directo al ADN de los ovocitos, lo que resulta en la activación de vías relacionadas con la apoptosis y / o autofagia. Los agentes alquilantes como la ciclofosfamida son los agentes quimioterapéuticos que se asocian a mayor riesgo de gonadotoxicidad, si bien habitualmente las pacientes con cáncer de mama reciben combinaciones de varios agentes de quimioterapia. Por el contrario, la terapia endocrina, como el tamoxifeno, no parece tener un efecto permanente sobre la fertilidad, pero su uso prolongado podría reducir la calidad de los ovocitos debido al envejecimiento. Se están utilizando nuevas terapias biológicas en la enfermedad localizada para las cuales aún es desconocido su efecto sobre la reserva ovárica, necesitándose un mayor desarrollo de la investigación en esta área. Los integrantes de equipos multidisciplinarios deben comprender el potencial impacto en la fertilidad de los tratamientos oncológicos y la importancia de una derivación temprana de las pacientes premenopáusicas a especialistas en medicina reproductiva con el objetivo de discutir todas las posibles estrategias para la preservación de la fertilidad.
The increasing number of cancer survivors with better long-term prognosis in recent decades have raised concerns about quality of life issues, including the impact of systemic cancer treatments on their future fertility. Many systemic cancer treatments that have shown the best survival outcomes in young women are associated with an increased risk of premature ovarian failure and infertility, as well as other side effects of estrogen deficiency such as cardiovascular disease, osteoporosis, and loss of libido.
Once oncological disease has been controlled, the possibility of conceiving a child becomes an important concern for many women, with studies showing that approximately 47%–63% of young female cancer survivors desire biological children1. In contrast, the growing tendency to postpone childbearing has led to an increase in the number of nulliparous women of reproductive age who are diagnosed with cancer2. In addition, women of reproductive age who survive an oncological disease have pregnancy rates approximately 40% lower than those of the general population (adjusted for age, level of education, and previous parity)3.
The potential loss of fertility not only has a psychological impact leading to emotional stress, anxiety, and depression but can also affect patients' treatment decisions, such as treatment adherence and selection of treatments associated with a lower risk of infertility despite inferior outcomes4–6. In this regard, it has been reported that approximately 30% of young women may not comply with their treatment plan because of its potential impact on fertility7. Previous research assessed 620 women with early breast cancer and showed that approximately 0.6% of patients decided not to undergo chemotherapy because of the risk of infertility, 1.9% opted for a different chemotherapy regimen to reduce such an impact, and up to 15.5% reported refusing or reducing the time of adjuvant hormone therapy for the same reason8.
In developed countries about 40% of breast cancer patients aged 40 and younger feel they have not yet completed their families8, so could be the candidates for a fertility preservation strategy. Despite this, a prospective study showed that 18.5% of women aged ≤40 years with early breast cancer diagnosis candidate to receive chemotherapy accepted to undergo a cryopreservation technique9.
As fertility is a key aspect to the quality of life of cancer survivors, all patients should be informed and counseled when making decisions related to cancer treatment and fertility preservation techniques for future family planning. In this regard, international guidelines also recommend early assessment at diagnosis (prior to cancer treatment) for fertility preservation in cancer patients10–12.
Regardless, the rate of documentation of fertility preservation discussions in the medical notes of women with breast cancer diagnosis aged 40 and younger have been reported up to 55%, and the rate of recall of this discussion by patients has shown rates up to 50%–68%8,13.
Here, we review the impact of systemic breast cancer treatments on the fertility of young women.
Breast cancer is the most frequently diagnosed cancer in women of childbearing age (20–39 years in most countries), but corresponds to less than 7% of all newly diagnosed tumors in the Western world14,15. Improvements in multimodal therapies have led to an increased chance of cure in approximately 70%–80% of patients worldwide. However, it is reported to be slightly lower in in patients aged <40 years, which is associated with a higher frequency of aggressive biological subtypes16,17.
It is essential that health-care professionals involved in breast cancer treatment acquire knowledge about the impact of systemic cancer treatments on future fertility so that they can correctly advise young women.
Referring patients earlier to oncofertility specialists allows for a better range of fertility preservation options such as oocyte, embryo, or ovarian tissue cryopreservation, and if one of these strategies could not be offered, an oocyte donation or adoption can be considered10. In addition, protection with gonadotropin releasing hormone agonists can be considered, not as a method to preserve fertility, but as a technique to preserve ovarian function18,19.
Factors determining the impact on fertilityThe impact of cancer treatments on fertility depends on several factors, including the type of cancer to be treated, the patient's age at the time of treatment, the type of treatment, the dose, and its duration. A history of previous infertility treatment, wherein age and the type of treatment are also important factors for risk assessment20.
With regard to the type of cancer, a Norwegian retrospective study analyzing 16,435 women aged 16–45 years who were diagnosed with cancer found that the pregnancy rates in women with melanoma or thyroid cancer were similar to those in the general population. However, the lowest rates were reported for women with breast cancer, stage I cervical cancer, and leukemia, with a decrease of approximately 65%–67%. Chemotherapy was not the only reason for women with breast cancer to have lower pregnancy rates; it was also the use of prolonged endocrine therapy in the case of sensitive endocrine tumors and the misconception of an increased risk of relapse with pregnancy after a breast cancer diagnosis21.
Females are born with approximately one million oocytes that are stored in the primordial follicles of the ovarian cortex. The number of follicles decline as women get older, beginning at approximately 500,000 oocytes at the age of menarche and decreases to approximately 25,000 oocytes by the age of 37–38 years. Since the loss accelerates over time, conception is harder to achieve until menopause is finally reached, when there are no oocytes left.
When the follicles are damaged due to chemotherapy, the result is temporary oligomenorrhea or amenorrhea, which can cause menopausal symptoms. If the number of primordial follicles falls below the minimum value necessary to achieve ovarian cyclicity, there is irreversible ovarian failure and menopause (>12 months without menstruation), which can occur either during chemotherapy or later, after several years of oligomenorrhea22–24. In young women, in whom amenorrhea occurs after treatment and menstrual cycle resume, ovarian age (as assessed by the ovarian reserve) is affected by premature aging of approximately 10 years compared to biological age25.
The risk of ovarian failure due to chemotherapy increases with the age of the patient at the time of treatment, since the number of follicles is lower at menopause than at puberty. A previous study found that a 40-year-old woman diagnosed with non-Hodgkin lymphoma was three times more likely to have ovarian failure than an 18-year-old woman who received the same treatment26. Therefore, fertility counseling should vary depending on the age of the patient.
Moreover, having a germline pathogenic variant in cancer susceptibility genes could influence the risk of treatment induced ovarian insufficiency because some of these pathogenic variants could impair DNA repair mechanisms. For younger breast cancer patients carrying BRCA mutations, the limited available evidence does not suggest an increased risk of treatment induced gonadotoxicity; however, for these patients, a lower reproductive potential before starting anticancer therapies cannot be excluded27,28. The potential added burden on the reproductive potential of these patients and fertility outcomes should be investigated promptly.
Effect of chemotherapy on fertilityThe effects of chemotherapy on fertility were first reported in the 1970s and were initially associated with the use of cyclophosphamide. The precise mechanisms underlying chemotherapy induced follicle loss remain unclear, and are still under investigation, with cell apoptosis emerging as the main cause24. Chemotherapy can cause direct DNA damage in oocytes due to its antitumoral mechanism of action (including inducing DNA double-stranded breaks, intra- and inter-strand crosslinking, intercalation between base pairs, and alkylation), which can result in the activation of apoptosis and/or autophagy-related pathways. In addition, chemotherapy can cause indirect DNA damage through increased oxidative stress or damage to the microvasculature of the ovary. Understanding these mechanisms is crucial for the development of research on fertility protective drugs29–31.
Chemotherapeutic agents are classified as cell cycle phase-specific or non-specific based on their antitumor effects.
For example, methotrexate and 5-fluorouracil exert their effects by blocking DNA synthesis; therefore, they are restricted to the S phase of the cell cycle (DNA replication phase), while vinca alkaloids and taxanes exert their action in the M phase (cell division phase). Phase non-specific chemotherapeutic agents cause damage in any phase of the cell cycle, including the G0 quiescent phase32.
During follicle development, proliferating granulosa cells can be damaged by all types of cytostatic agents, which can result in temporary amenorrhea. If the chemotherapeutic agent is phase-specific, the ovarian reserve of primordial follicles with immature oocytes (quiescent cells) should not be affected. Conversely, primordial follicles are more sensitive to phase non-specific chemotherapeutic agents, such as alkylating agents30.
It should also be noted that many clinical studies that have assessed the impact on fertility consider the duration of amenorrhea (with different definitions of time), which may not reflect the true impact of the treatment and would require studies with long-term follow-up. This form of fertility assessment would not seem accurate since the amenorrhea caused by the treatment is not always associated with infertility. On the contrary, the resumption of menstruation may not truly reflect an adequate ovarian reserve. Levels of anti-Mullerian hormone are considered a more reliable ovarian reserve marker in young women undergoing chemotherapy, although the evidence is still limited for its use in clinical practice33,34.
Chemotherapeutic agents have therefore been classified based on the risk of gonadotoxicity35.
a) High risk: alkylating agents (cyclophosphamide, ifosfamide, busulfan, chlorambucil, melphalan, procarbazine, and cyclophosphamide).
Alkylating agents: These chemotherapeutic agents are associated with the highest risk of gonadotoxicity. They act in all phases of the cell cycle, exerting cytotoxic effects on proliferating and non-proliferating cells. Alkylating agents have the potential to damage the DNA of immature oocytes of the primordial follicles, which constitute the ovarian reserve. The metabolites form crosslinks with DNA, triggering the inhibition of its synthesis and function, and double-stranded DNA breaks could lead to cell death by apoptosis24. Clinical studies have shown that the risk of infertility depends on both the cumulative dose of treatment and age of the patient. For breast cancer, the amenorrhea rate with the use of an alkylating agent (cyclophosphamide) as part of a polychemotherapy plan varies according to patient age, with a rate of 0%–15% in women aged 35 years, 30%–50% in women aged 36–60 years, and approximately 70% in women aged >70 years36–38.
b) Intermediate risk: platinum salts (cisplatin and carboplatin), anthracyclines (doxorubicin), taxanes (paclitaxel and docetaxel).
Platinum salts (cisplatin and carboplatin): These salts covalently bind to DNA to form intra- and inter-strand DNA crosslinks, leading to DNA breaks during replication. Although specific toxicity to primordial follicles has been demonstrated in animal studies, clinical evidence for ovarian toxicity with the use of cisplatin is limited. In general, patients receive cisplatin as part of a polychemotherapy regimen, which makes it difficult to determine its isolated gonadotoxic effect. In this regard, although platinum salts are considered to be a moderate risk treatment, this has only been reported in one clinical study using a polychemotherapy regimen that included cisplatin39. Other studies have not found this association, particularly after treatment of germ cell tumors; however, a potential gonadotoxic effect has been reported in males40,41.
Anthracyclines: These are also classified as having a moderate risk of gonadotoxicity. They interfere with DNA transcription by intercalating between DNA bases and inhibiting topoisomerase II, which causes double-stranded DNA breaks. Similar to platinum salts, it is difficult to determine the effect of anthracyclines on the ovary, since they are usually administered in combination with other chemotherapeutic agents. A study involving breast cancer patients reported that the incidence of amenorrhea with regimens containing adriamycin was 0% in women aged <30 years, 33% in women aged 30–39 years, and 96% in women aged 40–49 years42.
Taxanes: Phase-specific chemotherapeutic agents (M phase) bind to already formed microtubules and inhibit depolymerization so that the cell cannot divide and arrests. The impact of taxanes on the incidence of chemotherapy induced amenorrhea has not been well studied. Some reports have shown that the addition of a taxane is an independent risk factor for amenorrhea; however, there are other reports that do not support this finding43–46. The available data suggests that taxanes may contribute to the development of amenorrhea, although their absolute effect is likely small.
c) Low risk: vinca alkaloids (vincristine and vinblastine), antitumor antibiotics (bleomycin), antimetabolites (methotrexate and 5-fluoruracil).
Antimetabolites: Although available data on antimetabolites are limited, there is some evidence that they do not affect fertility. For example, the addition of methotrexate and 5-fluorouracil to alkylating agent regimens was not associated with an increase in amenorrhea after treatment47. Methotrexate has also been used to treat ectopic pregnancies without affecting subsequent fertility48.
Vinca alkaloids: These specifically bind to tubulin, which participates in microtubule formation, thus preventing the microtubule polymerization needed for mitosis. Animal models have shown high levels of aneuploidy in oocytes exposed to vinblastine. However, clinical studies have not reported an increased risk of ovarian failure49.
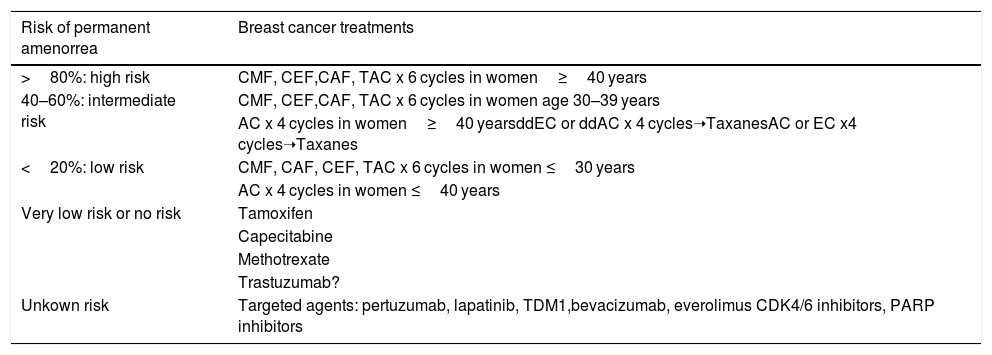
It is common for patients to receive combination cancer therapy involving several of the above drugs, making it difficult to accurately predict the risk of infertility. Information on amenorrhea rates for some chemotherapy plans has been obtained from published clinical studies. Table 1 shows the risk of amenorrhea with the use of chemotherapy regimens frequently indicated in patients diagnosed with breast cancer34.
Risk of permanent amenorrea with anticancer therapies for breast cancer. Modified from Lambertini M. et al.32.
| Risk of permanent amenorrea | Breast cancer treatments |
|---|---|
| >80%: high risk | CMF, CEF,CAF, TAC x 6 cycles in women≥40 years |
| 40–60%: intermediate risk | CMF, CEF,CAF, TAC x 6 cycles in women age 30–39 years |
| AC x 4 cycles in women≥40 yearsddEC or ddAC x 4 cycles➝TaxanesAC or EC x4 cycles➝Taxanes | |
| <20%: low risk | CMF, CAF, CEF, TAC x 6 cycles in women ≤30 years |
| AC x 4 cycles in women ≤40 years | |
| Very low risk or no risk | Tamoxifen |
| Capecitabine | |
| Methotrexate | |
| Trastuzumab? | |
| Unkown risk | Targeted agents: pertuzumab, lapatinib, TDM1,bevacizumab, everolimus CDK4/6 inhibitors, PARP inhibitors |
Regarding biological therapies, there is still insufficient information about their effects on ovarian reserve. However, no studies have shown direct toxic effects on the ovary. The prolonged use of any treatment could compromise fertility because the period of use is associated with lower ovarian reserve and lower oocyte quality due to patient aging. In the case of trastuzumab, an anti-human epidermal growth factor receptor 2 (HER2) antibody used in the treatment of HER2-positive breast cancer, clinical studies do not show an increased incidence of amenorrhea. However, it is recommended to wait for at least 7 months after finishing trastuzumab therapy before trying to conceive, given the increased risk of teratogenesis46,50. An unplanned analysis of a trial that explored the effectiveness of dual HER2 blockade and chemotherapy showed that either sequential or combined use of trastuzumab and lapatinib does not have a higher incidence of treatment amenorrhea than trastuzumab alone, although this trial lacked a control arm without anti-HER2 therapy50.
The potential effects on fertility of many new and effective anticancer therapies in non-metastastic breast cancer setting remain to be clarified, such as pertuzumab, neratinib, and TDM1, or those under clinical evaluation like CDK4/6 inhibitors, immunotherapy, or PARP inhibitors. To provide more adequate and accurate information to patients, reproductive health outcomes should be included in standard toxicity assessments of modern treatments in clinical trials as well as routine care51.
Effect of endocrine therapy on fertilityAdjuvant endocrine therapy for 5–10 years is the standard protocol for women with ER positive breast cancer. The use of hormone therapy with tamoxifen in premenopausal women with breast cancer after adjuvant chemotherapy is associated with menstrual alterations and a two-fold increase in the risk of amenorrhea36,46,52,53. Conversely, women aged 40 years or younger treated with tamoxifen alone may never develop amenorrhea53. Tamoxifen does not appear to have a permanent effect on fertility, but is teratogenic; therefore, the need to delay childbearing may pose a risk due to the aging of the ovaries. The effect of the suspension of normal menses with gonadotropin releasing hormone analogs (GnRH analogs) is 90% reversible before 40 years of age, but recovery is not always seen in older patients54. The effect of GnRH analogs combined with aromatase inhibitors is still unknown.
ConclusionsBreast cancer has a high survival rate that forces us to increasingly consider the potential long-term side effects and quality of life of survivors, particularly the infertility associated with systemic cancer treatments. The impact of potential loss of fertility is not only psychological, but it also affects patients' adherence to anticancer treatments. The precise mechanisms underlying chemotherapy induced follicle loss remain unclear, and the death of oocytes by apoptosis has emerged as the main cause. Alkylating agents, such as cyclophosphamide, are associated with the highest risk of gonad toxicity; platinum salts, anthracyclines, and taxanes are classified as having an intermediate risk of gonadotoxicity, whereas vinca alkaloids, antimetabolites, and antitumor antibiotics are of low risk. Most often, breast cancer patients are administered combinations of these drugs in adjuvant or neoadjuvant settings, which together with individual patient variability makes it difficult to predict the risk of infertility. Additionally, adjuvant endocrine therapy while it does not have permanent effects on fertility itself, the long-term use could lower oocyte quality due to patient aging. It is essential that health professionals who advise and provide therapeutic options to these patients are aware of the impact of these treatments. Early referral is essential for oncofertility counseling, clarifying possible gonadal effects, and discussing all possible preventive strategies.
FundingThe authors declare the did not receive funding for this study.
Ethical approvalNo clinical data has been used, so IRB approval is not required.
Conflict of interestThe authors declare they have no conflict of interest.
We thank Dr. Ronald Barr (Mc Master University) for useful discussions.