To examine the association between endogenous hormones with mammographic breast density, glandular volume, and breast volume in postmenopausal women.
Material and methodsA cross-sectional study among 363 postmenopausal women not using menopausal hormonal treatment. The following data were collected: age, age at menopause, smoking status, body mass index, adiposity, and physical activity. Plasma levels of follicle-stimulating hormone, estradiol, testosterone, dehydroepiandrosterone sulfate (DHEAS), ∆4 androstenedione, cortisol, insulin-like growth factor-1 (IGF-1), and 25-hydroxyvitamin D were evaluated. Directed acyclic graph was used for the selection of potential confounding variables, and the linear regression was adjusted for confounders to study the association between endogenous hormones and mammographic parameters. Results are reported as β-coefficients (β) and 95% confidence interval (95% CI).
ResultsMultivariable linear regression analysis adjusted for confounding variables showed that cortisol (β = 0.20; 95% CI: 0.02; 0.37), and ∆4 androstenedione (β = −1.90; 95% CI: −3.30, −0.39) were significantly associated with breast density. IGF-1 (β = −0.01; 95% CI: −0.20, −0.01) was the only hormone with significant association with glandular volume. No relationship was found between the studied hormones and breast volume.
ConclusionsHigher cortisol and lower ∆4 androstenedione levels are associated with higher breast density, and higher IGF-1 levels are associated with lower glandular volume in postmenopausal women.
Examinar la asociación entre las hormonas endógenas con la densidad mamaria mamográfica, el volumen glandular y el volumen mamario en mujeres postmenopáusicas.
Material y métodosEstudio transversal entre 363 mujeres postmenopáusicas que no utilizan tratamiento hormonal dela menopáusia. Se recogieron los siguientes datos: edad, edad de la menopausia, tabaquismo, índice de masa corporal, adiposidad y actividad física. Se evaluaron los niveles plasmáticos de hormona folíciloestimulante, estradiol, testosterona, sulfato de dehidroepiandrosterona (DHEAS), ∆4 androstenediona, cortisol, factor de crecimiento similar a la insulina-1 (IGF-1) y 25-hidroxivitamina D. Se utilizó un gráfico acíclico dirigido para la selección de posibles variables de confusión y la regresión lineal se ajustó con los factores de confusión para estudiar la asociación entre hormonas endógenas y los parámetros mamográficos. Los resultados se informan como coeficientes-β (β) e intervalo de confianza del 95% (IC del 95%).
ResultadosEl análisis de regresión lineal multivariable ajustado por variables de confusión mostró que el cortisol (β = 0,20; IC del 95%: 0,02; 0,37) y ∆4 androstenediona (β = −1,90; IC del 95%: −3,30, −0,39) se asociaron significativamente con densidad mamaria. IGF-1 (β = −0,01; IC del 95%: −0,20, −0,01) fue la única hormona con una asociación significativa con el volumen glandular. No se encontró relación entre las hormonas estudiadas y el volumen mamario.
ConclusionesLos niveles más altos de cortisol y de ∆4 androstenediona se asocian con una mayor densidad mamaria y los niveles más altos de IGF-1 se asocian con un volumen glandular más bajo en mujeres postmenopáusicas.
Mammographic breast density reflects the relative amounts of dense areas (stroma and epithelium tissues) with respect to non-dense areas (fatty tissue) in the mammogram. Women with a very high percentage of mammographically dense tissue have a 4–6-fold increased risk of developing breast cancer than women with predominately fatty breasts.1 Breast density adjusted for age and body mass index (BMI) is related to the risk of developing breast cancer and it is considered an independent risk factor. Furthermore, high mammographic density is associated with reduced sensitivity for the detection of breast cancer during the mammographic screening and it has been associated with more advanced tumor staging at the time of diagnosis, increased risk of local relapse and the appearance of second primary cancers. The variation in mammographic density is heritable and it is estimated that up to 60% of this variation is genetically determined.2 However, with aging a physiological process of lobular involution occurs in the breast, and subsequently a decline in breast density. In addition to age, this process can be influenced by certain lifestyle risk factors, such as body mass index, physical activity, and smoking status.3
Breast density is also influenced by hormonal factors, and sex hormones may be involved in the development of breast cancer due to their proliferative effect on breast tissue. This effect is evidenced by an increase in breast density. The influence of exogenous hormones on breast density can be observed in the association of conventional menopausal hormone treatment with increased breast density and risk of breast cancer.4 Nevertheless, the association between endogenous sex hormones with breast density in postmenopausal women remains uncertain.5–11 In postmenopausal women, the relationship between breast density and other hormonal factors, such as dehydroepiandrosterone sulfate (DHEAS), insulin-like growth factor-1 (IGF-1), and vitamin D has been investigated in some studies, but data are somewhat inconsistent.12–14
The objective of the present study was to investigate the relationship between mammographic breast density, glandular volume, and breast volume with circulating levels of ovarian steroids, adrenal hormones, IGF-1, and vitamin D in postmenopausal women, applying a directed acyclic graph (DAG) representation.15
Material and methodsStudy designThis cross-sectional study was approved by the Institutional Review Board and Ethics Committee of our Center, and the procedures provided for obtaining Informed Consent are adequate. This study used data from January 2022 to December 2022 at the Department of Obstetrics, Gynecology and Reproduction of our Center. Inclusion criteria were being naturally postmenopausal (amenorrhea of 1 year or more before the initiation of the study) and had a recent mammogram with serum hormone analysis. Women were excluded if: (i) they had early menopause (<45 years) or surgical menopause; (ii) were using menopausal hormonal treatment; or (iii) had breast implants, breast surgery or history of cancer. Finally, a group of 363 postmenopausal women aged 49–83 years participated in the study.
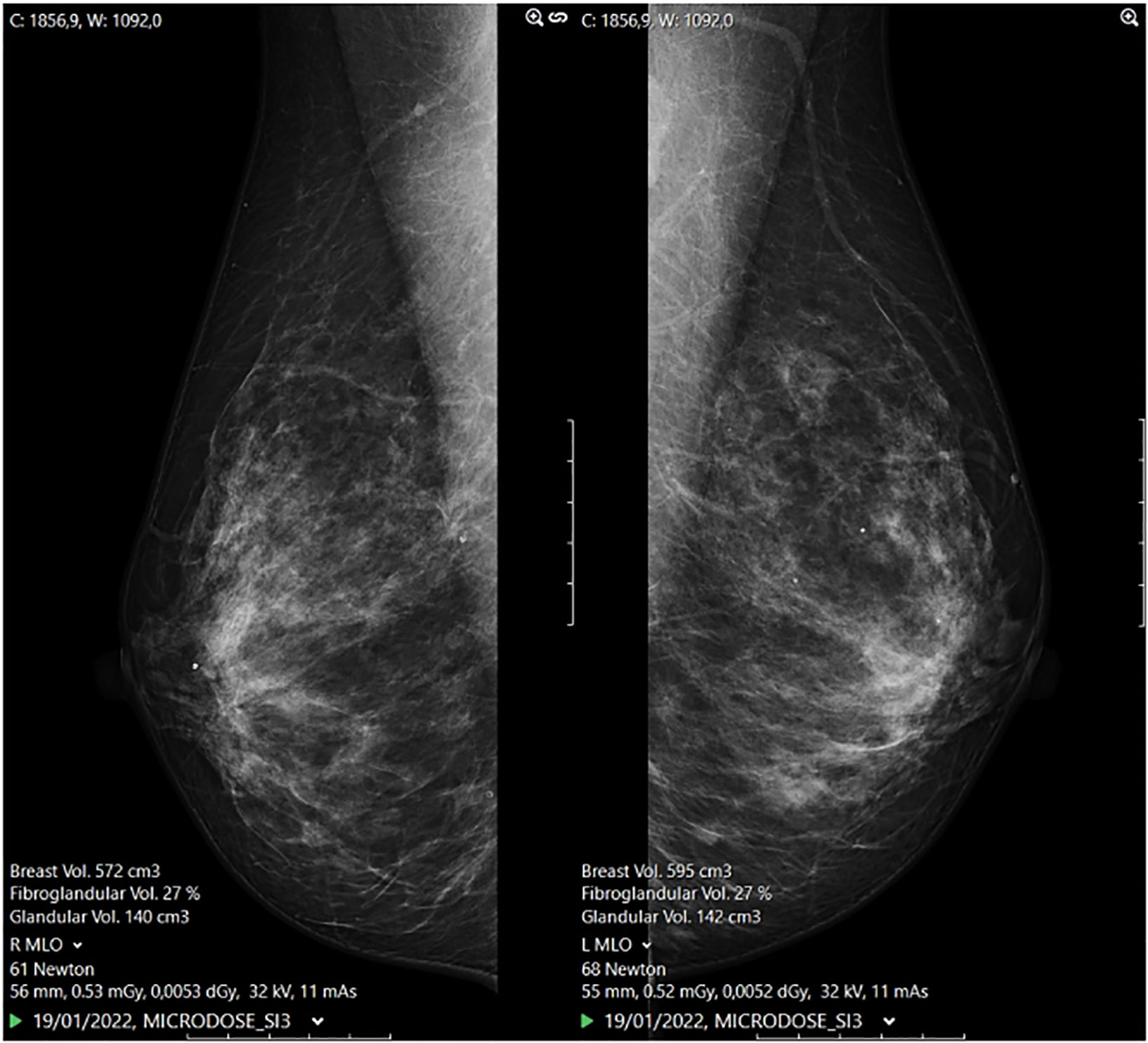
Mammographic measures and laboratory parametersMammographic measures were performed with photon-counting spectral mammography Phillips MicroDose SI (Philips Health Systems, Kista, Sweden) mammography units. Full-field digital images of mediolateral oblique and craniocaudal projections were obtained. Breast density (%), glandular volume (cm3), and breast volume (cm3) measures were automatically calculated from these views by combined multispectrable algorithms software, included in the equipment (Fig. 1).The quality control of the mammography units was performed daily by way of the measurement of a phantom's density. The phantom measurements showed stable results during the course of the study, and the coefficients of variation ranged from 0.23% to 0.38%.
Blood samples were collected after an overnight fast without any prior exercise or smoking. Hormonal parameters (follicle stimulating hormone [FSH], estradiol, testosterone, cortisol, DHEAS, ∆4 androstenedione, IGF-1, and 25-hydroxyvitamin D plasma levels) were determined by electrochemiluminescence immunoassay using Roche Elecsys reagents and measured by an automated COBAS® 8000 modular analyzer System (Roche Diagnostics: Pleasanton, CA, USA). Laboratory performs daily quality control of each parameter, and the coefficients of variation ranged from 1.2% to 1.7% for FSH, 1.1% to 1.8% for estradiol, 1.3% to 1.9% for testosterone, 1.5% to 2.2% for DHEAS, 1.7% to 2.1% for ∆4 androstenedione,1.5% to 1.7% for cortisol, 1.2% to 1.7% for IGF-1, and 2.3% to 3.1% for 25-hydroxyvitamin D.
Potential confoundersThe following categorical variables: age, age at menopause, BMI, adiposity, current smoking status, and physical activity were recorded. Body weight (kg) and height (cm) were measured without shoes, and BMI was obtained according to the formula weight divided by height square (kg/m2). Adiposity was assessed by bioelectrical impedance analysis (BIA) equipment Omron BF 306 monitor (Omrom healthcare Co. Ltd., Kyoto, Japan). The results were expressed as a percentage of fat. Information on smoking status was recorded and classified as never or smoker. The estimation of physical activity was assessed using the Spanish version of the short International Physical Activity Questionnaire. This tool includes 7-items that provide information about the average number of days per week, and the average time per day that subject spent on moderate and vigorous activities, walking, and sitting. With the final score obtained, the physical activity was classified as low, moderate, or high level.16
Covariate assessmentA DAG representation was used to select potential confounding factors adjusted in our analysis. A DAG with endogenous hormones as the main exposure and mammographic parameters (breast density, glandular volume, and breast volume) as the outcomes were generated to determine confounding variables. DAG includes a set of variables represented as nodes, and arrows (directed edges) connect the variables with a relationship. The arrowhead represents a direct effect of one variable over the other, but not the other way around.15 According to the minimal sufficiency set of adjustments, age, age at menopause, adiposity, BMI, smoking status, and physical activity were identified as confounders. Following the DAG, all these variables were considered in the regression model to assess associations between endogenous hormones and each of the mammographic measures (Fig. 2).
Statistical analysisContinuous variables were calculated as mean and standard deviation, whereas percentages and numbers were used for categorical variables. Pearson correlation was used to estimate the relation between numeric parameters with the mammographic measures. Finally, a multivariable linear regression analysis, adjusting for potential confounders identified by DAG, was modeled to determine the relationship between endogenous hormones and mammographic measures. Results are reported as β-coefficients (β) and 95% confidence interval (95% CI). All analyses were performed using the R software (R Core Team, 2019). The R package ‘dagitty’ was used. All the analyses were exploratory. No formal a priori sample size calculation was performed.
ResultsThis study included 363 postmenopausal women, a mean age of 63.1 ± 6.36 years, and a mean age at menopause of 50.2 ± 2.83 years. The mean BMI of the whole sample was 24.9 ± 3.72 kg/m2, and the mean adiposity was 40.5 ± 4.99%. The values of hormonal parameters and mammographic parameters pertaining to women, expressed as means ± SD, are reported in Table 1. Current smoking habits and regular physical activity were reported in 19% (69/363) and 63% (230/363) of the sample, respectively.
Hormonal parameters and mammographic measurements reported as mean ± standard deviation.
| Parameters | Estimate (n = 363) | 95% confidence interval | Reference ranges |
|---|---|---|---|
| FSH (mU/mL) | 77.53 ± 25.45 | 74.90, 80.16 | 40–116 |
| Estradiol (pg/mL) | 11.56 ± 6.77 | 10.86, 12.26 | 5–37 |
| Testosterone (ng(mL) | 0.33 ± 0.23 | 0.31, 0.36 | 0.02–0.40 |
| Cortisol (μg/dL) | 14.11 ± 4.89 | 13.61, 14.62 | 4.3–22.4 |
| DHEAS (μg/dL) | 82.37 ± 53.11 | 76.89, 87.85 | 35–430 |
| ∆4 Androstenedione (ng(mL) | 0.87 ± 0.67 | 0.80, 0.94 | 0.49–1.31 |
| IGF-1 (ng(mL) | 125.76 ± 40.60 | 121.57, 129. 95 | 44–241 |
| Vitamin D (ng/mL) | 31.82 ± 9.63 | 30.82, 32.81 | 30–100 |
| Breast density (%) | 17.58 ± 9.39 | 16.61, 18.55 | |
| Glandular volume (cm3) | 94.57 ± 38.11 | 90.64, 98.51 | |
| Breast volume (cm3) | 678.02 ± 307.46 | 646.29, 709.76 |
DHEAS, Dehydroepiandrosterone sulphate; FSH, Follicle stimulating hormone; IGF-1, Insulin-like growth factor.
The Pearson correlation coefficients (r) of the parameters pertaining to all the women are shown in Fig. 3. The present study observed that breast density was correlated negatively with age (r = −0.17), adiposity (r = −0.38), BMI (r = −0.50), and breast volume(r = −0.49), instead was correlated positively with FSH (r = 0.16), and glandular volume (r = 0.30). The glandular volume was correlated positively with age at menopause (r = 0.13), BMI (r = 0.22), breast density (r = 0.30), and breast volume (r = 0.55). We also observed that there were positive correlation between breast volume with adiposity (r = 0.30), BMI (r = 0.68), and glandular volume(r = 0.55), and negative correlation with FSH (r = −0.17), cortisol (r = −0.10), IGF-1 (r = −0.11), and breast density (r = −0.49).
A multivariable linear regression analysis was used to determine the relationship between endogenous hormones and mammographic parameters. After adjusting for confounding variables, cortisol (β = 0.20; 95% CI: 0.02, 0.37), and ∆4 androstenedione (β = −1.90; 95% CI: −3.30, −0.39) were significantly associated with breast density. No relationship was found between the other studied hormones and breast density. IGF-1 (β = −0.01; 95% CI: −0.20, −0.01) was the only hormone with significant association with glandular volume. No association was found between the studied hormones and breast volume Table 2.
Multivariable linear regression model to analyses the relationship between endogenous hormones and mammographic measurements adjusting for age, age at menopause, adiposity, BMI, smoking status, and physical activity.
| Breast density (%) | Glandular volume (cm3) | Breast volume (cm3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | β | 95% CI | P-value | |
| FSH | 0.01 | −0.02, 0.05 | .400 | 0.02 | −0.14, 0.17 | .800 | −0.18 | −1.20, 0.81 | .700 |
| Estradiol | 0.03 | −0.09, 0.16 | .600 | −0.12 | −0.70, 0.45 | .700 | 0.56 | −3.10, 4.20 | .800 |
| Testosterone | 1.20 | −2.80, 5.20 | .600 | 2.00 | −16.00, 20.00 | .800 | −48.00 | −163.00, 68.00 | .400 |
| Cortisol | 0.20 | 0.02, 0.37 | .030 | −0.08 | −0-09, 0.74 | .800 | −1.20 | −6.30, 4.00 | .700 |
| DHEAS | 0.01 | −0.01, 0.03 | .400 | 0.00 | −0.08, 0.09 | >.900 | −0.10 | −0.63, 0.43 | .00 |
| ∆4 Androstenedione | −1.90 | −3.30, −0.39 | .013 | −3.60 | −10.00, 3.10 | .300 | −6.30 | −49.00, 36.00 | .800 |
| IGF-1 | −0.01 | −0.03, 0.01 | .200 | −0.01 | −0.20, −0.01 | .033 | −0.49 | −1.10, 0.12 | .110 |
| Vitamin D | 0.03 | −0.06, 0.11 | .500 | 0.16 | −0.24, 0.56 | .400 | −0.24 | −2.70, 2.30 | .900 |
BMI, Body mass index; CI, Confidence Interval; DHEAS, Dehydroepiandrosterone sulphate; FSH, Follicle stimulating hormone; IGF-1 Insulin-like growth factor.
In the present study, the application of DAG showed that in our cohort of postmenopausal women, cortisol and ∆4 androstenedione was associated with breast density, and IGF-1 was associated with glandular volume. No association was observed between the other studied endogenous hormones and mammographic breast density, glandular volume, or breast volume.
To our knowledge, the present is the first study that analyzed the association between endogenous hormones and mammographic parameters in postmenopausal women using a DAG. We previously used DAG in specific clinical issues, such as handgrip strength in postmenopausal women.17 DAG is a graphical epidemiologic tool that connects with arrows the variables with associated dependence, and provides a simple way to visualize assumptions about the statistical relationships between the main exposure, outcome, and covariates.15 Photon-counting spectral mammography is a reliable volumetric method to measure breast density and its components, glandular volume and breast volume, with a precision of approximately 2 times greater than a non-spectral method. Our hospital had photon-counting spectral mammographs since 2014, and we used this technique to evaluate the breast density of the women in our study.
Menopause has effects on mammogram characteristics, causing a reduction in dense tissue area, an increase in non-dense area, and therefore a decrease in density. The maximal reduction of percent breast density occurs across the menopausal transition. During the menopausal transition, the decrease in plasma estradiol levels leads to a progressive increase in circulating FSH levels, which remain high after menopause. It has been suggested that FSH may influence breast cancer development and progression. Breast cancer cells express functional receptors for FSH, and this hormone promotes migration and invasion properties through regulatory actions on the actin cytoskeleton.18 Lifetime exposure to estradiol may influence breast density due to estrogens have a proliferative effect on breast epithelial tissue. However, the relationship between breast density and estrogens in postmenopausal women remains controversial. Some studies have observed a significant association between the levels of estradiol and breast density in postmenopausal women,6,7 while others, after adjusting for confounding covariates have reported no association.8–10 There are possible reasons to explain the difference in results between studies when analyzing the association of estradiol with breast density. For instance, it would be that in postmenopausal women, very low levels of estradiol may not exert a detectable effect on mammographic breast density.19 The association between sex hormone levels and mammographic breast density is strongly influenced by BMI,11therefore the association with estradiol may disappear after adjusting for BMI. Finally, exposure to exogenous hormones generates a steroidal environment that affects mammographic breast density differently than endogenous hormones.20 Our results show a lack of association between estradiol and breast density or glandular volume. In addition, FSH was not found to be associated with breast density.
Controversies still exist when it comes to assessing the relationship between androgens and mammographic breast density. Androgens, notably testosterone, act as pro-apoptotic and inhibitory effects on breast cancer cell growth via its action on androgen receptor.21 Previous studies reported that testosterone is not associated with breast density among premenopausal22 or postmenopausal women5,7,9 after adjustment for BMI, or even regardless of BMI adjustment.19 In postmenopausal women, with the decrease in estrogen levels, DHEAS is the dominant circulating androgen. DHEAS is itself inactive, but in peripheral tissues it is transformed inside the cells in estradiol and testosterone.23 DHEAS levels decline with aging, both in men and women. DHEAS has no affinity for the androgen receptor, and has been associated with breast cancer independently of mammographic breast density in postmenopausal women.12 Although previous studies reported that DHEAS is associated with mammographic breast density in premenopausal women,24 this association has not been observed in postmenopausal women.5 Our cohort of postmenopausal women reports similar results to previous studies, showing no association between testosterone or DHEAS and breast density. Some study showed that androstenedione was associated with breast cancer risk,25 but was not related with mammographic density in other studies.8,10 We found there is an association between ∆4 androstenedione and breast density.
Cortisol is a glucocorticoid hormone that plays a vital role in the physiological response to stress, with immunosuppressive, hyperglycemic, and adipogenic effects. Therefore, the possible relationship of cortisol with increased breast cancer risk has been investigated, but no significant association has been found. Regarding the association between cortisol with mammographic breast density, few studies are available.26 Interestingly, our current study found an association between cortisol and breast density.
IGF-1 is a polypeptide hormone with autocrine, paracrine, and endocrine effects that promotes proliferation and inhibits apoptosis of breast cells. Circulating IGF-1 levels gradually decrease with aging. Therefore, when studying the association between IGF-1 and mammographic breast density, age must be accounted for. There are several studies with controversial results regarding the relationship between circulating levels of IGF-1 and mammographic breast density. In postmenopausal women, the association between IGF-1 and breast density relationship has not observed,20 instead Bremnes et al.13 report a positive but weak association between plasma IGF-1 levels and both percent and absolute mammographic densities. This association was only statistically significant among postmenopausal women not currently using hormone therapy. Interestingly, in our study, the results showed significant association between IGF-1 and glandular volume.
Vitamin D is a prohormone synthesized in the skin by exposure to sunlight's ultraviolet B spectrum. In recent years, there has been a growing interest in pleiotropic functions of vitamin D, due to immunological and antiproliferative effects. In breast tissue, vitamin D may have an effect on inhibition of cellular proliferation, induction of differentiation and apoptosis. Also, vitamin D receptor contributes for mammary gland development from puberty to pregnancy and involution.27 It has been reported that women with higher vitamin D levels are associated with lower breast cancer risk, and results from observational studies shown that serum vitamin D levels of approximately 52 ng/mL are associated with a reduction of 50% in breast cancer incidence.28 The association between vitamin D and mammographic breast density remains unclear.14 Some studies that investigated the association between vitamin D levels with breast density reported an association among premenopausal women,29 while others have not observed any association in postmenopausal women.30 Our results did not find a significant association between vitamin D and breast density, glandular volume, or breast volume. Vitamin D may possibly influence the risk of breast cancer, but it is suggested that this effect would be mediated by pathways other than breast density.30
The results of our study do not allow immediate clinical application since the objective is descriptive the hormonal status and its current mammographic parameters.
Possibly, the elevated rate of ∆4 Androstenedione could explain the cases of breast cancer in women with polycystic ovary syndrome or the elevation of IGF-1 would be associated with the etiopathogenesis of breast cancer related to obesity. Further studies are needed to relate the increase of some hormones with the pathogenesis or treatment of breast cancer.
This study has certain limitations, including its cross-sectional design, the effect of the studied hormones on breast density could not be longitudinally assessed, and we did not measure sex hormone-binding globulin and progesterone levels. However, our study has several strengths. First, it includes a relatively large sample of postmenopausal women. Second, all mammograms were performed with the same photon counting technology and laboratory, with daily quality controls. Third, careful adjustments for likely confounders were performed in the regression model using DAG. Finally, we are not aware of a previous approach using DAGs to study mammographic parameters in postmenopausal women.
ConclusionsThe present study showed in postmenopausal women aged 49–83 years: (i) higher cortisol levels, and lower ∆4 androstenedione levels are associated with higher breast density, (ii) higher IGF-1 levels had a significant association with lower glandular volume, and (iii) there was no relationships between the other studied endogenous hormones and the absolute measure of mammographic breast density, glandular volume, or breast volume. Future studies are needed to assess whether our results are useful in clinical practice.
FundingThe authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Ethical disclosuresThis study was approved by the Institutional Review Board and the Hospital Ethics Committee of the QuirónDexeus University Hospital, Barcelona, Spain (2022/105-GIN-DEX).
Confidentiality of dataI confirm that the procedures provided for obtaining Informed Consent are adequate. This is a cross-sectional study and the research team requested from the ethics committee the exemption from obtaining informed consent from all participants, taking advantage of the exceptional nature included in article 14, paragraph 5b of the GDPR (General Data Protection Regulation) of the European Regulation 2016/679. In addition, it has ensured the confidentiality of all personal and research data in accordance with the current Spanish legislation.
Author contributionsPGA was the principal investigator and drafted and revised the manuscript. IR was responsible for the methodology of the study and statistical analysis. JLB revised the manuscript. RF revised the manuscript and provided scientific input. All authors interpreted, revised, and approved the final version of the manuscript.
This study has been done under the auspices of the Professorship in Obstetrics and Gynecological Research of the Hospital Universitario Dexeus, of the Universidad Autónoma de Barcelona.