Since 2006 breast cancer has been the leading cause of death by cancer in Mexican women. Recently several studies have shown the relationship of neutrophil-lymphocyte index (NLI) and breast cancer as a predictor of overall survival (OS) and disease-free survival (DFS).
MethodsThe study included 2711 patients of the Institute of Breast Diseases (FUCAM) treated since April 2006 to March 2011. The NLI and its relation with OS and DFS were evaluated. A receiver operating characteristic curve was used to obtain the cut-off point of greater sensitivity and specificity for the NLI. A multivariate analysis was used to analyze the clinical parameters of prognostic factor.
Results342 patients were included for the analysis. The follow-up time average of 80.1 months and the mean of NLI was 2.1. In this model, sensitivity was 66.7% (95% CI: 46–64) p=0.04. The Kaplan–Meier analysis was applied to evaluate OS and the DFS of all patients according to the NLI, comparing two groups: ≤2.1 and ≥ 2.1. No differences were reported between these groups. In triple negative patients (n=32), patients with NLI>2.1 had a shorter DFS in comparison to patients with NLI<2.1 (20 vs 55.6% in 5 years, p=0.04). The univariate Cox risk model revealed that a NLI>2.1 pretreatment, lymph node stage and Ki-67 were correlated independently with the DFS.
ConclusionsOur study suggests that there is a relationship between the NLI and the breast cancer DFS; especially in triple negative subtypes.
El cáncer de mama tiene un enorme impacto en la salud de las mujeres. En México, desde el año 2006, el cáncer de mama ha sido la principal causa de muerte por cáncer en las mujeres mexicanas, y que representa 14% de las muertes relacionadas con el cáncer. Existen diversos factores que representan un papel importante en el desarrollo, progresión y persistencia del cáncer; entre ellos se reconoce a la respuesta inflamatoria. Múltiples estudios han demostrado la relación del índice linfocito neutrófilo (INL) y el cáncer de mama como predictor de sobrevida global y período libre de enfermedad.
Material y métodosEl diseño de estudio fue retrospectivo, transversal. Se revisaron 2.711 expedientes de pacientes con cáncer de mama tratados en el Instituto de Enfermedades de la Mama, FUCAM, en el período de abril de 2006 a marzo de 2011; se evaluó el INL previo a recibir tratamiento, y se obtuvo la relación con el período libre de enfermedad y la sobrevida global. Análisis estadístico: para obtener el punto de corte de mayor sensibilidad y especificidad para el INL se utilizó una curva de característica operativa del receptor (COR). Para variables dicotómicas se utilizó la prueba de Chi-cuadrado, mientras que para sobrevida y periodo libre de enfermedad se utilizaron las curvas de Kaplan-Meier. Un análisis multivariable se realizó para analizar el factor pronóstico de los parámetros clínicos.
ResultadosDe la muestra inicial de 2.711, 342 cumplieron criterios de inclusión y fueron analizados. La media de edad al momento del diagnóstico fue de 51,38 años, un seguimiento promedio de 80,1 meses y la media del INL fue de 2,1. Se evaluó la sensibilidad/especificidad de la prueba para el INL, mediante la curva COR, la sobrevida libre de enfermedad y la sobrevida global. En este modelo, la sensibilidad fue del 66,7%. Al evaluar la sobrevida global y el periodo libre de enfermedad de todas las pacientes con cáncer de mama, de acuerdo al INL, comparando en 2 grupos, por INL, grupo 1, menor a 2,1 y grupo 2, ≥2,1 no se encontraron diferencias estadísticamente significativas. Se realizó otro análisis a una submuestra de pacientes con subtipo triple negativos (n=32), las que obtuvieron un INL>2,1 mostraron una menor supervivencia libre de enfermedad en comparación con los pacientes con IN<2,1 (a 5 años, 20 frente al 55,6%; p=0,04). Al realizarse el modelo de riesgo univariante de Cox se identificó que un INL>2,1 pretratamiento, el estadio nodular y Ki-67 fueron variables que correlacionaron de manera independiente con el periodo libre de enfermedad.
ConclusionesNo se encontraron diferencias estadísticamente significativas en periodo libre de enfermedad, ni en sobrevida global, en pacientes con cáncer de mama agrupados acorde a INL. En la submuestra de pacientes con subtipo triple negativo (n=32), el grupo con mayor INL (> 2,1), mostró una menor supervivencia libre de enfermedad en comparación con el que tuvo menor INL (<2,1). Las variables que mostraron correlación con el periodo libre de enfermedad en este subgrupo (triple negativo), fueron un INL>2,1 pretratamiento, el estadio nodular y Ki-67.
Breast cancer has a huge impact on women health. It is the most common type of cancer in women of reproductive age worldwide. Approximately 178,480 women are diagnosed with invasive breast cancer annually in the United States, which represents approximately 32% of all incidents of cancer among women.1,2 In Mexico, by 2012, according to figures from the National Institute of Statistics and Geography (INEGI) an incidence of 26.64 per 100,000 women over the age of 20 which corresponds to the second leading cause of death by cancer in that same age group with 15.4%.3
Breast cancer prognosis can be influenced by different risk factors and stimuli, including clinicopathologic characteristics (such as age of the patient, lymph node status and size of tumor) and biomolecular parameters (such as hormone receptors, receptor (HER2) 2 human epidermal growth factor). Thus, the lymph nodes are considered the most important prognostic marker, however, the final clinical behavior cannot be predicted in patients with the same lymphatic condition4–6; because cancer behavior involves numerous molecular processes including tumor microenvironment, particularly inflammatory response.7,8 Therefore, inflammation has been considered to play an important role in the development, progression, metastasis and tumor relapse.9
Several studies have demonstrated the association among hematological inflammatory components, including lymphocyte-monocyte index, platelet-lymphocyte index, and particularly the relationship between the high neutrophil-lymphocyte index (NLI) and the increase in mortality in different populations with cancer, including: Lung, colorectal, stomach, liver, pancreas and, recently, breast cancer.8,10–14
Currently the mechanism by which NLI is related to cancer prognosis is still uncertain,7 although there are several theories that recently described the importance of lymphocytes, reporting a relationship between increased intratumoral lymphocytes with a better response to chemotherapy and a better prognosis.15,16
NLI has been widely associated to the disease-free period in different studies in patients with operable disease and usually this relationship is positive only in the univariate analysis. However, it has not always been able to prevail as an independent prognostic factor. Many studies have failed to report the clinical relationship between the NLI and several variables such as tumor size or the affected lymph nodes in breast cancer.10,11,17,18
So far there are no studies of this type in mexican population. The proposed project, tried to get own results and conclusions regarding the relationship between the neutrophil-lymphocyte index, the disease-free survival and overall survival in patients with breast cancer.
MethodsThis retrospective, observational study included patients with diagnosis of breast cancer treated in the FUCAM Institute of Breast Diseases, including records from April 2006 to March 2011. The inclusion criteria were all women with breast cancer stage I–III with pretreatment complete blood cell count (any type of treatment), with full record and a minimum follow-up of 5 years from the time of diagnosis. Patients with cancer in situ, skin or chest wall affection (T4 including inflammatory carcinoma), documented infection, hematologic disorders, acute or chronic inflammatory diseases, autoimmune diseases, steroid therapy, long term anti-inflammatory drug intake, immune-modulating drug intake, diabetes, hypertension or heart disease, chronic renal disease, documented liver disease, metabolic syndrome and smokers were excluded.
All data were obtained from the clinical records: age, TNM/clinical stage (AJCC), histological grade, cancer histologic type, molecular subtype (luminal A, Luminal B, Triple negative, Her2, Not Available (NA)), surgery (mastectomy or conservative surgery), chemotherapy, endocrine, radiation therapy, Trastuzumab.
The NLI is defined as the absolute neutrophils divided by the absolute count of lymphocytes. The NLI is calculated based on a complete blood count routinely carried out immediately after the diagnosis of breast cancer and prior to the initiation of any form of treatment, including surgery.
Disease-free survival (DFS) was defined as the interval between the date of cancer diagnosis to the first failure in therapy (including locoregional relapse and relapse, distant, primary or secondary). Overall survival (OS) was of is defined as the interval between the histological diagnosis to death or the last follow-up visit.
Statistical analysisA (COR) receiver operating characteristic curve was used to obtain the cut-off point of greater sensitivity and specificity for the NLI. The end-point used in COR was DFS or OS. Dichotomous variables were analyzed with Chi-square test, while the survival and disease-free survival were analyzed using Kaplan–Meier curves. A multivariate analysis was used to analyze the clinical parameters of prognostic factor. The values of p<0.05 were considered statistically significant. Data was analyzed with IBM® SPSS® STATISTICS 23 software (IBM Corp., Armonk, NY, US).
ResultsWe evaluated 2711 patient records from April 2006 to March 2011, 342 were included for the final analysis. The exclusion criteria were: chronic diseases (such as diabetes, rheumatoid arthritis, hypertension, metabolic syndrome), stage 4 cancer, mechatronic and T4 tumors, smoking and anti-inflammatory drugs intake.
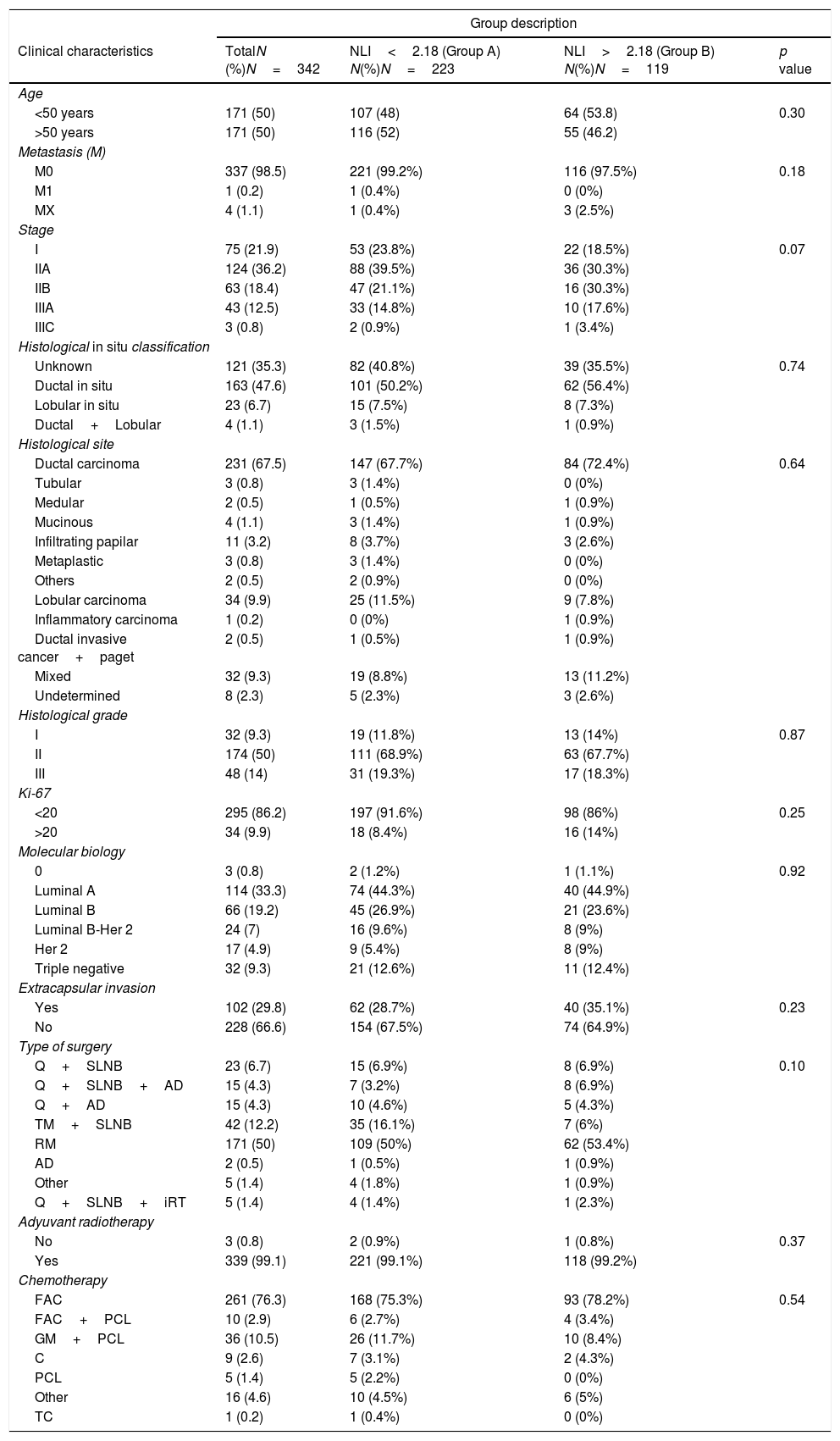
Socio-demographic variables (n=342) were taken at time of diagnosis and described. The mean age was 51.38 years (SD±9.6), weight 66.48kg (SD±11.4), height 1.54m (SD±6.4) and NLI 2.13 (SD±1.1). Clinical characteristics are described in Table 1. As shown in Table 1, there was no significant correlation between the NLI pretreatment and clinical factors such as: age, size of tumor, nodes, ki 67, histological type and grade, extra capsular invasion, tumor biology, surgery, radiotherapy, and chemotherapy used.
Clinical and socio-demographic characteristics.
| Group description | ||||
|---|---|---|---|---|
| Clinical characteristics | TotalN (%)N=342 | NLI<2.18 (Group A) N(%)N=223 | NLI>2.18 (Group B) N(%)N=119 | p value |
| Age | ||||
| <50 years | 171 (50) | 107 (48) | 64 (53.8) | 0.30 |
| >50 years | 171 (50) | 116 (52) | 55 (46.2) | |
| Metastasis (M) | ||||
| M0 | 337 (98.5) | 221 (99.2%) | 116 (97.5%) | 0.18 |
| M1 | 1 (0.2) | 1 (0.4%) | 0 (0%) | |
| MX | 4 (1.1) | 1 (0.4%) | 3 (2.5%) | |
| Stage | ||||
| I | 75 (21.9) | 53 (23.8%) | 22 (18.5%) | 0.07 |
| IIA | 124 (36.2) | 88 (39.5%) | 36 (30.3%) | |
| IIB | 63 (18.4) | 47 (21.1%) | 16 (30.3%) | |
| IIIA | 43 (12.5) | 33 (14.8%) | 10 (17.6%) | |
| IIIC | 3 (0.8) | 2 (0.9%) | 1 (3.4%) | |
| Histological in situ classification | ||||
| Unknown | 121 (35.3) | 82 (40.8%) | 39 (35.5%) | 0.74 |
| Ductal in situ | 163 (47.6) | 101 (50.2%) | 62 (56.4%) | |
| Lobular in situ | 23 (6.7) | 15 (7.5%) | 8 (7.3%) | |
| Ductal+Lobular | 4 (1.1) | 3 (1.5%) | 1 (0.9%) | |
| Histological site | ||||
| Ductal carcinoma | 231 (67.5) | 147 (67.7%) | 84 (72.4%) | 0.64 |
| Tubular | 3 (0.8) | 3 (1.4%) | 0 (0%) | |
| Medular | 2 (0.5) | 1 (0.5%) | 1 (0.9%) | |
| Mucinous | 4 (1.1) | 3 (1.4%) | 1 (0.9%) | |
| Infiltrating papilar | 11 (3.2) | 8 (3.7%) | 3 (2.6%) | |
| Metaplastic | 3 (0.8) | 3 (1.4%) | 0 (0%) | |
| Others | 2 (0.5) | 2 (0.9%) | 0 (0%) | |
| Lobular carcinoma | 34 (9.9) | 25 (11.5%) | 9 (7.8%) | |
| Inflammatory carcinoma | 1 (0.2) | 0 (0%) | 1 (0.9%) | |
| Ductal invasive cancer+paget | 2 (0.5) | 1 (0.5%) | 1 (0.9%) | |
| Mixed | 32 (9.3) | 19 (8.8%) | 13 (11.2%) | |
| Undetermined | 8 (2.3) | 5 (2.3%) | 3 (2.6%) | |
| Histological grade | ||||
| I | 32 (9.3) | 19 (11.8%) | 13 (14%) | 0.87 |
| II | 174 (50) | 111 (68.9%) | 63 (67.7%) | |
| III | 48 (14) | 31 (19.3%) | 17 (18.3%) | |
| Ki-67 | ||||
| <20 | 295 (86.2) | 197 (91.6%) | 98 (86%) | 0.25 |
| >20 | 34 (9.9) | 18 (8.4%) | 16 (14%) | |
| Molecular biology | ||||
| 0 | 3 (0.8) | 2 (1.2%) | 1 (1.1%) | 0.92 |
| Luminal A | 114 (33.3) | 74 (44.3%) | 40 (44.9%) | |
| Luminal B | 66 (19.2) | 45 (26.9%) | 21 (23.6%) | |
| Luminal B-Her 2 | 24 (7) | 16 (9.6%) | 8 (9%) | |
| Her 2 | 17 (4.9) | 9 (5.4%) | 8 (9%) | |
| Triple negative | 32 (9.3) | 21 (12.6%) | 11 (12.4%) | |
| Extracapsular invasion | ||||
| Yes | 102 (29.8) | 62 (28.7%) | 40 (35.1%) | 0.23 |
| No | 228 (66.6) | 154 (67.5%) | 74 (64.9%) | |
| Type of surgery | ||||
| Q+SLNB | 23 (6.7) | 15 (6.9%) | 8 (6.9%) | 0.10 |
| Q+SLNB+AD | 15 (4.3) | 7 (3.2%) | 8 (6.9%) | |
| Q+AD | 15 (4.3) | 10 (4.6%) | 5 (4.3%) | |
| TM+SLNB | 42 (12.2) | 35 (16.1%) | 7 (6%) | |
| RM | 171 (50) | 109 (50%) | 62 (53.4%) | |
| AD | 2 (0.5) | 1 (0.5%) | 1 (0.9%) | |
| Other | 5 (1.4) | 4 (1.8%) | 1 (0.9%) | |
| Q+SLNB+iRT | 5 (1.4) | 4 (1.4%) | 1 (2.3%) | |
| Adyuvant radiotherapy | ||||
| No | 3 (0.8) | 2 (0.9%) | 1 (0.8%) | 0.37 |
| Yes | 339 (99.1) | 221 (99.1%) | 118 (99.2%) | |
| Chemotherapy | ||||
| FAC | 261 (76.3) | 168 (75.3%) | 93 (78.2%) | 0.54 |
| FAC+PCL | 10 (2.9) | 6 (2.7%) | 4 (3.4%) | |
| GM+PCL | 36 (10.5) | 26 (11.7%) | 10 (8.4%) | |
| C | 9 (2.6) | 7 (3.1%) | 2 (4.3%) | |
| PCL | 5 (1.4) | 5 (2.2%) | 0 (0%) | |
| Other | 16 (4.6) | 10 (4.5%) | 6 (5%) | |
| TC | 1 (0.2) | 1 (0.4%) | 0 (0%) | |
Q – quadrantectomy, SLNB – sentinel lymph node biopsy, AD – Axillary Disection, TM – total mastectomy, RM – radical mastectomy, iRT – intraoperative radiotherapy, FAC – Fluorouracil, Adriamycin and Cyclophosphamide, PCL – Paclitaxel, C – Cyclophosphamide, GM – gemicitabine, TC – Taxotere and cyclophosphamide.
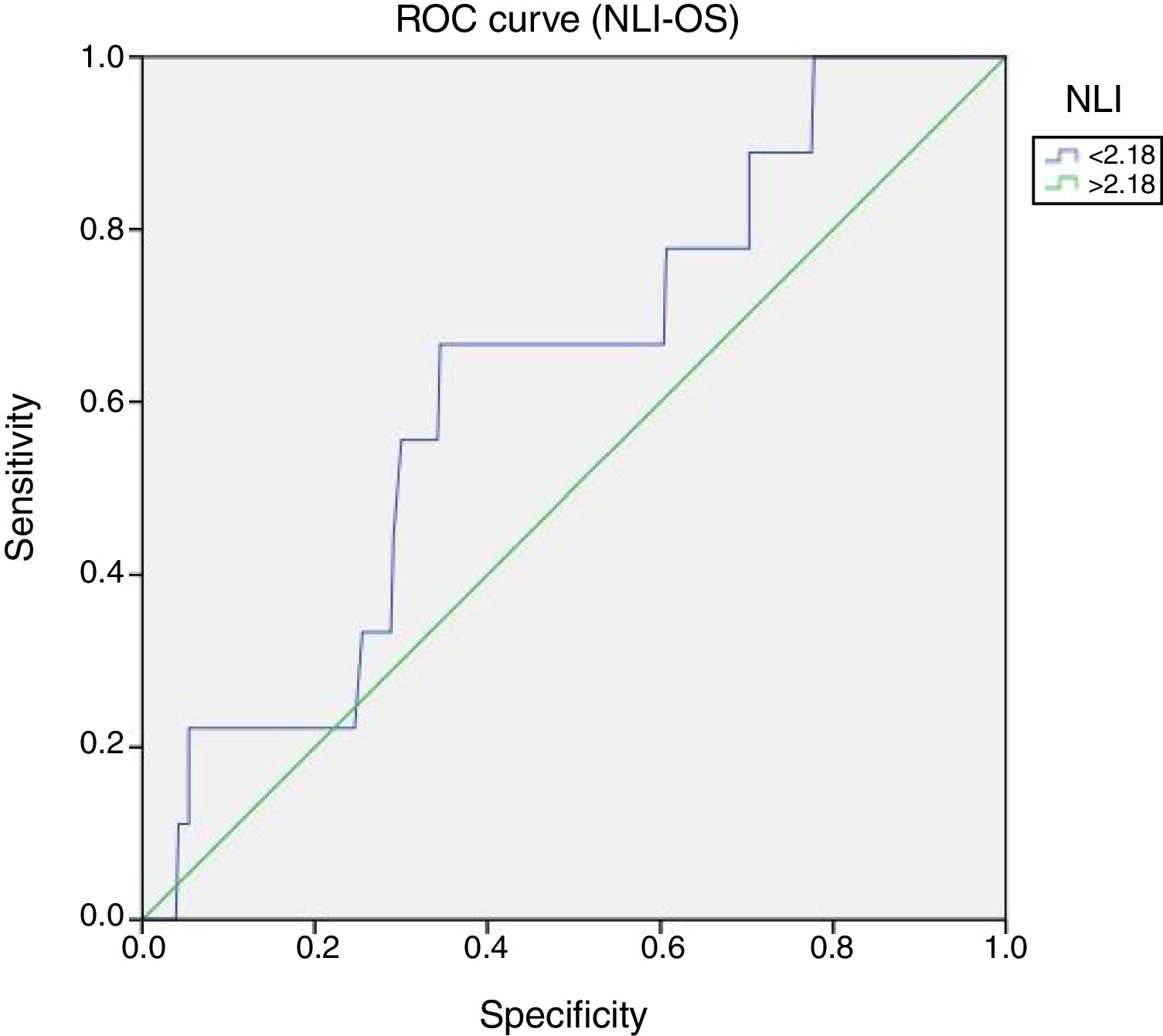
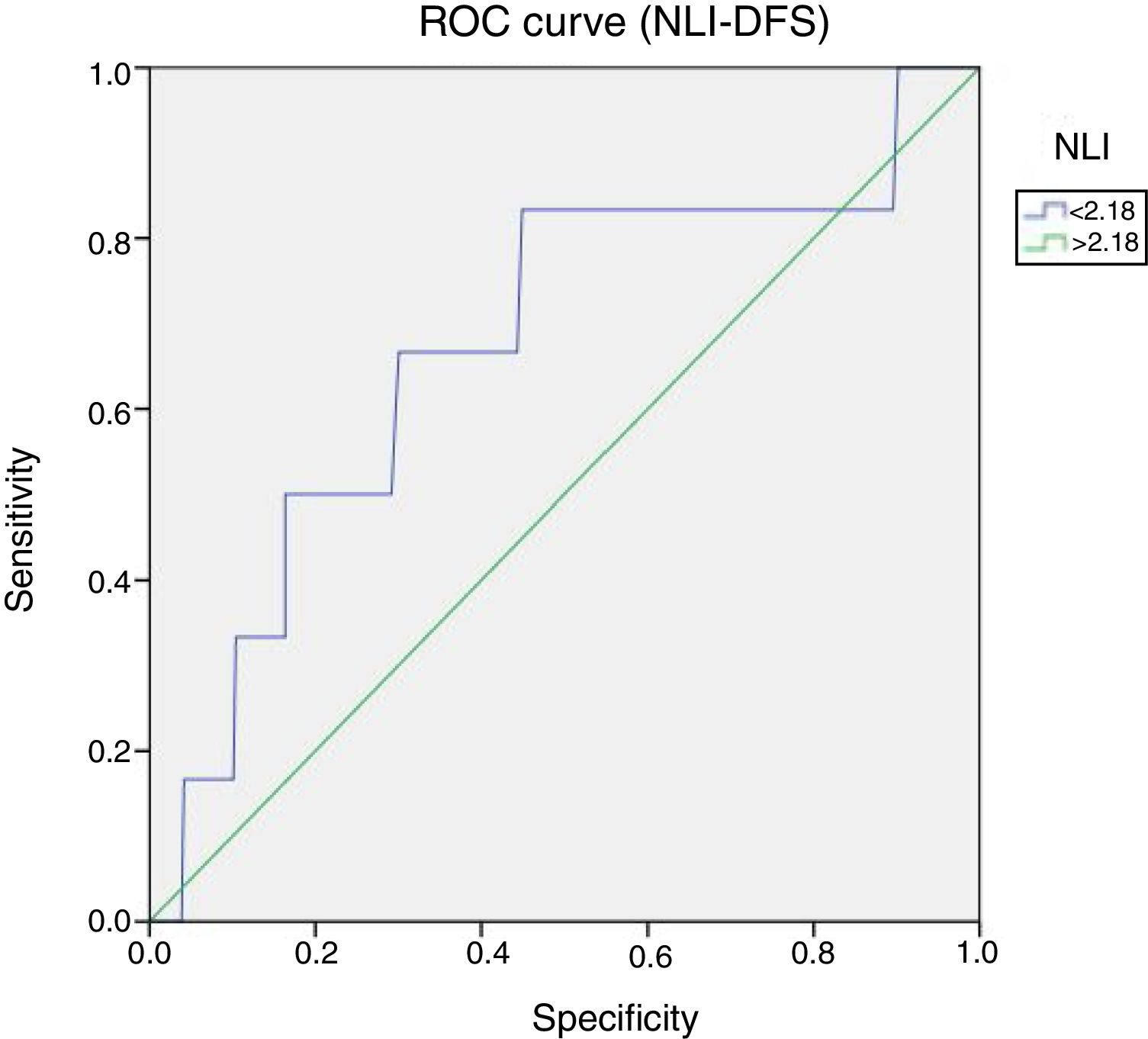
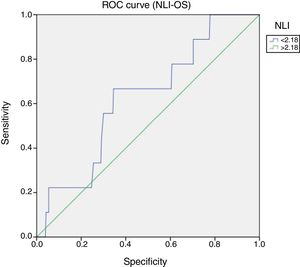
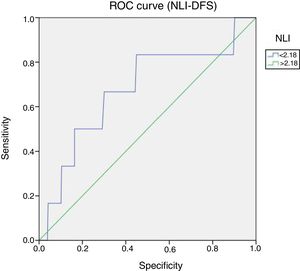
Cutoff point of NLI at 2.18 was defined on the maximum point (sensitivity+specificity) under the ROC curves (Receiver Operating Characteristic), sensitivity was 66.7% (95% CI: 46–64) p=0.04 (Figs. 1 and 2).
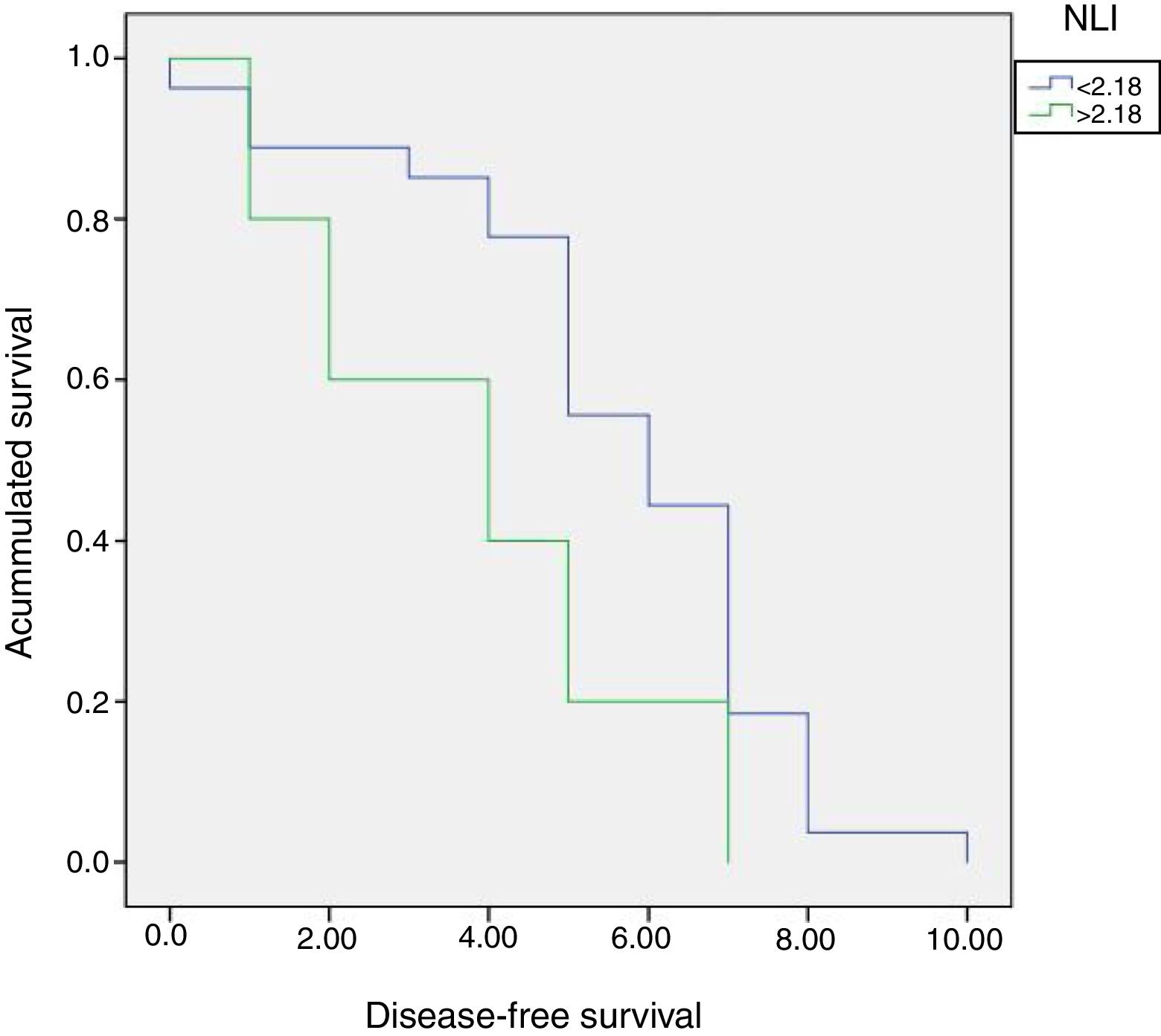
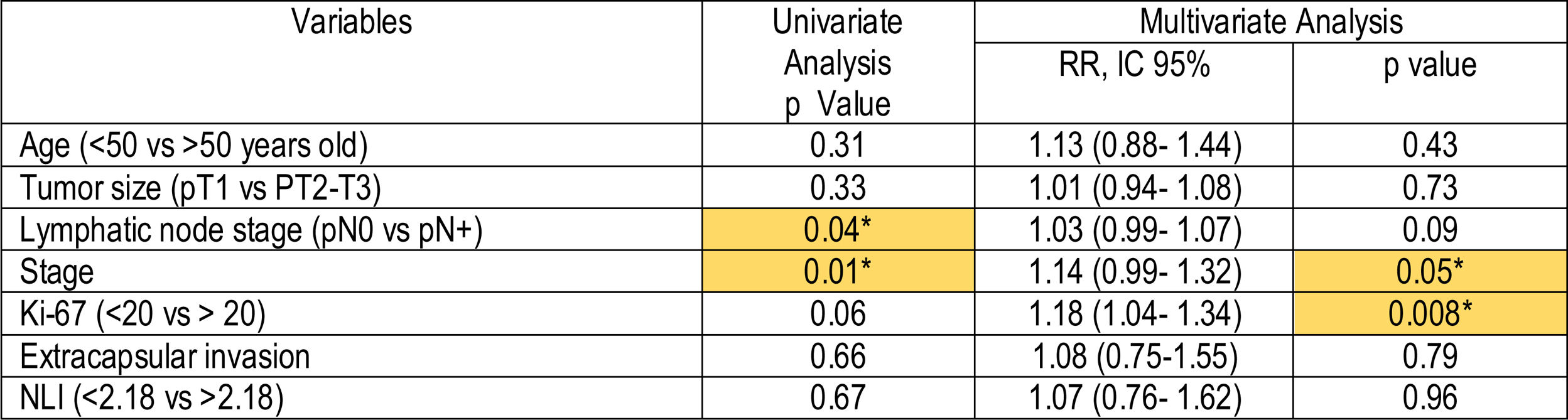
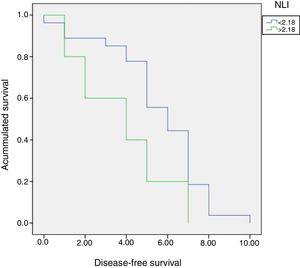
Multivariate Cox risk model revealed that NLI>2.18, was not independently correlated with the disease-free period (Table 2). Nevertheless, patients with NLI>2.18 showed shorter disease-free period in comparison with patients with NLI<2.18 (Table 3, Fig. 3).
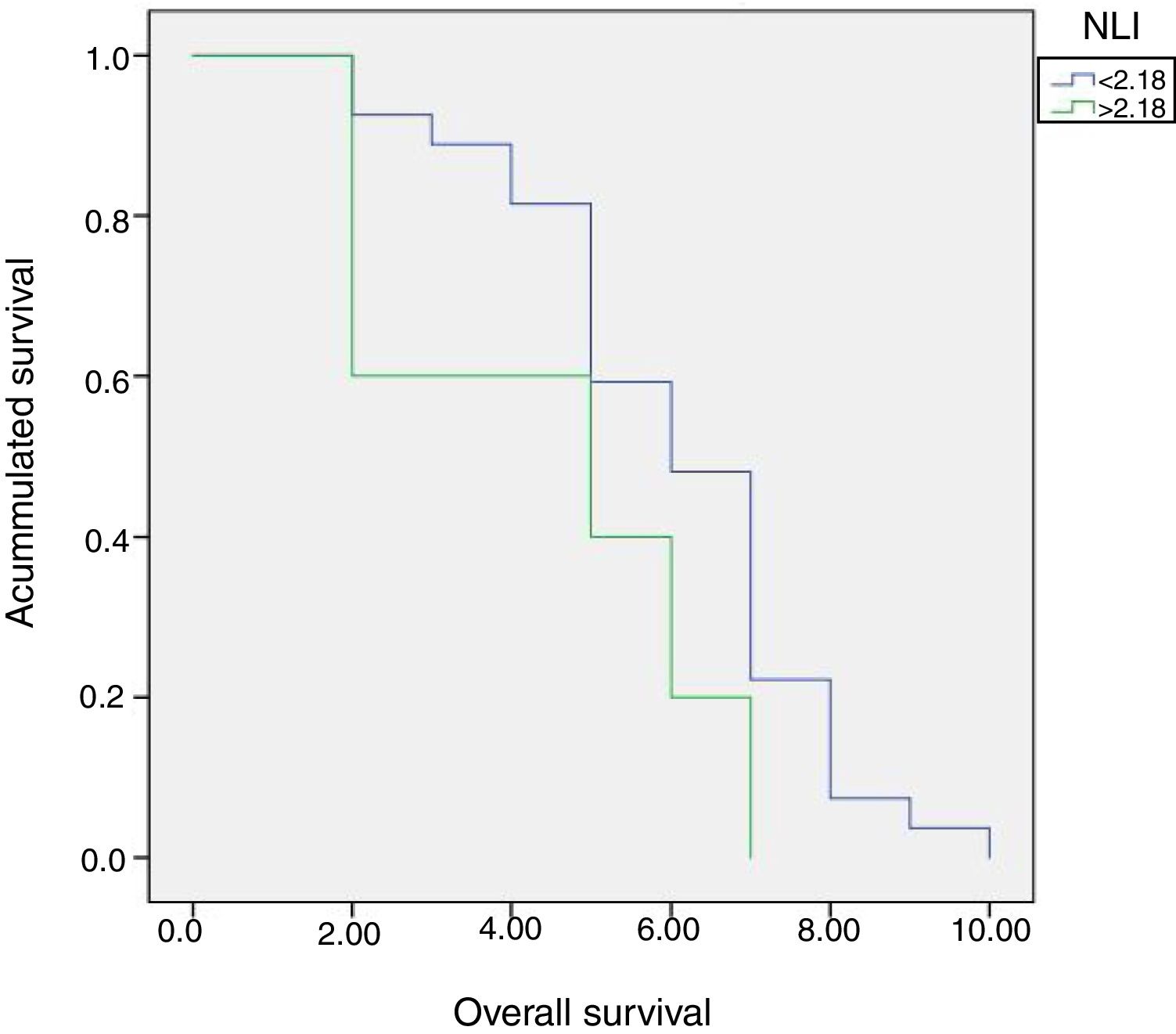
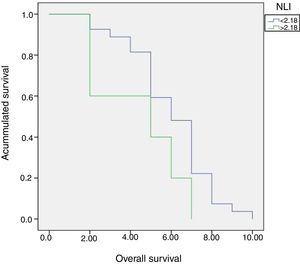
Patients were divided into two groups according to NLI, A (NLI≤2.18) and B (NLI>2.18). No significant differences were found between both groups in terms of global 5 years survival (40% versus 59.3%, p=0.11) (Fig. 4).
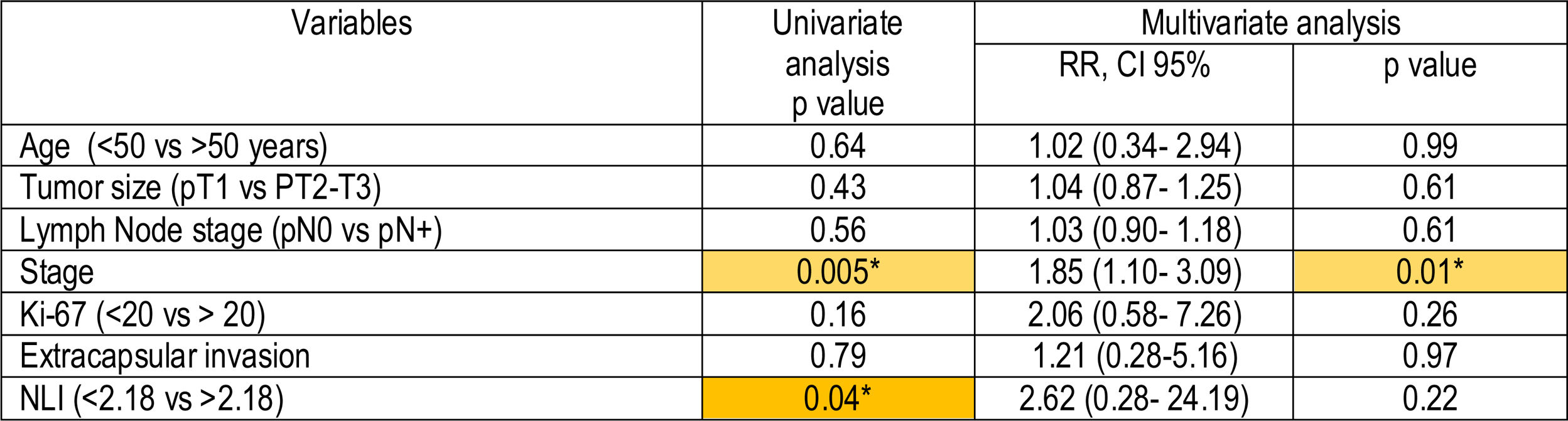
A specific univariate Cox risk model was applied in the subgroup of triple negative patients, the results revealed that in triple negative patients the NLI>2.18 and the stage were correlated independently to the disease-free SURVIVAL. Multivariate statistical analysis confirmed the stage as an independent prognostic variable which influenced disease-free survival (p=0.01; (RR=1.85, 95% CI 1.10–3.09), not the NLI>2.18, though (Table 4).
DiscussionIt has been previously described that inflammatory response plays an important role in prognosis, development and relapse of cancer.7–9 The inflammatory response is widely involved in angiogenesis, formation, maintenance and progression of breast cancer. In addition, there are signs that suggest the immune system is partly responsible for resistance to some treatments.17,19,20 Changes in the tumor microenvironment, stromal and peritumoral lymphocyte infiltration, as well as the lymphocyte response in general could have a predictive factor in various neo and adjuvant therapies in breast cancer; mainly in tumors with triple negative and with Her 2 expressed.21 Multiple studies have investigated the relationship between systemic inflammation and survival in breast cancer patients; some of these markers are: serum amyloid, reactive C protein, Interleukin 6, hypoalbuminemia, and increased levels of certain lymphocytes.1–3
The neutrophil-lymphocyte index (NLI), an inflammation marker, has received special attention because of its role as an independent prognostic factor in coronary artery disease, hypertension, chronic kidney disease, diabetes and peripheral arterial disease.22,23 It has been demonstrated that NLI is affected by alcohol intake, smoking, hypercholesterolemia, and metabolic syndrome, in addition to undocumented infection or the use of steroid drugs. Its role in abdominal sepsis has as well been studied.24–26
The NLI has been evaluated in different malignant tumors, proving a negative influence and a poor prognosis in most cases.27,28 Templeton and colleagues, recently demonstrated in a meta-analysis with 40,559 patients, that a cutoff point of 4 for the NLI was a factor adverse prognosis in global survival in several types of cancer (cholangiocarcinoma, pancreas, ovary, sarcoma, among others).29 Recently, many studies have shown the relationship between NLI and breast cancer in all its stages4,7,28,30 even in metastatic breast cancer.27 Azab and collaborators were the first to report the NLI as a predictor of mortality in breast cancer. In this study the index was correlated with mortality in short and long term (one and five years, respectively).10 Jin Hong reported the preoperative NLI as independent prognostic factor for disease-free survival, especially in patients with triple negative cancer.33 Jie Chen published a meta-analysis including 4239 subjects, and highlighted the relevance of NLI as prognostic factor in global mortality and disease-free survival.31
However, the real value of the NLI and its relationship as a prognostic factor in breast cancer is still in study. Results have not been conclusive, due to its relation in terms of the period free of disease which is positive only in the univariate analysis. Nevertheless, it has not always been able to prevail as an independent prognostic factor; a large number of studies failed to report the clinical relationship between the NLI and several variables such as tumor size or the affected lymph node.10,11,17,18 In a cohort of 442 patients, NLI greater than 2.5 was reported to be predictor of a poor prognosis only in patients with luminal A subtypes,32 on the other hand, Yuka Asano showed that a NLI under 3 could predict a good pathological response to chemotherapy in patients with triple negative breast cancer.33 Results consistent with the Italian group of Pistelli, who published that a NLI of 3 or more predicted a shorter global survival and disease free period.17
We investigated the prognostic role of pre-treatment NLI in Mexican patients with all molecular subtypes of breast cancer, especially in triple negative tumors. We investigated the role of neutrophil index forecast – lymphocyte pre-treatment in Mexican patients with breast cancer molecular subtypes, especially in tumors in triple negative. Present study shows results without statistical significance and curves without finding a strong association between overall survival, disease-free survival and the NLI in general analysis. However, in a sub-analysis in patients with triple negative subtype (n=32), those who obtained a NLI>2.18 showed a lower free of disease survival compared with patients with NLI<2.18 (5 years, 20% vs. 55.6%). Correlation was found only in the univariate analysis in the triple negative subtype, relation was lost in the multivariate analysis. However, there seems to be a slight tendency, which could be corroborated with a longer follow-up or a larger number of patients. Our results are consistent with other studies; Jin Hong reported in 2015 a cut-off point of 1.93 (in Chinese patients), with a mean follow-up of 55 months, a worse prognosis in terms of PLE in patients with NLI higher than 1.93, however this was only true for the triple negative subtypes.
There were no significant differences in the patients luminal or overexpression of Her-2 and no statistical significance between NLI high or low for overall survival in to any subtype of breast cancer.30 Sabine Krenn-Pilko and collegues, in a large cohort of 762 patients with a NLI cut-off point of 3 evidenced a significant association with the free-disease period, but not in global survival patients.34 On the other hand, Weijuan Jia in 2015, at 10-year follow-up found correlation between the NLI and the period free of disease and overall survival in patients with triple negative breast cancer and not for the rest of subtypes.4 Interestingly, the cutoff point varies considerably depending on the study, from 1.9 to 4, suggesting a wide range of associations.
Regardless of the results, we are aware of the limitations of the study. It is a retrospective analysis of a single institution and although patients were systematically selected, there are variables that could affect the results; for example, the use of non-steroidal anti-inflammatory drugs (not reported in medical history), hypercholesterolemia, or abnormal thyroid function, studies not routinely requested for all the patients. In addition, bacterial or viral infections, not detected at the time of the sampling of peripheral blood, can affect the leukocyte count. Nevertheless, it is important to emphasize that, to our knowledge, this is the first analysis with pretreatment NLI in Mexican patients. Other limitation is study sample, since original sample was 2711 patients, due to exclusion criteria, such as cancer in situ, skin or chest wall affection (T4 including inflammatory carcinoma), documented infection, hematologic disorders, acute or chronic inflammatory diseases, autoimmune diseases, steroid therapy, long term anti-inflammatory drug intake, immune-modulating drug intake, diabetes, hypertension or heart disease, chronic renal disease, documented liver disease, metabolic syndrome and smokers, which are variables that can affect leukocyte count. These exclusion criteria affected study sample, but was determined in order to increase control of external variables.
This study analyzes general survival, in this type of survival, death produced due to cancer, and death by other causes are not differentiated. A specific death cause analysis (due to cancer or not) should be included in future research.
With a longer follow-up, we could be able to confirm the tendency described on the NLI and disease-free period in triple negative patients to give a clinical application. The analysis of neutrophil lymphocyte index is a low-cost, easily reproducible source, which can be integrated with other well described prognostic factors.
This study presents the first approach to analyze the relation between pre-treatment neutrophil-lymphocyte index and breast cancer in Mexican women. It is necessary, make prospective studies and on larger scale to validate the use of the NLI in our field. Considering our findings and with the somewhat conflicting international information, we believe that the NLI should be evaluated in the context of other prognostic factors and so far we believe that there is not enough evidence to justify its isolated use in clinical practice.
ConclusionsIn our study population, NLI>2.18, was not independently correlated with the disease-free survival, nevertheless, patients with NLI>2.18 showed shorter DFS period in comparison with patients with NLI<2.18.
No significant differences were found between groups ((NLR≤2.18) and (NLR>2.18)) in terms of global 5 years survival (40% versus 59.3%).
In the subgroup of triple negative patients, the NLI>2.18 and the stage were correlated independently to the disease-free period. Multivariate statistical analysis confirmed the stage as an independent prognostic variable which influenced disease-free survival, though NLI was not.
Confidentiality of dataThe authors declare that they have followed the protocols of their Center on the publication of patient data.
Conflict of interestThe authors declare that they have no conflict of interest.