To study the management of patients with ductal carcinoma in situ (DCIS) and detect the predictors of recurrence and of missing an invasive component in the preoperative biopsy, aiming at guiding tailored treatment of these cases.
Materials and methodsA total of 123 cases of DCIS, pure/with invasion, were retrieved from the database of a tertiary cancer hospital in the period from February 2007 to February 2018. Clinical, radiologic & pathologic characteristics and its impact on the surgical management were analyzed.
ResultsThe mean age of the patients was 50.5±12.4 years. The commonest presentation was a palpable mass in 82.9% of the cases. Conservative breast surgery was successfully performed in 15 cases and mastectomy in 108 cases. Recurrence was reported in 11 cases. The underestimation rate in core needle biopsy was 48.9% missing invasive component within diagnosed malignant lesions and 19.6% missing the diagnosis of malignancy. On the other hand, overtreatment was noted as regard surgical procedure and adjuvant therapies.
ConclusionsMastectomy still the most common surgical treatment of DCIS and unfortunately sentinel lymph node biopsy is still underused. Underestimation of invasive component can occur in at least 1/4 of the patients, complexing the treatment plan. Overtreatment with axillary surgery, chemotherapy or radiotherapy needs governance.
Estudiar el manejo de pacientes con carcinoma ductal in situ (CDIS) y detectar los predictores de recaída y de ausencia de un componente invasivo en la biopsia preoperatoria, con el objetivo de orientar el tratamiento a medida de estos casos.
Materiales y métodosSe recuperó un total de 123 casos de CDIS, puro/con invasión de la base de datos de un hospital de cáncer terciario en el período de febrero de 2007 a febrero de 2018. Se analizaron las características clínicas, radiológicas y patológicas, así como su impacto en el manejo quirúrgico.
ResultadosLa edad media fue de 50,5 ± 12,4 años. La presentación más común fue masa palpable en el 82,9% de los casos. Se realizó cirugía de mama conservadora con éxito en 15 casos y mastectomía en 108 casos. Se informó de recaída en 11 casos. La tasa de subestimación en la biopsia con aguja fue de 48,9% sin componente invasivo en lesiones malignas diagnosticadas y 19,6% sin diagnóstico de malignidad. Por otra parte, se observó un exceso de tratamiento con relación al procedimiento quirúrgico y las terapias adyuvantes.
ConclusionesLa mastectomía sigue siendo el tratamiento quirúrgico más común del CDIS y desafortunadamente no se utiliza aún la biopsia de ganglio linfático centinela. La subestimación del componente invasivo puede ocurrir en al menos el 25% de los pacientes, complejizando el plan de tratamiento. Debe gestionarse el sobretratamiento con cirugía axilar, quimioterapia o radioterapia.
Ductal carcinoma in situ (DCIS) is a proliferative neoplastic heterogeneous breast disease originating from the epithelial cell-lining of the ducto-lobular system.1 DCIS lacks the power to invade stroma, lymphatic, or blood vessels as being restricted by both ductal myoepithelial cells and the basement membrane; however, it possess non-imperative inherited tendency of progression to invasive carcinoma.2
Over the years there was a dramatic increase in the incidence of newly diagnosed DCIS from 5% to around 25% due to the wide use and improvement of mammographically based screening programs and other imaging modalities.1–3
Only 13–50% of cases will progress to invasive breast cancer over a period of 10 years or more with difficult prediction which lesions will eventually progress.4
Due to the increased incidence of newly detected lesions, the non-obligatory tendency to progress, the high rate of invasive recurrence, and the low associated mortality rate, different treatment modalities were followed to both prevent the progression and reduce the risk of recurrence of DCIS.1,5
The current dilemma as regard management of DCIS is due to both potential under-estimation and over-diagnosis/over-treatment in a large number of patients.
Reported under-estimation at core needle biopsy (CNB) (i.e., missed invasive component at CNB compared with detected invasion on the result of excision specimen) ranges from 0% to 59%.6,7 The over-diagnosis (i.e. a lesion diagnosed by screening in an asymptomatic woman that would not have been detected during the woman's lifetime in the absence of screening)8 rate of DCIS is approximated to be 61% for low-grade, 57% for intermediate grade, and 45% for high-grade lesions.9
The perspectives of this study were to detect the predictors of presence of an associated invasive component within DCIS, examine the effectiveness of different preoperative biopsy techniques, delineate the factors affecting recurrence, and highlight the impact of over-treatment in these patients, aiming at guiding tailored treatment.
Patients and methodsPatient cohort and study designThe clinical, radiological, pathological, and surgical data, together with survival information of a total of 123 consecutive patients with histological-proven DCIS (either pure or with micro/macro-invasion on final pathological report) were retrieved from the database of a tertiary cancer center in the period between February 2007 and February 2018. The follow up visits were reviewed till January 2020. Patients were followed for a median of 42 months (range: 0–153 months).
The primary outcome was the rate of under-estimation (defined as missing an associated invasive focus) and its predictors. The secondary outcomes were exploration of incidence of over-treatment of DCIS cases, the accuracy of different biopsy techniques, and the predictors of recurrence.
The predictors of recurrence and disease-free survival were measured in the whole cohort including those with detected invasion in the final pathology.
Clinical routine work-upPatients were subjected to full clinical examination at their first outpatient clinic (OPC) visit, followed by routine radiological investigations in the form of ultrasonography (US)/mammography with or without magnetic resonance imaging (MRI) according to indications and availability at the time. Biopsy was taken to obtain pre-operative diagnosis and where pre-operative biopsy could not achieves diagnosis an excisional biopsy or intraoperative frozen section was arranged; however, some cases were presented at their first OPC visit by pre-operative excision biopsy performed out of our center (with or without pre-biopsy imaging) which was revised by expert pathologists to confirm the diagnoses then the remaining investigations were performed as usual. Microinvasion was defined as association of invasive focus measuring ≤1mm, while macroinvasion is any associated invasive focus>1mm.
Statistical analysisData were analyzed on a personal computer running SPSS© for windows (Statistical Package for Social Science) release 22. For descriptive statistics of qualitative variables, the frequency distribution procedure was run with the number of cases and percentages. For descriptive statistics of quantitative variables, the mean, median, range and standard deviation were used to describe central tendency and dispersion. Comparisons were done using Chi-square test for nominal variables with Fischer exact test for those with less than 5 squares. In addition, Mann–Whitney and student-t-tests were used for continuous variables according to their pattern of distribution. Disease free survival analyses were calculated by the Kaplan–Meier curve with significance measured according to log rank test. P-value was considered statistically significant if value was ≤0.05.
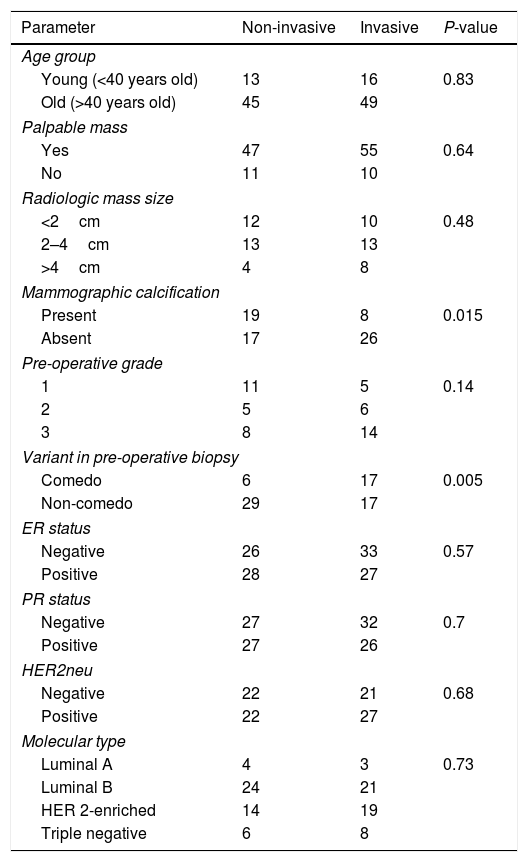
ResultsPatients’ characteristics (Table 1)Clinico-radiologic parametersIn this study 123 cases were enrolled. The mean age of the patients was 50.5±12.4 years. Palpable mass was detected in 102 patients (82.9%) followed by nipple discharge in 31 (25.2%) patients and (bloody in 13 patients).
Showing the clinico-epidemiological, radiological, and pathological characteristics.
| Parameter | Data |
|---|---|
| Age mean±SD | 50.5±12.4 (21–86) |
| Presentation | |
| Screening | 1(0.8%) |
| Discharge | 31 (25.2%) |
| Palpable mass | 102 (82.9%) |
| Palpable axillary LNS | 12 (9.8%) |
| Nipple erosion/deformation | 13 (10.6%) |
| Mammography (N=74 patients) | |
| Calcifications | 27 (36.5%) |
| Parenchymal distortion | 14 (18.9%) |
| Mass | 34 (45.9%) |
| Enlarged nodes | 20 (27%) |
| Ultrasound (N=105 patients) | |
| Calcifications | 36 (34.3%) |
| Parenchymal distortion | 11 (10.5%) |
| Mass | 69 (65.7%) |
| Enlarged nodes | 72 (68.6%) |
| Biopsy (N=117) | |
| FNAC | 8 (6.8%) |
| CNB | 56 (47.9%) |
| Incisional | 14 (12%) |
| Excisional | 36 (30.8%) |
| Exfoliative cytology | 3 (2.8%) |
| Preoperative biopsy (N=114) | |
| Benign | 11 (9.6%) |
| Pure DCIS | 58 (50.9%) |
| DCIS with invasion | 31 (27.2%) |
| Malignant with invasion undetermined | 14 (12.3%) |
| Preoperative grade (N=49) | |
| 1 | 16 (32.7%) |
| 2 | 11 (22.4%) |
| 3 | 22 (44.9%) |
| Variant in preoperative biopsy (N=69) | |
| Comedo | 23 (33.3%) |
| Non-comedo | 46 (66.7%) |
| Frozen section pathology (N=48) | |
| No invasion | 26 (54.2%) |
| Invasion | 18 (37.5%) |
| Undetermined | 4 (8.3%) |
| Frozen safety margin (N=34) | |
| Infiltrated | 20 (58.8%) |
| Free | 14 (41.2%) |
| Surgery (N=123) | |
| CBS | 15 (12.2%) |
| Mastectomy | 108 (87.8%) |
| Reconstruction (N=20) | |
| LDF | 17 (85%) |
| Implant | 1 (5%) |
| LDF & implant | 1 (5%) |
| Omental flap | 1 (5%) |
| Axillary surgery | |
| ALND | 113 (91.9%) |
| SLNB | 7 (5.7%) (2 proceeded to ALND) |
| No | 5 (4.1%) |
| Final pathology (N=123) | |
| Pure DCIS | 58 (47.2%) |
| DCIS with invasion | 65 (52.8%) |
| Type of invasion (N=65) | |
| Micro-invasion | 47 (72.3%) |
| Macro-invasion | 18 (27.7%) |
| Median pathologic size (range) | 4.25 (0–18) cm |
| Nodal status (N=118) | |
| N negative | 103 (87.3%) |
| N positive | 15 (12.7%) |
| DCIS type (N=114) | |
| Comedo | 66 (57.9%) |
| Non-comedo | 48 (42.1%) |
| Spread of DCIS in other quadrants(N=108) | |
| No | 88 (81.5%) |
| Yes | 20 (18.5%) |
| Final grading | |
| 1 | 15 (12.2%) |
| 2 | 14 (11.4%) |
| 3 | 72 (58.5%) |
| Staging | |
| 0 | 58 (47.2%) |
| I | 43 (35%) |
| II | 16 (13%) |
| III | 4 (3.3%) |
| Adjuvant therapy | |
| chemo | 63 (51.2%) |
| Radio | 26 (21.1%) |
| Hormonal | 62 (50.4%) |
| Recurrence | |
| No | 112 (91.1%) |
| Yes | 11 (8.9%) |
| Site of recurrence (N=11) | |
| Local | 1 (9.1%) |
| Isolated distant | 6 (54.5%) |
| Multiple sites | 4 (36.4%) |
| Local, nodal & distant | 3 (27.3%) |
| Nodal & distant | 1 (9.1%) |
| Site of distant recurrence (N=10) | |
| Bone | 5 (50%) |
| Lung | 2 (20%) |
| Multiple sites | 3 (30%) |
FNAC: fine needle aspiration cytology, CNB: core needle biopsy, DCIS: ductal carcinoma in situ, CBS: conservative breast surgery, LDF: latissimus dorsi flap, ALND: axillary lymph node dissection, SLNB: sentinel lymph node biopsy.
The commonest mammographic finding was mass in 34 patients (45.9%), followed by calcifications in 27 (36.5%), then parenchymal distortion in 14 (18.9%) patients. As regard ultrasonography (US), mass was detected in 69 (65.7%), followed by calcifications in 36 (34.3%), and parenchymal distortion in 11 (10.5%) patients. Lymph nodes were better detected by US than mammography, 68.6% and 27%, respectively. In a minority (10 patients) MRI was used.
PathologyPreoperative biopsy was performed for 117 patients and the most common method was CNB in 56 (47.9%). Frozen section was implemented in 48 cases for either diagnosis (when preoperative biopsy failed to diagnose) or in the context of performing CBS. Overall, of the 117 patients who underwent biopsy, the pathology reports of 114 patients could be retrieved, with benign disease diagnosed in 11 (9.6%) patients, pure DCIS in 58 (50.9%) patients, DCIS with invasion in 31 (27.2%) patients, and finally malignant invasion cannot be determined in 14 (12.3%) patients (10 with cytology and 4 with CNB).
In the postoperative pathology, no residual tumor was found in 17 out of the 36 patients with unplanned excisional biopsy (47.2%). Invasive component was detected in paraffin sections in 65 (52.8%) patients, mostly microinvasion in 47 (72.3%) patients. Of importance is that foci of DCIS were encountered in other quadrants in 20 patients (18.5%). In 15 (12.7%) patients there were spread to axillary nodes, 2 cases were SLNB positive. Of the 15 cases with positive ALND, 8 cases were DCIS with micro-invasion, 3 cases were DCIS with macro-invasion, and 4 cases were pure DCIS (which could be explained by missed invasive foci during specimen sectioning).
Treatment modalitiesConservative breast surgery was initially attempted in 36 patients and was successfully completed in only 15 (12.2%) patients, while mastectomy was performed in a total of 108 (87.8%) patients. One patient was reported with infiltrated depth margin by DCIS despite re-excision and refused mastectomy. Of note, the 11 cases with preoperative benign pathology, but with suspicious radiological criteria underwent frozen section which revealed malignant lesions in all cases. Axillary lymph node dissection (ALND) was done in 113 (91.9%) patients, while only 7 (5.7%) patients offered sentinel lymph node biopsy (SLNB) initially with 2 of them proceeding to ALND.
Thereafter, 63 (51.2%) patients received adjuvant chemotherapy as following; 36 were DCIS with microinvasion, 10 DCIS with macroinvasion, and 17 pure DCIS. Of the 36 cases of DCIS with microinvasion who received chemotherapy, the hormonal profile was 2 luminal A, 11 luminal B, 12 HER2neu-enriched, and 4 triple-negative.
As regard adjuvant radiotherapy, 26 (21.1%) patients received external beam radiation (2 were DCIS with macro-invasion, 13 DCIS with micro-invasion, and 11 pure DCIS), and according to surgical procedure 10 cases had the radiation following CBS, and 16 cases following mastectomy.
Hormonal treatment was received in total 62 (50.4%) patients (9 DCIS with macro-invasion, 22 DCIS with micro-invasion, and 31 pure DCIS).
RecurrenceRecurrence occurred in a total of 11 (8.9%) patients. In addition, isolated distal recurrence occurred in 6 patients representing the commonest recurrence pattern.
Predictors of associated invasive component (Table 2)Only the absence of mammographic calcifications and preoperative biopsy showing comedo type DCIS were significant predictors of invasion in the final pathology, P-value=0.015 and 0.005, respectively.
Showing the predictor of associated invasive component.
| Parameter | Non-invasive | Invasive | P-value |
|---|---|---|---|
| Age group | |||
| Young (<40 years old) | 13 | 16 | 0.83 |
| Old (>40 years old) | 45 | 49 | |
| Palpable mass | |||
| Yes | 47 | 55 | 0.64 |
| No | 11 | 10 | |
| Radiologic mass size | |||
| <2cm | 12 | 10 | 0.48 |
| 2–4cm | 13 | 13 | |
| >4cm | 4 | 8 | |
| Mammographic calcification | |||
| Present | 19 | 8 | 0.015 |
| Absent | 17 | 26 | |
| Pre-operative grade | |||
| 1 | 11 | 5 | 0.14 |
| 2 | 5 | 6 | |
| 3 | 8 | 14 | |
| Variant in pre-operative biopsy | |||
| Comedo | 6 | 17 | 0.005 |
| Non-comedo | 29 | 17 | |
| ER status | |||
| Negative | 26 | 33 | 0.57 |
| Positive | 28 | 27 | |
| PR status | |||
| Negative | 27 | 32 | 0.7 |
| Positive | 27 | 26 | |
| HER2neu | |||
| Negative | 22 | 21 | 0.68 |
| Positive | 22 | 27 | |
| Molecular type | |||
| Luminal A | 4 | 3 | 0.73 |
| Luminal B | 24 | 21 | |
| HER 2-enriched | 14 | 19 | |
| Triple negative | 6 | 8 | |
It is worth noting that most cases of pure DCIS were low-grade variant. There was no statistically significant difference as regard lesion palpability between the two groups, however being slightly higher in the invasive group.
Sensitivity and specificity of preoperative pathology & frozen section in detection of invasionThe preoperative biopsy had a sensitivity of 56.5%, specificity of 87.8% and accuracy of 71.3% (excluding cases with false benign biopsy and those with malignant smear).
While the intraoperative frozen section had a sensitivity of 72.7%, specificity of 90.9% and accuracy of 81.8% (excluding 4 cases were frozen could not give a conclusion asking for paraffin wait).
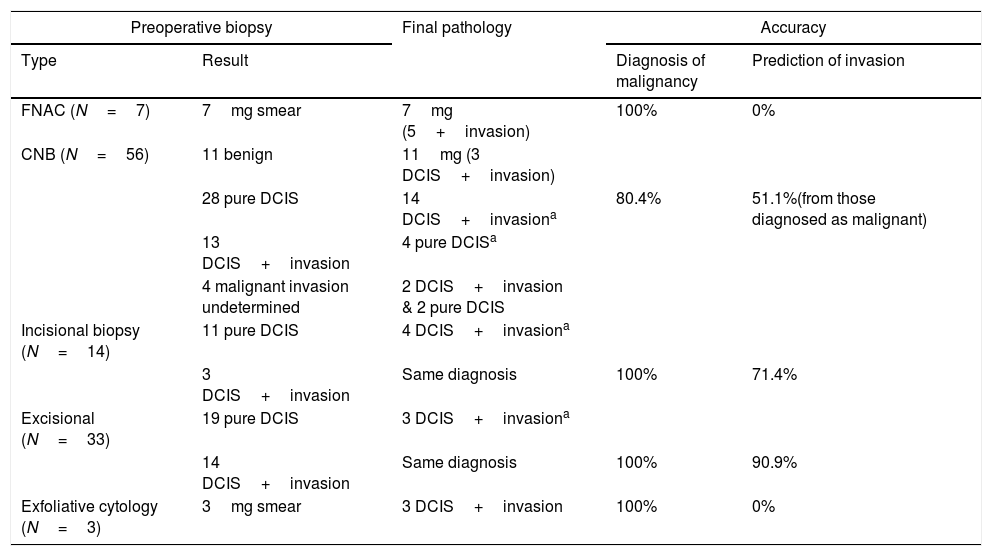
Under-estimation and over-treatment (Table 3)As regard the under-estimation rate of different biopsy techniques, excisional biopsy was of the least rate (3/33 cases), followed by incisional biopsy (4/14 cases), while CNB showed the highest rate (25/56 cases) cases. On the other side over-estimation in CNB occurred in 4 out of 56 cases.
Showing the accuracy of different biopsy types in diagnosis of DCIS and detection of invasive component.
| Preoperative biopsy | Final pathology | Accuracy | ||
|---|---|---|---|---|
| Type | Result | Diagnosis of malignancy | Prediction of invasion | |
| FNAC (N=7) | 7mg smear | 7mg (5+invasion) | 100% | 0% |
| CNB (N=56) | 11 benign | 11mg (3 DCIS+invasion) | ||
| 28 pure DCIS | 14 DCIS+invasiona | 80.4% | 51.1%(from those diagnosed as malignant) | |
| 13 DCIS+invasion | 4 pure DCISa | |||
| 4 malignant invasion undetermined | 2 DCIS+invasion & 2 pure DCIS | |||
| Incisional biopsy (N=14) | 11 pure DCIS | 4 DCIS+invasiona | ||
| 3 DCIS+invasion | Same diagnosis | 100% | 71.4% | |
| Excisional (N=33) | 19 pure DCIS | 3 DCIS+invasiona | ||
| 14 DCIS+invasion | Same diagnosis | 100% | 90.9% | |
| Exfoliative cytology (N=3) | 3mg smear | 3 DCIS+invasion | 100% | 0% |
aThe remaining cases retained the same pathology as preoperative biopsy.
Over-treatment was noted in 30/63 (47.6%) cases who received adjuvant chemotherapy (17 of pure DCIS and 13 cases of DCIS with microinvasion and hormonal profile luminal A, B). Also, adjuvant radiotherapy was given to 7 cases of pure DCIS following mastectomy with negative axilla in 4 cases. Furthermore, of the 113 procedures of ALND, 98 (86.7%) of cases had negative axillary nodes.
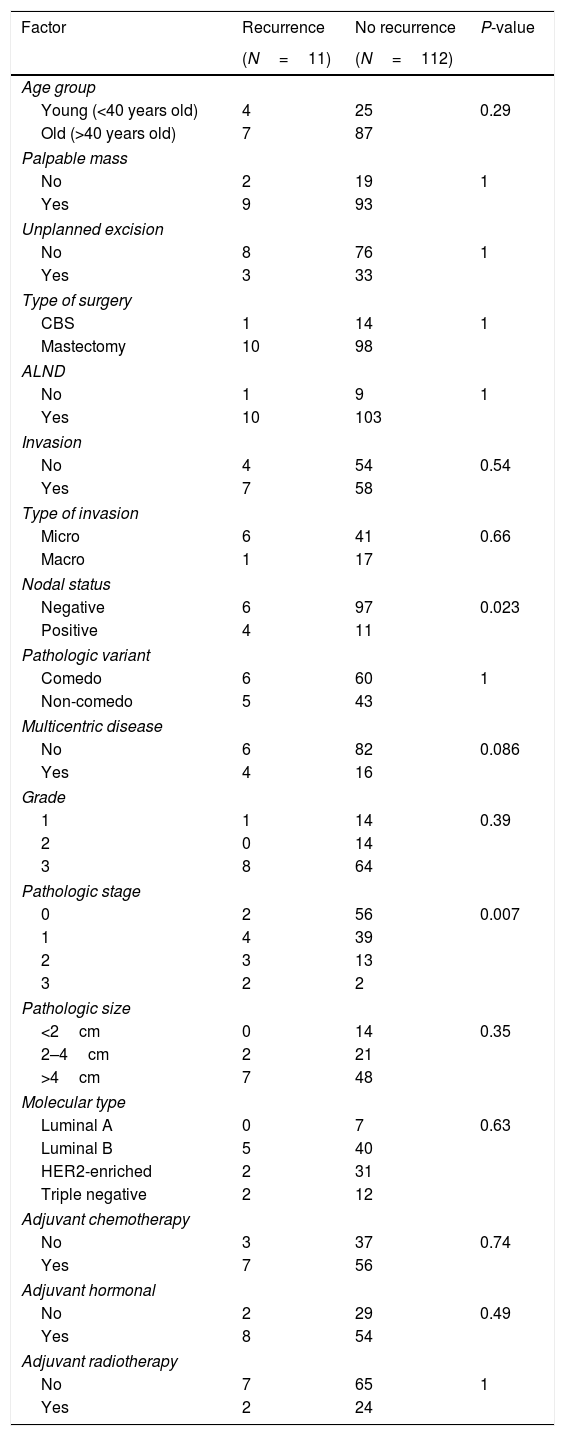
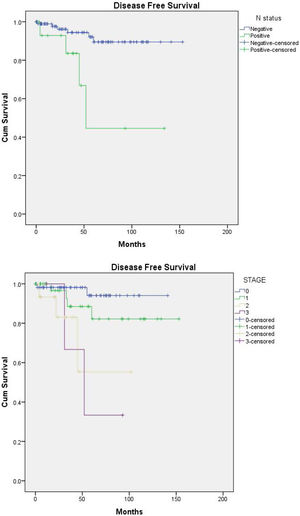
Factors contributing to recurrence (Table 4)Node positive disease and advanced stage were the only significant factors increasing the risk of recurrence, P-value=0.023 and 0.007, respectively.
Showing the predictors of recurrence after surgery.
| Factor | Recurrence | No recurrence | P-value |
|---|---|---|---|
| (N=11) | (N=112) | ||
| Age group | |||
| Young (<40 years old) | 4 | 25 | 0.29 |
| Old (>40 years old) | 7 | 87 | |
| Palpable mass | |||
| No | 2 | 19 | 1 |
| Yes | 9 | 93 | |
| Unplanned excision | |||
| No | 8 | 76 | 1 |
| Yes | 3 | 33 | |
| Type of surgery | |||
| CBS | 1 | 14 | 1 |
| Mastectomy | 10 | 98 | |
| ALND | |||
| No | 1 | 9 | 1 |
| Yes | 10 | 103 | |
| Invasion | |||
| No | 4 | 54 | 0.54 |
| Yes | 7 | 58 | |
| Type of invasion | |||
| Micro | 6 | 41 | 0.66 |
| Macro | 1 | 17 | |
| Nodal status | |||
| Negative | 6 | 97 | 0.023 |
| Positive | 4 | 11 | |
| Pathologic variant | |||
| Comedo | 6 | 60 | 1 |
| Non-comedo | 5 | 43 | |
| Multicentric disease | |||
| No | 6 | 82 | 0.086 |
| Yes | 4 | 16 | |
| Grade | |||
| 1 | 1 | 14 | 0.39 |
| 2 | 0 | 14 | |
| 3 | 8 | 64 | |
| Pathologic stage | |||
| 0 | 2 | 56 | 0.007 |
| 1 | 4 | 39 | |
| 2 | 3 | 13 | |
| 3 | 2 | 2 | |
| Pathologic size | |||
| <2cm | 0 | 14 | 0.35 |
| 2–4cm | 2 | 21 | |
| >4cm | 7 | 48 | |
| Molecular type | |||
| Luminal A | 0 | 7 | 0.63 |
| Luminal B | 5 | 40 | |
| HER2-enriched | 2 | 31 | |
| Triple negative | 2 | 12 | |
| Adjuvant chemotherapy | |||
| No | 3 | 37 | 0.74 |
| Yes | 7 | 56 | |
| Adjuvant hormonal | |||
| No | 2 | 29 | 0.49 |
| Yes | 8 | 54 | |
| Adjuvant radiotherapy | |||
| No | 7 | 65 | 1 |
| Yes | 2 | 24 | |
In those patients who did mastectomy, 20 patients were found to have occult foci of DCIS in other quadrants. On analyzing the different epidemiologic, radiologic, and pathologic factors, the only factor that was significantly associated with this multi-centricity was presence of parenchymal distortion in mammography (P-value=0.01). Also, it was marginally significant as to presence of residual tumor after excision biopsy/trial conservation (P-value=0.094) and presence of positive axillary nodes (P-value=0.065) and molecular type (more in luminal type) (P-value=0.06).
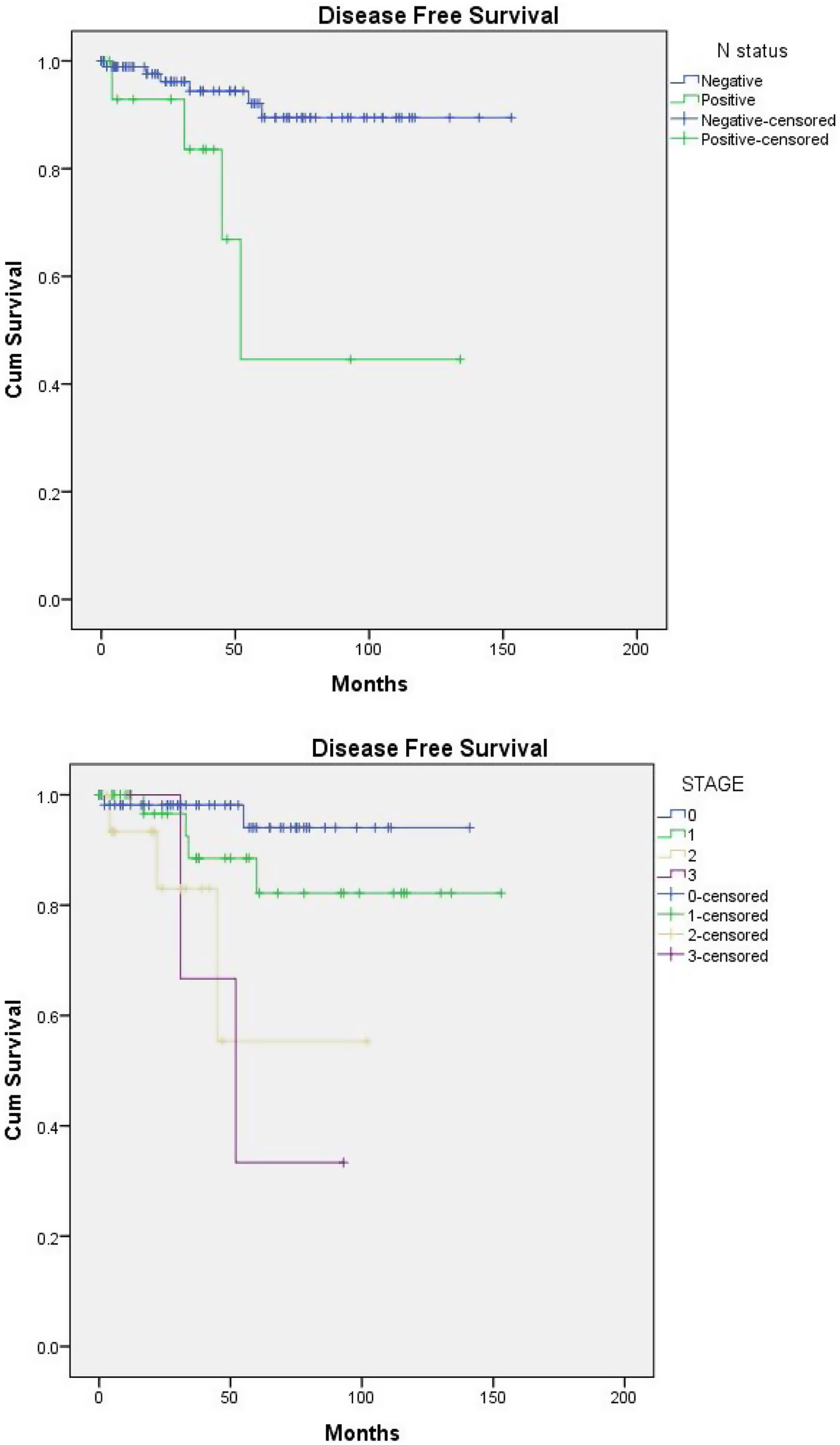
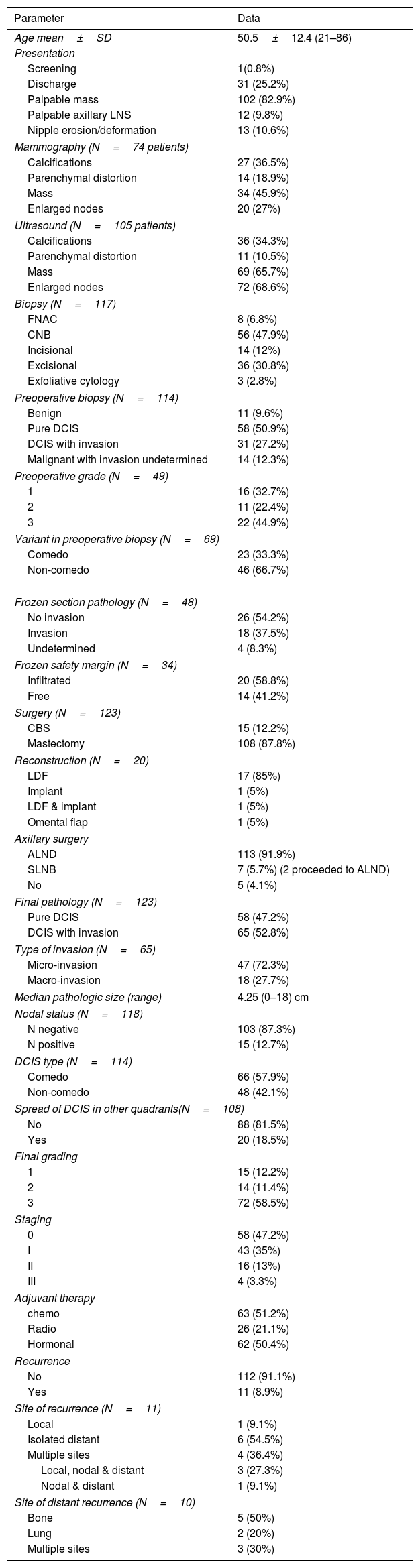
Disease free survival (DFS) (Fig. 1a and b)Nodal spread and advanced stage were the only determinant factors of a short DFS in patients with DCIS, P-value=0.003 for both. With node negative versus positive estimated mean DFS (140.9 vs. 82 months), as regard staging: estimated mean DFS for stages 0,1,2,3 was 134.9, 132.8, 71.4, 58.7 months, respectively.
DiscussionUltrasonography (US) has been generally considered less effective for DCIS detection due to its low sensitivity for identification of microcalcifications. The primary use of US is further assessment for a mass, increase likelihood of detection of an associated invasive component and obtains US guided biopsy for pathological diagnosis.10
Mass lesion with different shapes and margins is the most common presentation of DCIS using US accounts for 44–72%.11 Our series reported higher number of patients imaged primarily by US with mass presentation being the commonest finding in 69 (65.7%) patients.
Mammographically detected microcalcifications is by far the most common presentation of DCIS accounting for approximately 70–80%,12 and is associated with clinically palpable mass in 10%.13 However, in our series a smaller number of patients underwent mammography which could be explained by the less availability of screening programs in early years coupled with lack of knowledge in DCIS with trend of excisional biopsy among general non-breast surgeons without proper preparation which made it difficult to use mammography for evaluation after that. In addition to the patient preference for mastectomy due to fear, lack of awareness and understanding of such disease, and poor previous experience, the same factors could be attributed to the use of MRI in our series with higher impact.
Unscreened populations usually presents by a palpable breast lesion, nipple discharge or Paget's disease of the nipple,14 this explains the high number of palpable breast mass presentation in our series (102, 82.9%), as these patients usually diagnosed outside the screening programs.
A recent SEER analysis on identification of pure DCIS by various techniques showed that 44% of lesions were detected by combined US/mammography, 46% mammography alone, 8% by US alone, and 2% were not detected by either.3
Our results showed under-estimation rate of CNB of 48.9% missing invasion from those diagnosed with malignant lesion and 19.6% missing diagnosis of malignancy. In addition, our data shows that excisional and incisional biopsies were more accurate compared with CNB. This does not justify the increasing use of incisional/excisional biopsy as they entail second operative procedure with more trauma for the patient and cost for the health system, together with difficulties in planning CBS if not previously planned by the same oncoplastic surgeon.
Many preoperative factors had been linked with under-estimation/up-staging of CNB in DCIS lesions, including: palpable lesion, symptomatic cases, mass at mammography, BIRADS 4 or 5, larger lesion size (>20mm at imaging), clinical and US guided biopsy compared to stereotactic guided biopsy, high-grade features at CNB.6 Schulz et al.,7 also reported ≥50% of histologically affected ducts and diameter ≥50mm as a risk factors on univariate analysis with clinically palpable lesion as the only independent predictor after multivariate analysis. Marques et al.,15 in addition reported comedo-necrosis as the independent predictor of invasion after multivariate analysis.
Elmore et al.,16 reported a low level of diagnostic agreement between expert pathologists and those with lesser expertise for DCIS when compared with invasive lesions (84% vs. 96%), with possible under or over-interpretation for DCIS as of 3% incidence of over-interpretation of DCIS to IDC, 13% incidence of under-interpretation of DCIS to atypia and 17% incidence of over-interpretation of atypia to DCIS. As such Hong et al.,17 recommended obtaining a second opinion to avoid the risk of unnecessary treatments.
Due to the increased rate of mammographically detected DCIS lesions during screening campaigns a large number of indolent DCIS that would not progress to invasive carcinoma during the lifetime of the patient had been diagnosed,18 which in turn leads to over-treatment thus exposing larger portion of patients to unnecessary treatment complications. What supports this claim is the high prevalence of DCIS in autopsy cases of non-symptomatic non screened patients who died of other causes.1,19 Another support is that not every case of DCIS will progress to invasive carcinoma during life time, only 13–50% of cases4, and only 30% of DCIS will have an invasive recurrence.20
A SEER database analysis reported the utilization of different options for treating DCIS with a significant increase of lumpectomy with radiotherapy (RT), bilateral mastectomy and no treatment, and for SLNB in both mastectomy and lumpectomy over the time.21 In our series, 108 (87.8%) patients underwent mastectomy due to difficult assessment following unplanned excisional biopsy, palpable mass presentation in most cases, and patient/surgeon preference over CBS due to fear of recurrences, other causes are lack of deep knowledge of different biological behavior of DCIS.
Stuart et al.,22 reported an adjusted meta-regression local recurrence (LR) rate of 2.6% for mastectomy; 13.6% for CBS with RT; 25.5% for CBS without RT and 27.8% for biopsy-only (residual predominately low-grade DCIS following inadequate excision). Because of its low LR rate, total mastectomy is still a very valid option for any patient with DCIS, when properly selected, as it provides overly aggressive treatment for many women. In the current study, the overall recurrence rate was 8.9% with no significant difference between those who underwent CBS and those who had total mastectomy.
Cases with pure DCIS will show nodal metastasis in only 1–2% of cases, obviously from missed invasive component,23 as we found 4 cases of pure DCIS with positive axillary nodal status.
Investigating the incidence of positive SLNB in 470 high-risk DCIS patients, researchers at Memorial Sloan-Kettering reported positivity in 9% with extensive disease demanding mastectomy and the presence of necrosis were associated with an increased risk.24 The risk of lymphedema after SLNB is not negligible (5.3%),25 but still better than that following ALND (14.1%).26 This should raise concern about the extensive use of ALND by surgeons in our series, and the low incidence of positive nodes (12.7%) detected should discourage this approach toward SLNB or not touching the axilla.
It is well recognized that adjuvant RT post CBS will significantly reduce the incidence of LR, and distant metastasis free survival, but with no improvement in overall survival.27,28
Hughes et al.,29 reported that the 5-year rate of in breast tumor recurrence in the low/intermediate grade patients was 6.1% compared to 15.3% for patients with high-grade DCIS with a median follow-up of 6.7 years. However, at 12 years follow up both groups showed increased incidence of IBTR with 14.4% for low/intermediate grade and 24.6% for high grade DCIS.30
Considering the previous data, the current guidelines5 recommend an individualized approach based on patient preference and other important factors.
Examples of over-treatment: treatment applied for many indolent over-diagnosed lesions not expected to progress, the aggressive treatment approaches used such as total mastectomy for cases candidate for CBS, the use of ALND in cases indicated for SLNB, or the use of SLNB in non-indicated cases, and the overuse of different adjuvant therapies. The current study showed different patterns of overtreatment as (1) adjuvant chemotherapy for 17 patients with pure DCIS and another 13 cases of DCIS with microinvasion with luminal tumors, (2) adjuvant radiotherapy was given to 4 cases of pure DCIS following mastectomy with negative axilla, and (3) 98 unnecessary procedures of ALND in patients of negative axillary nodal status.
Over-treatment of DCIS could be attributed to high rate of over-diagnosis, its heterogeneous biological behavior and lack of predictive pathologic and clinical markers for progression, high incidence of under-estimation of CNB, fear of the high rate of invasive recurrence, and the psycho-mental impression given to both surgeons and patients by the misleading use of the term carcinoma coupled with lack of efficient knowledge as regard different aspects of DCIS.
Limitations of the studyThis is a retrospective study conducted on a relatively small patient cohort over a long time. Also, the heterogeneous nature of the cohort as regard; presence of invasion and the management process should be considered when interpreting these results.
ConclusionsMastectomy is still commonly used for surgical treatment of DCIS and unfortunately SLNB is still underused. Detection of occult multicentric disease is infrequent and its affection of recurrence is unknown, as such this should not prohibit breast conservation. Under-estimation of the presence of an invasive component can occur in at least a quarter of DCIS patients, complexing the treatment plan. Survival is nearly hundred percent, and recurrence is low as such radiotherapy should be used accurately in a personalized way.
Ethical approvalWe conducted this study in compliance with the principles of the Declaration of Helsinki. This study was approved by IRB of the Mansoura Faculty of Medicine under the number R/16.01.140.
FundingNo funding was received.
Conflict of interestThe authors declare that they have no conflict of interest.