The most frequent malignant tumour affecting women throughout their reproductive years is breast cancer, which is also the most often identified cancer worldwide.The purpose of this literature review is to examine current treatments for this condition, assess how they affect pregnancy outcomes, and assess how pregnancy affects the course and prognosis of the condition itself via this narrative review.
La neoplasia que afecta con mayor frecuencia a las mujeres a lo largo de su vida reproductiva es el cáncer de mama, siendo también el tipo de cáncer más identificado a nivel mundial. El objetivo de esta revisión de la literatura es examinar los tratamientos actuales para esta situación, evaluar cómo afecta a los resultados del embarazo, así como el modo en que afecta al curso y pronóstico de la enfermedad en sí, a través de esta revisión de la narrativa.
Worldwide,Breast Cancer (BC) is the most prevalent cancer in women and the main factor in female cancer mortality.1–3 BC that is detected during pregnancy, the postpartum period, or lactation is known as Pregnancy-Associated Breast Cancr (PABC.4 The length of the post-partum period, which can last anywhere from one to five years, is debatable, however most researches only take into account the first year following birth.5–8 The only other malignancy that affects pregnant women more frequently than PABC is cancer of the cervix.9 The prognosis for women with PABC is worse than for those with BC unrelated to pregnancy, according to growing evidence from numerous research.10–12
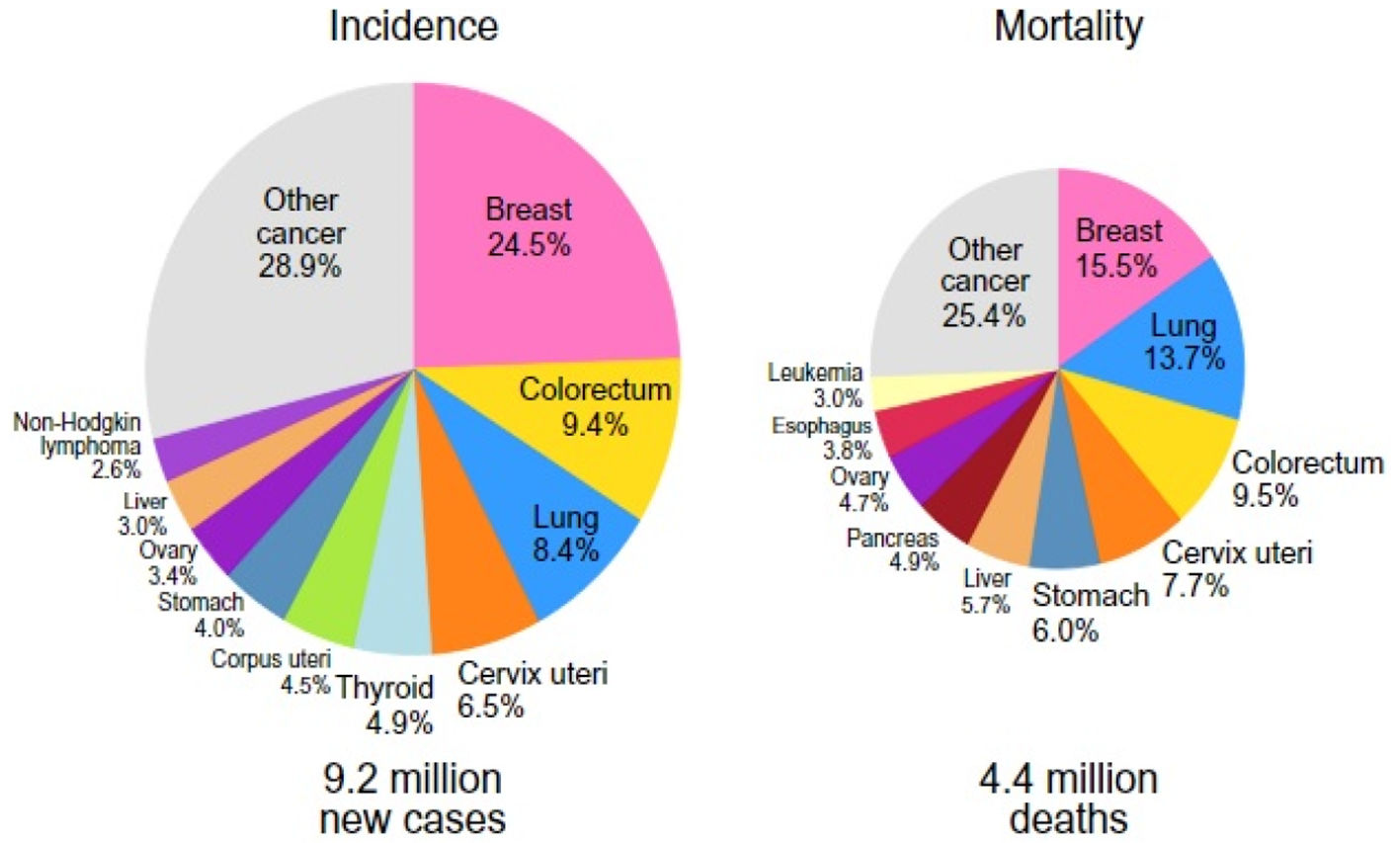
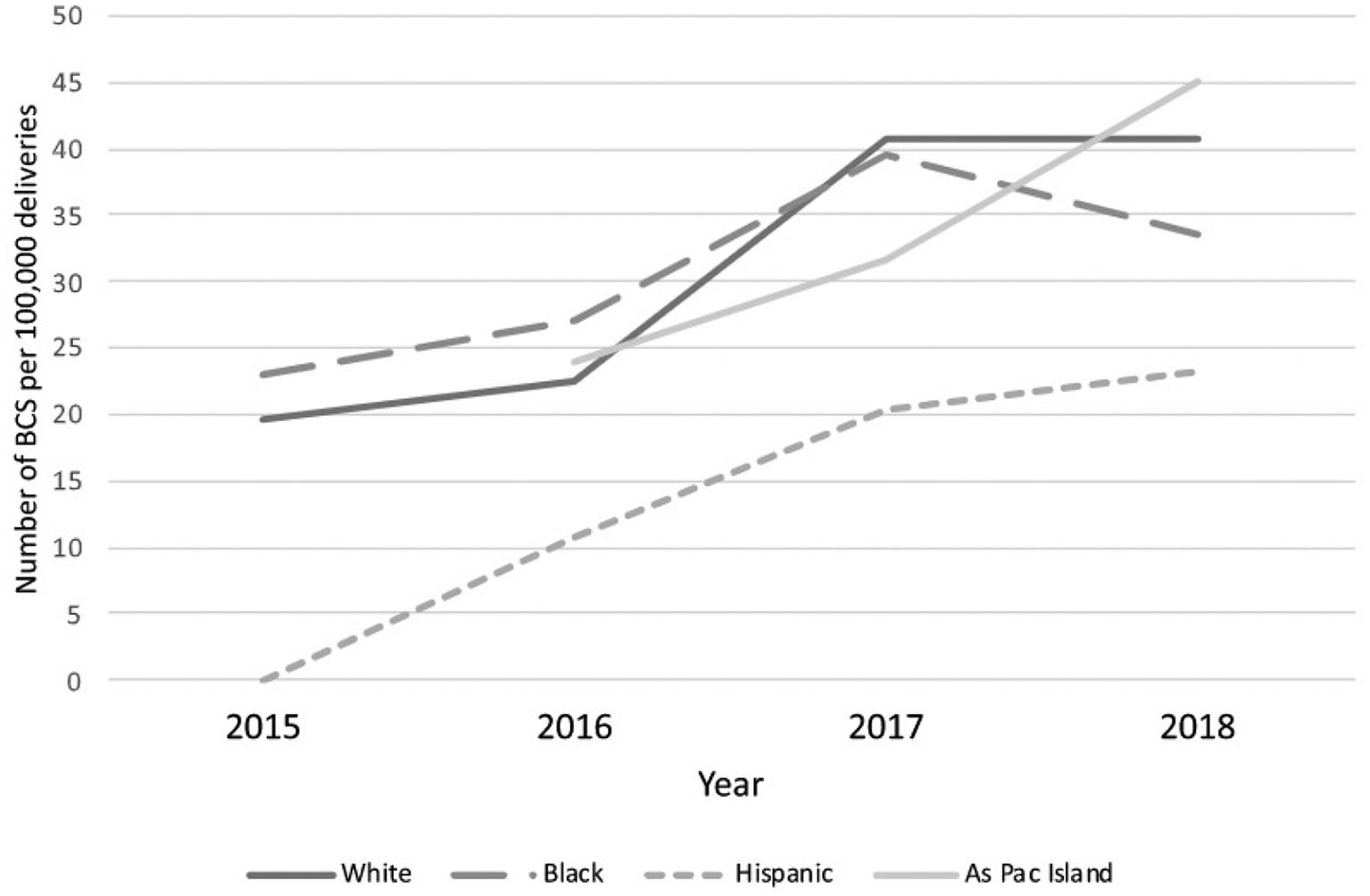
Incidence, epidemiology and associated risk factors of PABCBC is the most common malignant tumour affecting women during their childbearing period of age and is the most frequently diagnosed cancer worldwide (in 154 countries out of 185).13 Female breast cancer has now surpassed lung cancer as the leading cause of global cancer incidence in 2020 (24.5%), and it is also the most frequently diagnosed cancer with an estimated 2.3 million new cases, representing 11.7% of all cancer cases, according to the Global Cancer Observatory(GLOBOCAN) data 2020(published in 2021). (Fig. 1).1,14 According to several studies, the incidence of PABC ranges from 1:10,000 to 1:3000 of all pregnancies, and the rate of PABC ranges from 2.6% to 6.9% among women under the age of 45, but it climbs to 15.6% of all BC among women under the age of 35.15–17 Long-standing dogma, however, has suggested that being pregnant protects against breast cancer.18 However, a 2019 publication that revealed a genuine increase in breast cancer chances for over 20 years after a pregnancy event before turning back to negative, raised questions about this idea.19 According to research conducted between 1988 and 1992, Caucasians were found to have the highest overall incidence of breast cancer in the United States, followed by Afro-Americans, Asians, Hispanics, and American Indian women. This trend held true for all age groups, with the exception of those under 40, where Afro-Americans had the highest incidence rate.20,21 Furthermore, O.Kaidar-Person et al. in a population-based cohort study(2022) assessed the number of women who are BC survival at the time of childbirth admission per 100,000 births over the study period per ethnic groups (Fig. 2) and discovered that the proportion of BC Surs who were of Hispanic origin was lower than that of the other ethnic groups [OR 0.5, 95% CI 0.39–0.65, p < 0.001].22 Nevertheless, according to Breast Cancer Statistics (2022) the highest incidence rates of hormone receptor (HR)-positive/human epidermal growth factor-2 (HER-2)-negative breast cancers are reported to be among white American women (141 cases per 100,000), followed by American Indian/Alaska Native (AIAN) and Black women (112/100,000), adjusted for the age group of 20–49 years.23 In a case–control study Helena C.B. Jernström, Oskar T. Johannsson, Niklas Loman, Ake Borg, and Håkan Olsson,24 found that parous BReast CAncer gene1(BRCA1)/ BReast CAncer gene2(BRCA2) carriers have a higher chance of developing a BC by the age of 40 than nulliparous carriers, with the risk increasing with the number of pregnancies. Additionally, the chance of avoiding breast cancer increases by 10% with each successive pregnancy.25,26 In a pooled analysis of 20 studies, Clavel-Chapelon and M Gerber27 evaluated the relationship between parity and the risk of premenopausal breast cancer. They estimated that each term pregnancy is linked to a 3% lower risk of premenopausal breast cancer. Additionally, this protective impact will last much longer into postmenopausal life, with an estimated 12% lower risk for every full-term pregnancy.27 The chance of developing PABC is related to the age at menarche as well. According to Kim et al. (2017) research, there is a statistically significant link between the occurrence of menarche at a young age (≤13 years) and the risk of PABC.28 The same research also revealed that women with Body Mass Index (BMI) ≥23 kg/m2 and those who experience their first pregnancy at an older age (≥30 years) are more likely than controls to develop PABC.29,30 In addition to having been proven in numerous studies to have a substantial preventive impact against basal-like tumours, breastfeeding is known to have a protective effect against breast cancer.31,32
A pie chart shows the distribution of cases and deaths for the top 10 most common female cancers in 2020 (Source: GLOBOCAN 2020)1.
Shows trends in the number of women who are BCSur at time of childbirth admission per 100,000 deliveries over the study years per ethnic group. (Adapted from O. Kaidar-Person et al.2022.22).
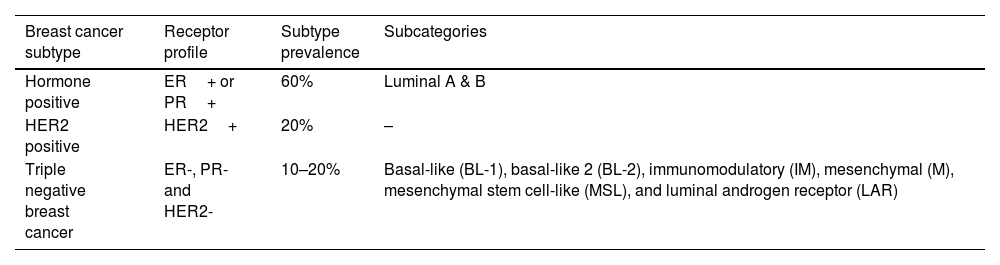
The evaluation of physical breast examination findings in association with pregnancy or lactation, presents a significant diagnostic challenge because the physiological changes in the breasts brought on by pregnancy hormones will result in an increase in the size, density, firmness, and nodularity of the breasts.20 It has been discovered that patients with PABC experience a diagnóstico delay of 5 to 10 months as opposed to 1 to 4 months in non-pregnant patients.33 Additionally, unusual presentations might happen; in one case study published by Shunya Sugai, Eiko Sakata, Takumi Kurabayashi,34 an advanced PABC manifested for the first time with low-back pain caused by multiple low spinal compression fractures.34 For the evaluation of a breast mass during pregnancy, breast ultrasonography is the preferred imaging method because it is non-invasive, poses no radiation risk to the mother or foetus, and is effective at differentiating between benign and malignant breast lesions16,20,35 with a reported sensitivity of up to 100% to detect breast cancer.16 The main benefits of mammography in the trustworthy diagnostic work-up of PABC are to analyse the contralateral breast and to detect any atypical calcifications as well as to determine the degree of the disease, which is necessary for the treatment plan.16,20 However, compared to ultrasonography, it has a lesser sensitivity of 78 to 86% to identify breast cancer.16 Notably, the mammography-associated radiation dose to the foetus with abdominal shielding is estimated to be 0.001–0.01 milligray (mGy) (when taken into account over two views), which is significantly lower than the 200 mGy minimum threshold for unfavourable foetal effects (typically involving the foetal central nervous system during organogenesis,up to 10 weeks of gestation). Additionally, using appropriate shielding is linked to a 50% reduction in radiation risk. The gold standard test for the diagnosis of a breast lesion is a histopathological assessment, with a high sensitivity of 98%, core needle biopsy or excisional biopsy are the first-choice techniques used. Even yet, Fine Needle Aspiration Cytology (FNAC) has a reduced sensitivity and is unable to offer data on cellular markers. However, it has the benefits of limited tissue invasion, speed, and low expense. In addition, FNAC can evaluate the axillary lymph nodes, which is a diagnostic required to plan the subsequent primary management, which may involve chemotherapy or surgery.24 Based on both molecular and histological data, BC might be divided into three groups: BC expressing human epidermal receptor 2 (HER2+), BC expressing hormone receptors (PR+ or ER+), and Triple Negative Breast Cancer (TNBC) (ER-,PR-,HER2-).36,37 Additionally, the TNBC is categorised into six categories: basal-like1(BL-1), basal-like-2(BL-2), immunomodulatory(IM), mesenchymal(M), mesenchymal stem cell-like(MSL), and luminal androgen receptor (LAR) (Table 1)37. The treatment strategies should be based on the BC molecular characteristics.38 Due to the radiation risks to the embryo, a comprehensive staging of the tumour with imaging radiation is not advised during pregnancy (organ malformation and mental retardation in childhood) thus, the multidisciplinary team should decide on an individual basis how to stage the tumour however, liver ultrasonography and chest x-rays can be done safely during pregnancy.24 Due to the use of the teratogenic chemical gadolinium contrast, MRI is not advised as a diagnostic method for breast cancer during pregnancy. However, the doses of contrast excreted in breast milk are negligible, and even the risks related to direct toxicity or allergy are very low. This makes the use of Magnetic Resonance Imaging (MRI) with contrast safe while breast-feeding. However, it is advised against breastfeeding for 12 to 24 h after gadolinium administration, as in the concern of the mother.33,39
Breast cancer subtypes category.
| Breast cancer subtype | Receptor profile | Subtype prevalence | Subcategories |
|---|---|---|---|
| Hormone positive | ER+ or PR+ | 60% | Luminal A & B |
| HER2 positive | HER2+ | 20% | – |
| Triple negative breast cancer | ER-, PR- and HER2- | 10–20% | Basal-like (BL-1), basal-like 2 (BL-2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem cell-like (MSL), and luminal androgen receptor (LAR) |
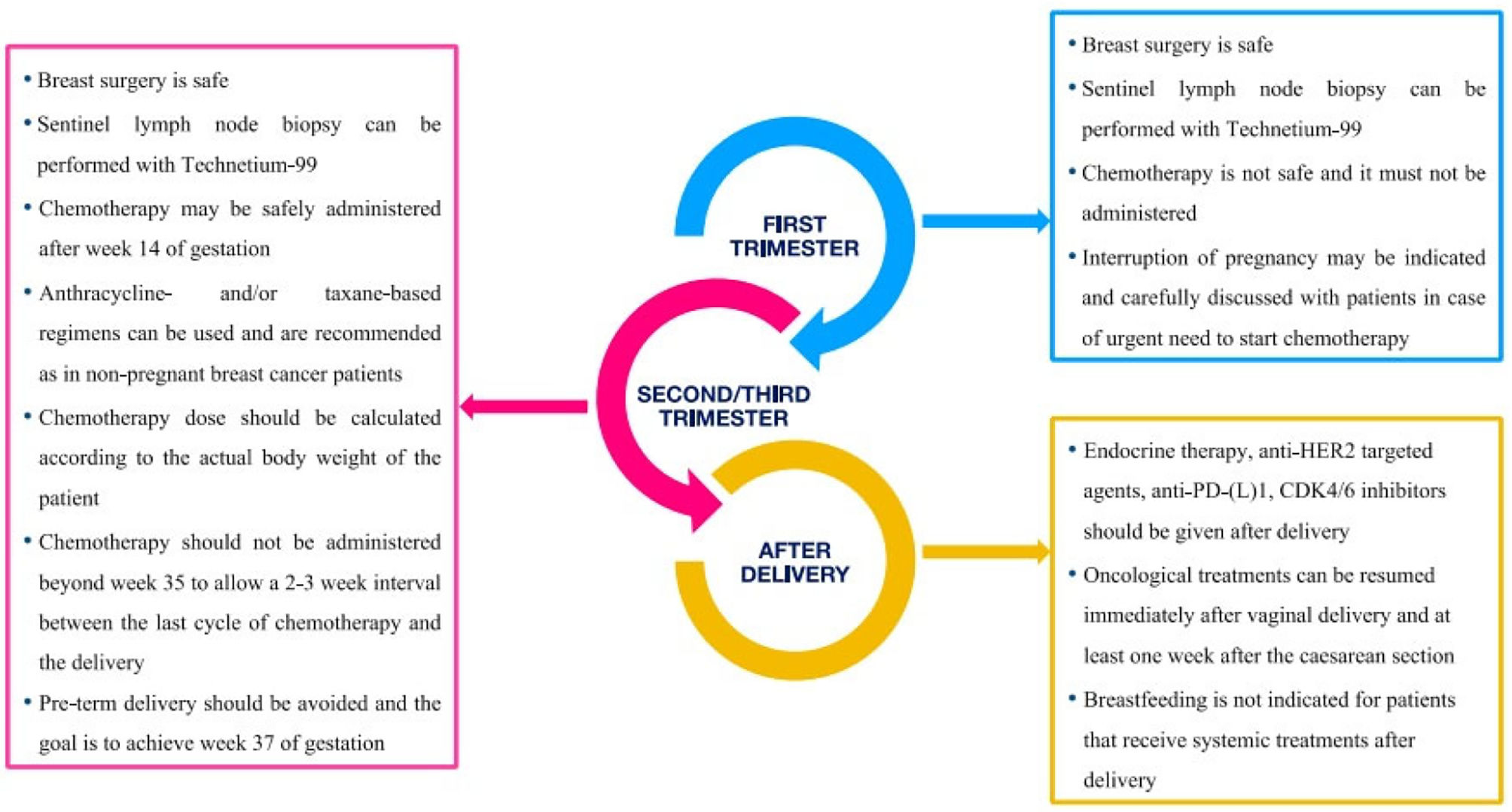
A multidisciplinary team composed of a general obstetrician, a specialist in fetal-maternal medicine, a medical oncologist, a gynaecological oncologist, a pathologist, a radiotherapy specialist, anesthesiologists, medical geneticists, and psychologists should provide PABC treatment. Once a patient has been diagnosed with PABC, tailored treatment should begin as soon as possible until delivery (Tables 2,3 & Fig. 2). When indicated, surgery for PABC is considered the first-line treatment and can be done through out the entire course of pregnancy. In this context, radical surgery is considered the first-choice treatment and preferred to breast conserving surgery, which necessitates additional radiotherapy. The decision for surgical choice will be influenced by the gestational age of the foetus, where lumpectomy with post-operative radiotherapy will come inferior to radical surgery during the first and second trimesters of pregnancy. However, this breast preserving surgery would be a viable and appropriate surgical option by the time the foetus reaches the third trimester of pregnancy.40 For patients with clinically positive nodes, an axillary lymphadenectomy is advised; however, individuals with nodes-negative disease may benefit from sentinel lymph node biopsy (SLNB), which uses a low-dose exposure technique,considered safe to pregnancy and estimated by most researchers to be as little as 1.1 μ Gy. In order to further decrease the amount of time and day that people are exposed to radiation, the radiotracer should be injected on the same day as surgery. It should be noted that facilities and the Caesarean delivery team should be prepared to deliver the patient if necessity arises. Breast reconstructive surgery should be postponed until after puerperium in order to reduce the operating time with the associated increased blood loss and anaesthetic hazards and, secondly, to allow healing and the restoration of the breast tissues to their pre-pregnancy state hence,improve cosmesis.16,20 It should be noted that all approaches for delayed breast reconstruction are feasible and won't interfere with a future pregnancy, including the Transverse Rectus Abdominis Myocutaneous (TRAM) flap method, which involves harvesting rectus abdominis muscle.41,42 Systemic chemotherapy is contraindicated in the first trimester of pregnancy due to worries about foetal teratogenicity during the period of organogenesis (approximately between 3–12 weeks of gestation). Systemic chemotherapy is indicated either as a neoadjuvant treatment in locally advanced cases of PABC or as a post-operative adjuvant treatment where it should be started within 3 weeks post-operatively. However, chemotherapy is thought to be safe during the second and third trimesters of pregnancy, with the exception of the final three to five weeks prior to delivery, when it is advised to delay chemotherapy administration to allow recovery from the mother's bone marrow depression while reducing the likelihood of haemorrhage and sepsis risks for both the mother and the baby. Notably, during the first trimester, the risk of foetal malformations associated with the use of chemotherapy is estimated to be up to 14% (and even higher with combination regimens), while during the second and third trimesters, the risk is estimated to be about 3%, which is comparable to that of the general population. However, chemotherapy should be administered in cycles with 3 weeks intervals.20 The most widely used and still the first-choice chemotherapeutic drugs during pregnancy are anthracycline-based, with no evidence that their usage increases the risk of foetal cardiotoxicity or has adverse effects on short- or long-term survival, teratogenicity, or neurocognitive impairment.43 When anthracyclines are contraindicated or when treating metastatic disease, taxanes represent the second-choice chemotherapeutic drugs. Adverse foetal effects of chemotherapy have been linked to preterm delivery and intra uterine growth restriction, with incidence rates ranging from 5% to 8%.24 Pregnancy is a contraindication for radiotherapy, so patients who chose breast conserving surgery or whose PABC requires post-mastectomy radiotherapy should postpone their radiation therapy until after giving birth to prevent foetal exposure to the risks of irradiation. Notably, a timely treatment strategy is crucial since adjuvant radiation needs to start between 8 and 12 weeks after surgery to benefit from the disease-free survival period and lower the chance of local recurrence.20 Tamoxifen and luteinizing hormone-releasing hormone analogues, which are frequently used in non-PABC, are contraindicated during pregnancy due to the possibility of an association with birth defects in up to 20% of exposures (primarily cranio-facial defects and genital deformities). Tamoxifen should also not be used during lactation due to the lack of information regarding its excretion into breast milk.16,24 The related negative foetal effects make biological medicines like Trastuzumab which interfers with amniotic fluid production leading to oligo- or anhydramnios and Bevacizumab which may endanger the embryonic development, inappropriate for PABC treatments and their administration should be postponed until after delivery.24 Supportive care during pregnancy is necessary to help patients tolerate side effects of the treatment, especially the chemotherapeutic agents. Metoclopramide is the first choice antiemetic and ondansetron can be used safely during pregnancy. Granulocyte-Colony Stimulating Factor (G-CSF) is recommended with chemotherapy to reduce the potential maternal and foetal effects secondary to chemotherapy-induced neutropenia, Erythropoietin can be used safely during pregnancy, but bisphosphonates are advised to be used as soon as possible after delivery. Antibiotics like penicillins, cephalosporins, carbapenems, and the majority of macrolides are classed as category C drugs and are therefore permitted to be used.16,44
Summary of current recommendations for the treatment of pregnancy-associated breast cancer.
| Treatment | Recommendation |
|---|---|
| Surgery | |
| Breast | Mastectomy is the definitive choice but there is a trend towards breast-conserving surgery. |
| Axilla | Sentinel lymph node sampling as a choice is not discouraged, as in the non-pregnant state.Avoid blue dye. |
| Reconstruction | After delivery. |
| Chemotherapy | Anthracycline-based regimen is the most common with taxane as an alternative.Dose-dense therapy can be considered.Avoid chemotherapy 3 weeks before delivery or after 35th week. |
| Hormonal therapy | Contra-indicated during pregnancy and breastfeeding. |
| Monoclonal antibodies | Contra-indicated due to adverse fetal outcome.Also contra-indicated during breastfeeding. |
Key points and contraindications for breast cancer treatment during pregnancy.
Key points
|
Contraindications
|
Abbreviations: HER2 human epidermal growth factor receptor 2; MRI, magnetic resonance imaging.
As a high-risk pregnancy, pregnancies complicated by breast cancer should be followed up on by an obstetrician or, preferable, a foetal maternal medicine specialist as part of the multidisciplinary management team. Ultrasonography should be used to regularly assess the foetal well-being every three to four weeks or prior to any treatment interventions, The following additional foetal surveillance techniques may be useful: foetal growth charts to detect any foetal growth restriction, umbilical artery Doppler to evaluate placental blood flow, Doppler of the foetal middle cerebral artery to rule out foetal anaemia, cardiotocography (when gestational age is 24 weeks) with the use of foetal cardiotoxic drugs like anthracyclines, and amniotic fluid volume assessment, which can be affected reversibly by some medications. The goal is to deliver the baby at 37 weeks, unless an indication(obstetric or oncologic) arises; however, patients receiving chemotherapy should have completed their last round at least 3 weeks before considering delivery. The majority of births are vaginal unless there is another obstetric reason for Caesarean delivery.44
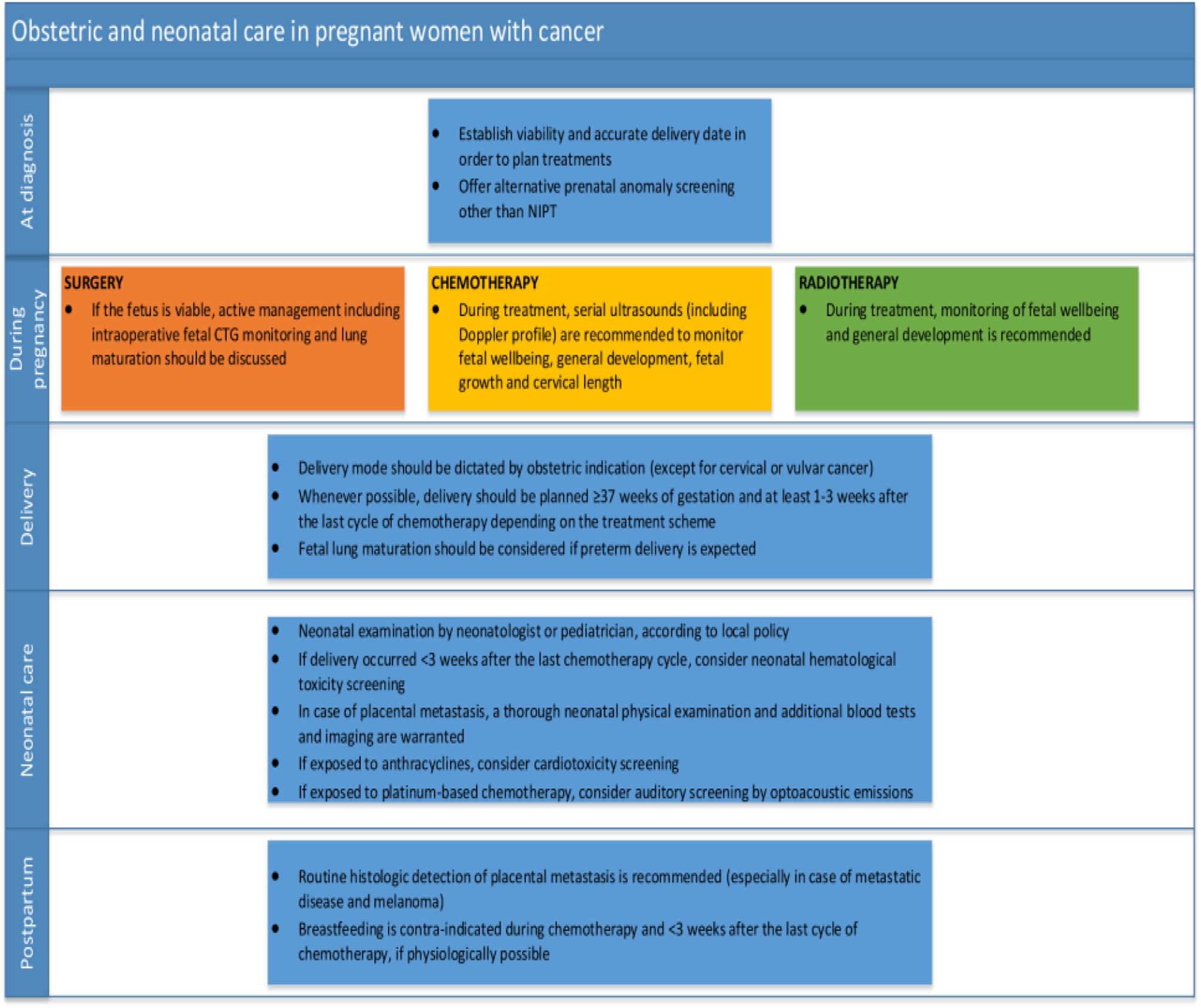
Obstetric outcomes in patients with PABCAn analysis of the obstetric outcomes of pregnant women treated for primary breast cancer at this tertiary centre was conducted in a retrospective German study that took place at a university centre for women's health between 2000 and 2009, with results being published in 2018. The study focused on obstetric outcomes in patients with PABC. The study discovered that there were no small-for-gestational-age newborns, but there was a high rate of caesarean deliveries (55%) among PABC patients, a low gestational age at delivery (median gestational age was 34.5 weeks), and relatively low birth weight neonates (median birth weight 2945 grammes) born to some of the PABC patients (11%). More than half of the newborns (53%) were delivered preterm, and some neonates' Apgar scores indicated foetal distress, especially for measurements taken at 1 and 5 min, according to the same study's analysis of neonatal problems.46Wolters V et al.(2021) summarised the obstetric and neonatal care in pregnant women with cancer at different stages of management (Fig. 3)47 (See Fig. 4).
Summary of management's recommendations according to pregnancy trimesters and postpartum period.
(Adapted from Poggio et al. 2020.45)
Obstetric and neonatal care in pregnant women with cancer.
Abbreviations; CTG, cardiotocography; NIPT, non-invasive prenatal testing. (Adapted from Wolters V et al. 202147).
One of the cancers most frequently related with pregnancy is breast cancer. As the condition poses a complex challenge in terms of diagnóstico, therapy, and prenatal monitoring, a multidisciplinary care strategy is advised. Surgery is considered the first-choice treatment when indicated and can be performed during all trimesters of pregnancy,with the radical mastectomy being superior and preferred over the local excision, which requires post-operative irradiation and is not advised during pregnancy due to the potential risks of foetal malformations, axillary lymphadenectomy is indicated for clinically nodes positive disease, while sentinel lymph node biopsies are used for nodes negative disease. While first trimester administration of systemic chemotherapy is contraindicated due to the possibility of foetal abnormalities, it is safe to provide it during the second and third trimesters of pregnancy. Due to the negative foetal consequences, radiotherapy, hormonal therapy, and biological treatments are only administered after delivery. Regular ultrasound scans, foetal growth charts, amniotic fluid indices, and foetal Doppler tests should all be used to monitor the foetus. Vaginal delivery is the norm, and caesarean delivery is only used when there are other obstetric indications. Delivery is intended to occur at 37 weeks, unless a reason for an earlier delivery develops.
FundingThis work has not received any funding.
Ethical considerationsNot applicable as the work does not involve the use of human subjects.