Quantitative analysis of tumor-infiltrating lymphocytes (TILs) is currently considered as a prognostic factor in several malignant tumors. The aim of our study was to assess the prognostic value of TILs in breast cancers and its association with other clinicopathological prognostic factors in non-specific type (NST) breast carcinoma in Tunisian population.
MethodsRetrospective study included 53 women with NST breast carcinoma. The data were collected over a period of 13 months with a follow-up of 40 months for all the patients. The TILs were evaluated according to the 2014 recommendations of the international working group on TILs.
ResultsTILs level was between 3% and 60% with mean of 21%. Ten patients had lymphocyte predominant breast cancer (LPBC). Statistical analysis had shown that the TILs level ≤50% was associated with the presence of vascular emboli and the absence of HER2 amplification. Inflammatory-type carcinoma and HER2 amplification significantly worsened OS. Presence of vascular emboli, lymph node metastases, inflammatory type of carcinoma, TILs ≤50%, and absence of estrogen receptors (ER) were associated with reduced RFS. In multivariate analysis, the presence of vascular emboli was an independent factor for OS. TILs ≤50%, inflammatory type of carcinoma and presence of vascular emboli were independent risk factors for RFS.
ConclusionThis Tunisian pilot study showed higher level of TILs in NST breast carcinomas is associated with improved RFS. The therapeutic implications will benefit from multiple research studies including ours on the predictive value of TILs for neoadjuvant or adjuvant treatment.
El análisis cuantitativo de los linfocitos infiltrantes de tumor (TIL) se considera actualmente como un factor pronóstico en varios tumores malignos. El objetivo de nuestro estudio fue evaluar el valor pronóstico de los TIL en los cánceres de mama y su asociación con otros factores pronósticos clinicopatológicos en el carcinoma de mama de tipo no específico (NST) en la población tunecina.
MétodosEn un estudio retrospectivo se incluyó a 53 mujeres con carcinoma de mama NST. Los datos se recogieron durante un período de 13 meses, con un seguimiento de 40 meses para todas las pacientes. Los TIL se evaluaron según las recomendaciones de 2014 del grupo de trabajo internacional sobre TIL.
ResultadosEl nivel de TIL estuvo entre el 3% y el 60% con una media del 21%. Tenían cáncer de mama con predominio de linfocitos (LPBC) 10 pacientes. El análisis estadístico mostró que el nivel de TIL ≤ 50% se asociaba a la presencia de émbolos vasculares y a la ausencia de amplificación de HER2. El carcinoma de tipo inflamatorio y la amplificación de HER2 empeoraron significativamente la SG. La presencia de émbolos vasculares, las metástasis en los ganglios linfáticos, el carcinoma de tipo inflamatorio, los TIL ≤ 50% y la ausencia de receptores de estrógeno se asociaron a una menor SSR. En el análisis multivariante, la presencia de émbolos vasculares fue un factor independiente para la SG. Los TIL ≤ 50%, el tipo de carcinoma inflamatorio y la presencia de émbolos vasculares fueron factores de riesgo independientes para la RFS.
ConclusiónEste estudio piloto tunecino mostró que un mayor nivel de TIL en los carcinomas de mama NST se asocia a una mejor RFS. Las implicaciones terapéuticas se beneficiarán de múltiples estudios de investigación, incluido el nuestro, sobre el valor predictivo de los TIL para el tratamiento neoadyuvante o adyuvante.
During the last couple of decades, the role of the immune system in the development, progression, and prognosis of some cancers has been well established. With the rise of immunotherapies, study of the immune infiltrate in cancers is quickly becoming a new challenge for pathologists.1 For breast cancer, evaluating the density of the inflammatory intratumoral infiltrate, by the quantification of tumor-infiltrating lymphocytes (TILs), has been the subject of several studies, some of which aimed at integrating this marker in current practice both for diagnosis and prognosis. These studies were carried out following the early publication of the international recommendations, offering the possibility of quantifying TILs in a standardized and reproducible method.2,3
The objective of this study was to assess the prognostic value of TILs in breast cancers through survival analysis and to study the association between the TILs density and other clinicopathological prognostic factors in breast cancer.
Materials and methodsClinicoepidemiological studyIt was retrospective, descriptive, and prognostic study, involving women with invasive carcinomas of no special type (NST), either biopsied in the Radiology Department, or operated on in the Obstetrics and Gynecology Department at Mongi Slim University Hospital. Pathological analysis was performed in the pathological laboratory at the same hospital. Fifty-three patients were included over a 13-month period, from December 2013 to March 2015, with a follow-up period of 40 months for all the patients. Collection of the clinical, biological, and radiological data was based on the patients’ records. The archived microscopic slides of 26 breast fine needle biopsies and 27 surgical specimens (16 lumpectomies and 11 mastectomies with axillary dissection) were reviewed.
Pathological studyThe tumor size was calculated on imaging for patients having only breast micro-biopsy and on surgical specimens for those who underwent breast surgery. Histological examination was based on reviewing the Hematoxylin–Eosin (HE) stained biopsy slides and those of the surgical excision specimen by one pathologist (DB). The main objective of the review was to quantify the TILs in tumor stroma. For this quantification, the 2014 recommendations of the International TILs Working Group were used.2 The TIL score in core needle biopsy specimens is a reliable value that reflects the TIL status of breast cancer in resected specimens.4
For the patients’ tumor stage, the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging was used.2 The correlation between the TILs level and the other prognostic factors was calculated according to a TILs threshold value of 50%. Lymphocyte-predominant breast cancer (LPBC) was defined as tumors having high TIL levels (≥50%). For patients having a breast micro-biopsy without surgery, the lymph node extension was assessed on imaging. However, for those having a surgical resection, the size and the lymph node extension were evaluated on macroscopic and histological examinations, respectively.
So far, no recommendations are available to validate the quantification of TILs on tissue samples after chemotherapy (CT). For this reason, specimens that were subjected to CT treatment were not included in our series.
Immunohistochemistry and in situ hybridizationThe immunohistochemistry (IHC) study was performed in all patients using antibodies directed against estrogen receptors (ER) (Leica® PA0151), and progesterone receptors (PR) (Leica® PA0312) and HER2 (Leica® lot 6033877).
The HER2 profile at IHC and Fluorescence In Situ Hybridization (FISH) was evaluated according to the recommendations of CAP/ASCO2013 and GEFPICS 2014.5,6
Statistical analysisData were entered and analyzed using Statistical Package for the Social Sciences (SPSS)® version 25. Proportions were presented with a 95% confidence interval.
In all statistical tests, the significance level was set to 0.05.
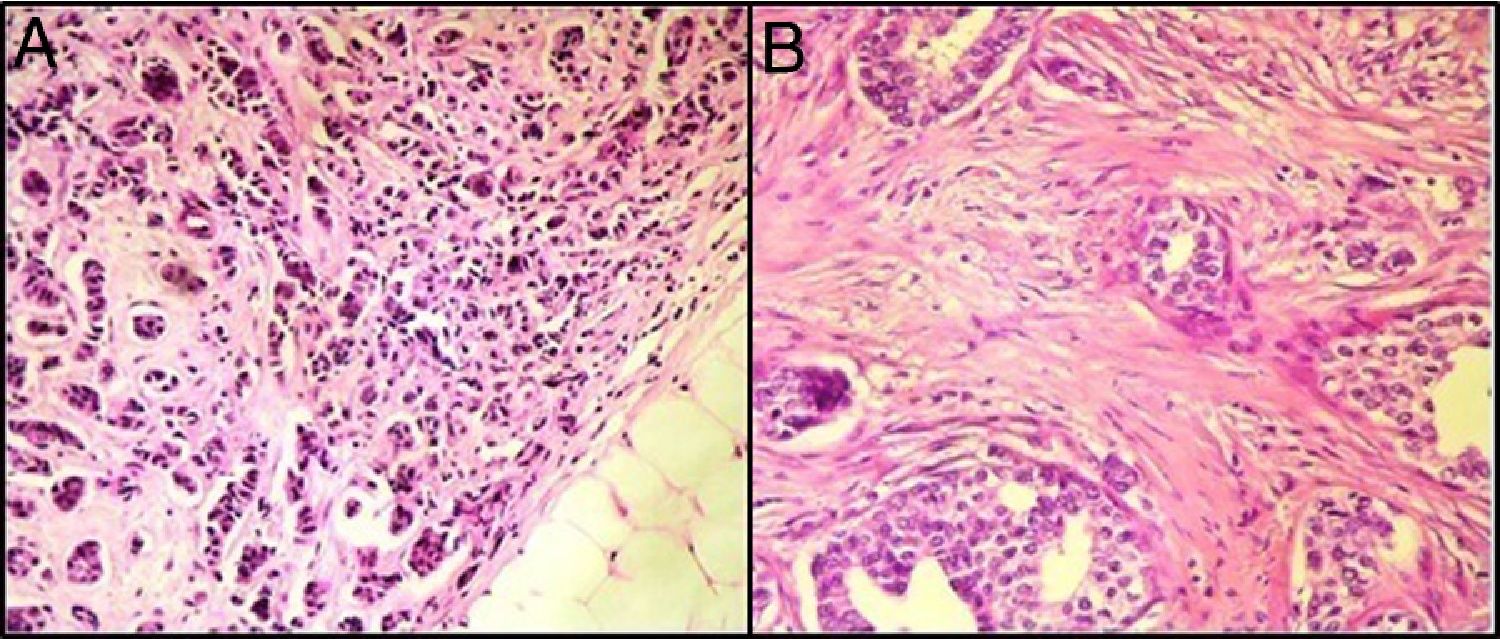
ResultsBreast TILs analysisOn histopathological examination, 19 patients (36%) had a TILs level<10% (Fig. 1), fifteen patients (28%) had a TILs level between 10% and 29%, nine patients (17%) had a TILs level between 30% and 50%, and 10 patients (18%) had a TIL level>50%.
Correlation between the TILs level and the other prognostic factorsIn our series, a level of TILs ≤50% was correlated with the presence of vascular emboli and the presence of HER2 amplification.
However, this level was not correlated with the age <40 years, tumor size> 2cm, inflammatory breast carcinoma, high number of mitoses, poorly-differentiated character of the tumor, presence of marked atypia, ER-negative and PR-negative profile, high-grade carcinoma, and lymph node and distant metastases (Table 1).
Distribution of patients according to the prognostic parameters and the TILs level.
| Parameters | TILs≤50% | TILs>50% | p |
|---|---|---|---|
| Age <40 years | 4 | 0 | 0.67 |
| Inflammatory breast carcinoma | 3 | 1 | 0.83 |
| Tumor size >2cm | 22 | 7 | 0.16 |
| SBR Grade | 29 | 4 | 0.27 |
| Carcinomatous emboli | 15 | 0 | 0.04* |
| Lymph node metastases+ | 19 | 4 | 0.94 |
| Distant metastases+ | 5 | 0 | 0.18 |
| ER-negative | 16 | 5 | 0.71 |
| PR-negative | 31 | 6 | 0.71 |
| HER2-amplified | 20 | 9 | 0.03* |
The follow-up period was 40 months for all the patients. Relapse free survival (RFS) was measured starting from the date of primary treatment (surgery or chemotherapy) for breast cancer to the date of recurrence or death. The mean overall survival (OS) and RFS were 34.3±1.4 months and 32.4±1.7 months, respectively. The average of RFS levels were 73%. Local recurrence was noted in three patients (5%) after an average of 17 months, and recurrence in the controlateral breast was noted in one patient. Two patients received revision surgery with adjuvant CT and two patients had CT alone.
One patient developed a distant bone metastasis during the follow-up period (after a follow-up of 28 months) and received palliative care.
Univariate analysisIn our series, univariate analysis showed that HER2 amplification and inflammatory breast carcinoma were significantly associated with decreased OS. As for the TILs level≤50% and other factors of poor prognosis (age <40 years, tumor size>2cm, presence of marked atypia, number of high mitoses, high-grade tumors, positive lymph node (N+), ER-negative and PR-negative profile), they tended to decrease OS, but without significant differences (Table 2).
Univariate analysis of OS and RFS in the 53 patients.
| Parameters | OS | RFS | ||
|---|---|---|---|---|
| Mean±SD (months) | p | Mean±SD (months) | p | |
| Age (years) | ||||
| ≤40 | 34.2±1.5 | 0.92 | 26.5±6.7 | 0.28 |
| >40 | 35.5±4.5 | 32.9±1.7 | ||
| Inflammatory breast carcinoma | ||||
| Yes | 23.5±4.5 | 0.01* | 33.5±1.7 | 0.03* |
| No | 35.6±1.4 | 14.4±2.1 | ||
| Tumor size (cm) | ||||
| ≤2 | 36±1.7 | 0.26 | 33.7±2.5 | 0.88 |
| >2 | 32.1±2.3 | 32.1±2.4 | ||
| SBR Grade | ||||
| Grade I and II | 36±1.5 | 0.11 | 33.2±1.8 | 0.41 |
| Grade III | 30.7±3.5 | 29.3±4.3 | ||
| Vascular emboli | ||||
| Present | 34.2±3.2 | 0.91 | 26±4 | 0.03* |
| Absent | 35.6±1.6 | 34.2±1.8 | ||
| Lymph nodes metastases | ||||
| Present | 30.7±2.4 | 0.51 | 26.2±3 | 0.01* |
| Absent | 35.5±1.6 | 37±1.6 | ||
| Distant metastases | ||||
| Present | 16.6±5.6 | 0.04* | 14.3±2.8 | 0.52 |
| Absent | 36.1±1.2 | 27.3±1.4 | ||
| ER status | ||||
| Positive | 33.6±2 | 0.607 | 36.8±1.7 | 0.02* |
| Negative | 35±2 | 28.2±2.7 | ||
| PR status | ||||
| Positive | 35±2.3 | 0.89 | 33.8±3 | 0.57 |
| Negative | 34±1.7 | 31.8±2.1 | ||
| HER2 status | ||||
| Amplified | 31.5±2.2 | 0.03* | 30.2±2.7 | 0.24 |
| Not amplified | 37.5±1.4 | 34.9±2 | ||
| TILs 50% | ||||
| ≤ 50% | 33.1 | 0.06 | 30.71±2 | 0.05* |
| >50% | 40 | 40 | ||
The presence of carcinomatous emboli was an independent factor for OS. Inflammatory carcinoma, presence of vascular emboli, and a TILs level≤50% were independent risk factors for tumor recurrence (Table 3).
Multivariate analysis of OS and RFS in the 53 patients.
| Parameters | p | OR | 95% CI |
|---|---|---|---|
| Multivariate analysis of OS in the 53 patients | |||
| Tumor emboli | 0.03* | 1.26 | 1.4–7.8 |
| Poorly-differentiation | 0.10 | 6.31 | 0.6–59.6 |
| ER-negative | 0.41 | 2.29 | 0.13–16.95 |
| PR-negative | 0.074 | 5.58 | 0.83–40.79 |
| Multivariate analysis of RFS in the 53 patients | |||
| Inflammatory breast carcinoma | 0.029* | 9.62 | 1.36–28.8 |
| Tumor emboli | 0.045* | 5.08 | 1.14–14.85 |
| TILs≤ 50% | 0.001* | 1.97 | 1.22–7.4 |
The prognostic value of TILs in breast cancer has been the subject of several studies, having variable results. This would be mainly related to the low reproducibility of the TILs evaluation, as mentioned in the last consensus of Saint Gallen.6 Among these immunological parameters, TILs are considered to be a prognostic factor for several types of cancer, such as colorectal, lung, or ovarian cancer.7,8 For breast cancer, the study of the prognostic value of TILs was particularly anticipated in the early publication of the International TILs Working Group recommendations for the quantification of these lymphocytes.2 The composition of TILs in breast cancer was recently analyzed by two studies showing that TILs are composed of T lymphocytes (75%), B lymphocytes (<20%), monocytes (<10%), as well as NK and NK-T cells (<5%).9
Several studies have shown a good correlation between intratumoral and stromal TILs measurement.2 In our series, we assessed stromal TILs whose quantification proved to be the most reliable and the most reproducible with less variability between pathologists (correlation coefficient 0.74).
Quantification of TILs is considered as a continuous variable and it is expressed as a percentage in the observed area. It is sometimes considered as a binary variable (high versus low) according to studies.10,11 However, it is essential to use a real reliable and valid method for several reasons. The method should be able to compare the results of multiple studies. Thus, quantification of TILS in breast cancer would be practical for implementation in the pathologists’ daily practice and for future clinical trials.10
Several methods have been used for the quantification of TILs in breast cancer and in other cancers. They involve histology, IHC, flow cytometry, and several types of automatons. The histological method follows the recommendations of the International TILs Working Group in breast cancer: Due to its simplicity and subjectivity, this evaluation method has raised questions with regard to the reliability and reproducibility of its results. Several studies have tackled this issue by studying inter-observer variability in the evaluation of TILs. However, the majority of these studies have failed to demonstrate a statistically significant variability between readers.11,12 This homogeneity of the results concerns only stromal TILs. For other types of TILs, the results remain heterogeneous.11,13
The level of TILs is different depending on the subtypes of breast cancer. This level varies from less than 5% in HR-positive tumors to 30% in triple-negative tumors. According to one of the largest published cohorts,14 the percentage of stromal TILs is 10% for ER-positive/HER2-negative tumors, 15% for HER2-positive tumors, and 20% for “triple-negative” tumors. In our series, the level of TILs varied between 3% and 60%, with an average of 21%. It was <30% in the majority of cases (64.15%).
The prognostic value of TILs has been mainly studied in the form of retrospective series based on the calculation of OS and RFS, and in the form of meta-analyses, especially during the last 5 years.15–20 The majority of the series have focused on the study of “triple-negative” and “HER2-positive” breast cancer.15–17 Several concordant studies confer the prognostic value on TILs in “triple-negative” and “HER2-positive” breast cancers, independently of the classic clinical and histological factors.10
Seven meta-analyses, including 96 studies with a total of 53,453 patients with breast cancer, were reviewed.15–21 Two studies analyzed the prognostic value of TILs involving OS and RFS as well as their predictive value for complete histological response to neoadjuvant or adjuvant treatment.16,17 In some studies, TILs were quantified on tissue samples after CT treatment.22 In our series, a TILs level≤50% was an independent risk factor for RFS (p=0.001). This factor also tended to decrease OS, but without significant differences (p=0.06).
Several studies have shown that a high TILs density (between 40% and 60%) is associated with a better prognosis in terms of OS and RFS compared to tumors with low TILs.15,19,21
Concerning the correlation between TILs and other histo-prognostic factors, in our series, a TILs level of ≤50% was found to be correlated with the presence of vascular emboli (p=0.047) and the presence of HER2 amplification (p=0.03). According to Lee, a high TILs level is correlated with the absence of vascular emboli, which is in agreement with our results, but this level is also correlated with the ER−/HER2+ status.22 In the same study, the amount of TILs (OR=2.256; 95% CI: 1.072–4.747; p=0.032) was an independent prognostic factor in the study population, which is consistent with our results. The results of our series were inconsistent with those in the literature concerning the correlation between TILs and the amplification status of HER2. Indeed, our results showed a correlation between TILs >50% and the HER2- status, while most of the series found a correlation between high TILs and “triple-negative” or HER2-amplified tumors.19
Other disparities between the results of our series and those of few other studies were observed,12 particularly the correlation between the TILs levels and other histo-prognostic factors, such as the histological grade and the status of hormone receptors. This could be explained by the variation in the techniques used for quantifying TILs, especially in studies carried out before 2014, the variation in hormone receptor positivity thresholds (1% or 10%), and the material availability (better analysis on surgical resection parts than on breast micro-biopsies).
Few studies have analyzed the implications of the immunophenotypic composition of the TILS, but the results are contradictory and inconclusive. However, IHC staining can be costly and the interpretation time-consuming. There is no international recommendation for TILs immunophenotyping. The global TILs count, obtained on Hematoxylin & Eosin, reflects well the net tumor immunogenicity and represents a robust independent prognostic biomarker in breast cancer.23
It should be noted that most of the studies investigating the correlation between the TILs levels and other clinical and biological prognostic factors were conducted at an early stage of research on this parameter before the advent of the International TILs Group of Work recommendations in 2014. It also worth noting that current data indicates that the prognostic value of TILs is specific to “triple-negative” and HER2- amplified tumors, independently of the other usually considered clinical, histological, and biological factors.
ConclusionsLymphocytes infiltrating tumors or TILs have been the subject of recent researches. According to many studies including this study, the TILs rates could be a prognostic factor for breast cancer, regardless of their sub-molecular types, mainly concerning RFS. Indeed, for tumors with TILs >50%, it would be particularly advantageous, due to a pre-existing immune response, to resort to immunotherapies which reactivate the lymphocytes of the inflammatory infiltrate of which the immune checkpoint inhibitors are currently. In contrast to poor TIL tumors, it would be preferable to use an adoptive cell therapy for the induction of anti-tumor immune response and establish close surveillance to detect early recurrence. The therapeutic implications will benefit from multiple research studies on the predictive value of TILs for neoadjuvant or adjuvant treatment.
Confidentiality of dataThis study was approved by the Institutional Research Ethics Committee of Mongi Slim University Hospital, La Marsa. It was conducted in accordance with the Declaration of Helsinki. All patient data are protected in accordance with the Act Respecting the Protection of Personal Information.
FundingThere was no funding from any organization or institute.
Conflicts of interestThe authors declare that there is no conflict of interest.