Metastasis from Triple-negative breast cancer (TNBC) is a significant cause of morbidity and death and also presents a diagnostic challenge. There are significant therapeutic implications for making a correct and timely diagnosis of metastatic TNBC because the tumors may respond to chemotherapy. PELP1 expression has been reported recently in BC but is poorly studied in the diagnosis of primary and metastasis of TNBC.
ObjectivesThe aim of the present study is to assess the diagnostic utility of PELP1and compare it with GATA3, the most commonly used in our practice for breast cancer.
MethodsPELP1 and GATA3 were assessed by immunohistochemistry in formalin fixed paraffin embedded tissue blocks of 30 cases of primary TNBC and 15 cases of metastatic TNBC at the Pathology department, Zagazig University, Egypt.
ResultsThe immunohistochemical expression of PELP1 revealed a higher frequency of expression than GATA3 in both primary and metastatic TNBC. PELP1 revealed a (96.67%) positive expression rate in primary TNBC and a (86.67%) positive in metastatic TNBC. In comparison to GATA3, revealed (53.33%) positive expression rate in primary TNBC and (60%) positive in metastatic TNBC. Furthermore, the majority of the PELP1 positive cases showed diffuse strong staining, making observation of the staining easy and suggested that PELP1 may be a molecular target for TNBC therapy. We predict that this analysis will shed light on PELP1's significance in TNBC.
ConclusionIn comparison to GATA3, PELP1 protein expression is substantially higher in diagnosis of primary and metastatic TNBC.
La metástasis del cáncer de mama triple negativo (TNBC) es una causa importante de morbilidad y muerte y también presenta un desafío diagnóstico. Existen importantes implicaciones terapéuticas para hacer un diagnóstico correcto y oportuno de TNBC metastásico porque los tumores pueden responder a la quimioterapia. La expresión de PELP1 se ha informado recientemente en BC, pero está poco estudiada en el diagnóstico de metástasis primaria y de TNBC.
ObjetivosEl objetivo del presente estudio es evaluar la utilidad diagnóstica de PELP1 y compararlo con GATA3, el más utilizado en nuestra práctica para el cáncer de mama.
MétodosPELP1 y GATA3 se evaluaron mediante inmunohistoquímica en bloques de tejido embebidos en parafina fijados con formalina de 30 casos de TNBC primario y 15 casos de TNBC metastásico en el departamento de Patología de la Universidad de Zagazig, Egipto.
ResultadosLa expresión inmunohistoquímica de PELP1 reveló una mayor frecuencia de expresión que GATA3 en TNBC tanto primaria como metastásica. PELP1 reveló una tasa de expresión positiva (96,67%) en TNBC primario y un (86,67%) positivo en TNBC metastásico. En comparación con GATA3, reveló (53,33%) tasa de expresión positiva en TNBC primaria y (60%) positiva en TNBC metastásica. Además, la mayoría de los casos positivos de PELP1 mostraron una tinción fuerte difusa, lo que facilitó la observación de la tinción y sugirió que PELP1 puede ser un objetivo molecular para la terapia de TNBC. Predecimos que este análisis arrojará luz sobre la importancia de PELP1 en TNBC.
ConclusiónEn comparación con GATA3, la expresión de la proteína PELP1 es sustancialmente mayor en TNBC primaria y metastásica.
According to data from GLOBOCAN 2020, breast cancer (BC) is the second most often diagnosed cancer in the general population, accounting for around 11.7% of all new cancer cases. It is, in fact, the major cause of cancer-related mortality in women throughout the world.1,2
Triple-negative breast cancer (TNBC) refers to tumors that lack ER and PR expression as determined by immunohistochemistry, as well as HER2 expression as determined by immunohistochemistry and/or in-situ hybridization tests. It accounts for 15%–20% of all breast cancers and has the worst 5-year survival rate of any kind of BC.3 Visceral metastasis to the lung (40%) and brain (30%), rather than lymph nodes, bone, or liver, is more common early in the disease course.4 Because about 20%–30% of TNBC responds to chemotherapy, achieving an accurate and timely diagnosis of metastatic TNBC has significant therapeutic consequences.5 Thus, it is believed that accurate and quick detection of metastatic TNBC, as well as prompt therapy, will enhance patient survival.
Immunohistochemistry (IHC) is a critical aspect of breast pathology diagnosis. IHC can probably prove the origin of primary or metastatic carcinomas in the breast. However, no immunostain is ideal in terms of sensitivity or specificity.6 Proline, glutamic acid and leucine rich protein 1 (PELP1) labeling of TNBC was recently described. However, there are few publications, with just one comparing their performance to GATA3 in primary and metastatic TNBC.7
PELP1 is a new nuclear hormone receptor (NR) co-regulator that has a function in breast cancer progression and metastatic potential.7,8 Co-regulators are multiprotein complexes that act as “master regulators” of NR activity. PELP1 also affects the activities of many NRs, including the ER-related receptor, the glucocorticoid receptor, and the androgen receptor, all of which have recently been discovered to have key roles in the biology of TNBC and non-TNBC.9–11 PELP1 oncogenic signaling is implicated in progression of several cancers not only BC but also endometrial, ovarian, salivary, prostate, lung, pancreas, and colon.10
GATA3 is a zinc finger transcription factor that plays a role in the differentiation of a variety of tissues, including mammary luminal epithelial cells.12 A more recent review demonstrated that GATA3 is more sensitive for TNBC, with labeling commonly reported in over 50–83%.13
In this study, we investigated the PELP1 expression by immunohistochemistry in primary and metastatic TNBC in human tissues and compared its expression with GATA3, a novel sensitive diagnostic marker for TNBC, to explore its potential diagnostic utility in metastatic TNBC.
Material and methodsPatients and tissue specimensThis is a retrospective, cross-sectional study. A total of 30 cases of selected females with primary TNBC, 10 lymph nodes with metastasis (related to select 10 cases of TNBC) and 5 cases of distant metastasis of TNBC were collected in the period from February 2019 to December 2020 at the Pathology department, Faculty of Medicine, Zagazig University, Egypt. These specimens were collected after taking an approval by the local ethical committee Institutional review board (IRB). Tru-cut biopsy, modified radical mastectomy, or cell block were used to collect the samples.
The clinicopathological and histopathological data, including, patient age, tumor size, lymph nodes involvement, ER, PR, and Her2 status, and ki67 index, were retrieved from pathology reports available with the tissue specimens. For ER and PR expression, moderate to strong nuclear staining in ≥1% of tumor cells were considered positive. HER2 was positive if complete intense, circumferential membranous staining within >10% of tumor cells were found (According to ASCO/CAP HER2Testing Guideline Update, 2018).14 The tumors were graded (modified Bloom and Richardson) and TNM staged (8th d AJCC).15
Immunohistochemistry procedureImmunohistochemical staining (IHC) is carried out using the polymer Envision detection system; the Dako EnVision™ kit (Dako, Copenhagen, Denmark). 3–5 μm tissue sections were deparaffinized in xylene and rehydrated in graded alcohol. Slides were incubated for 10 min in 3% hydrogen peroxide to block endogenous peroxidase. Dako target antigen retrieval solution (pH 6.0) was applied for 20 min. Then the slides were incubated for 60 min with a GATA3: a mouse monoclonal antibody (dilution 1:100, clone L50-823, Biocare Medical, USA); PELP1 polyclonal antibody (1:100 dilutions, clone A13414, ABclonal). The reaction was visualized by incubating the sections with diaminobenzidine for 15 min after that Mayer's hematoxylin was used. The study complied with the local ethics committee guidelines at pathology department, Zagazig university hospitals.
Interpretation of immunostainingThe immunoreactivity of nuclear PLEP1 and GATA3 was assessed separately and blindly. Percentage of tumor cells were scored based on extent of nuclear staining as a cutoff 5% is used to define PELP19 and GATA316 positivity for diagnosis TNBC cases.
ResultsThe clinicopathological and histopathological data of the studied casesThe clinicopathological data of the cases enrolled in this study were summarized in Table 1. The age of patients of the primary TNBC cases (n = 30) at the time of initial diagnosis ranged from 27 to 75 years. The mean and median ages were 48.3 ± 13.31 years and 46.5 years respectively. The majority of cases were grade 3 (60%), stage III (83.33). All clinical information was gathered from the patient's medical records.
clinicopathological and histopathological parameters of primary TNBC (N = 30)
| Variable | (N = 30) | ||
|---|---|---|---|
| Age (year) | Mean ± SD | 48.3 ± 13.31 | |
| Median (Range) | 46.5(27–75) | ||
| Variable | No. | % | |
| Pathological type | IDC NST | 27 | 90 |
| lobular | 1 | 3.33 | |
| special | 2 | 6.67 | |
| Grade | Grade II | 12 | 40 |
| Grade III | 18 | 60 | |
| Tumor size (mm) | Mean ± SD | 46.8 ± 14.46 | |
| Median (Range) | 42.5 (15–70) | ||
| Lymph node | Negative | 3 | 10 |
| Positive | 27 | 90 | |
| Stage | Stage I | 1 | 3.33 |
| Stage II | 4 | 13.33 | |
| Stage III | 25 | 83.33 | |
Continuous variables were expressed as mean ± SD & median (range), Categorical variables were expressed as number (percentage).
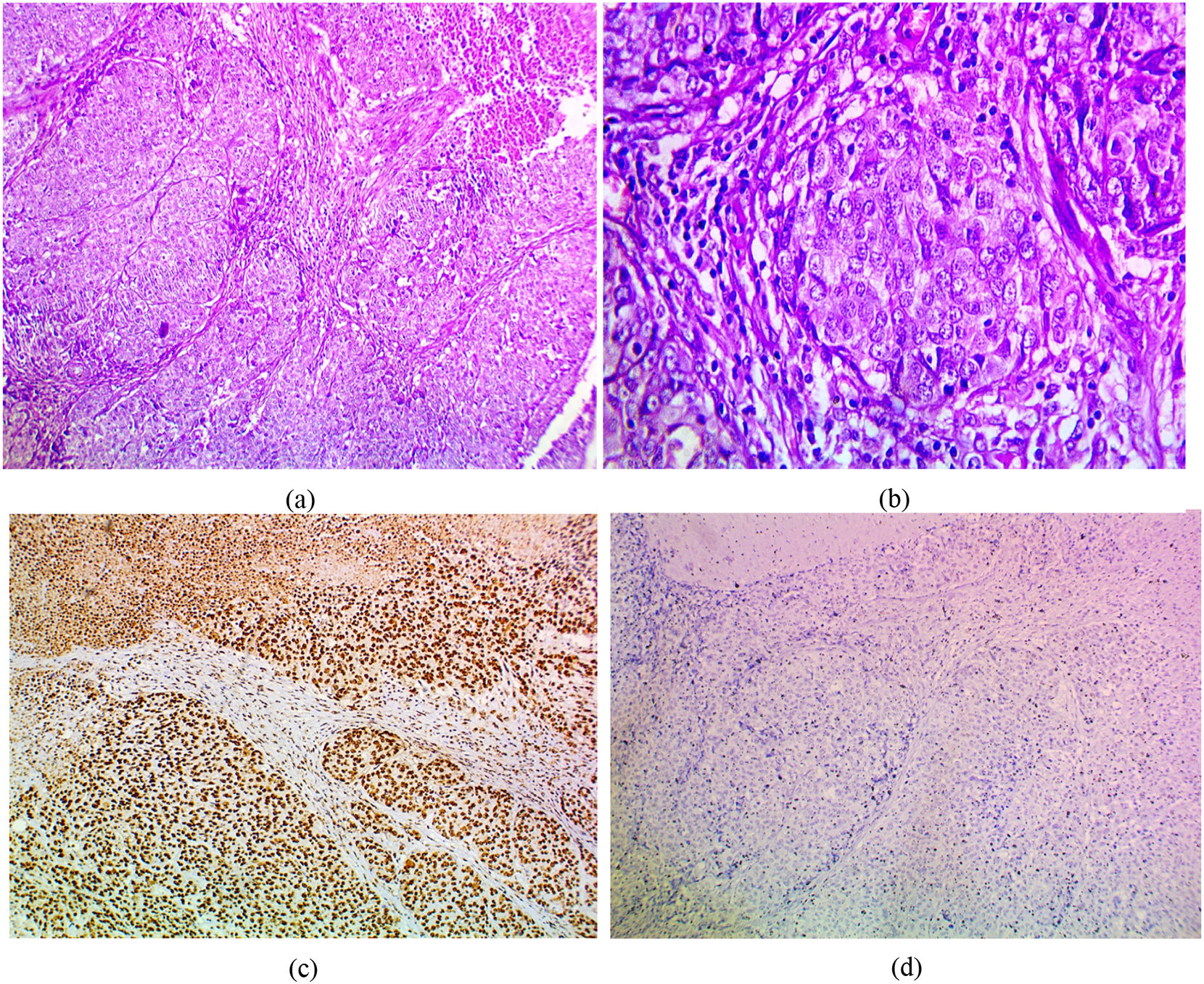
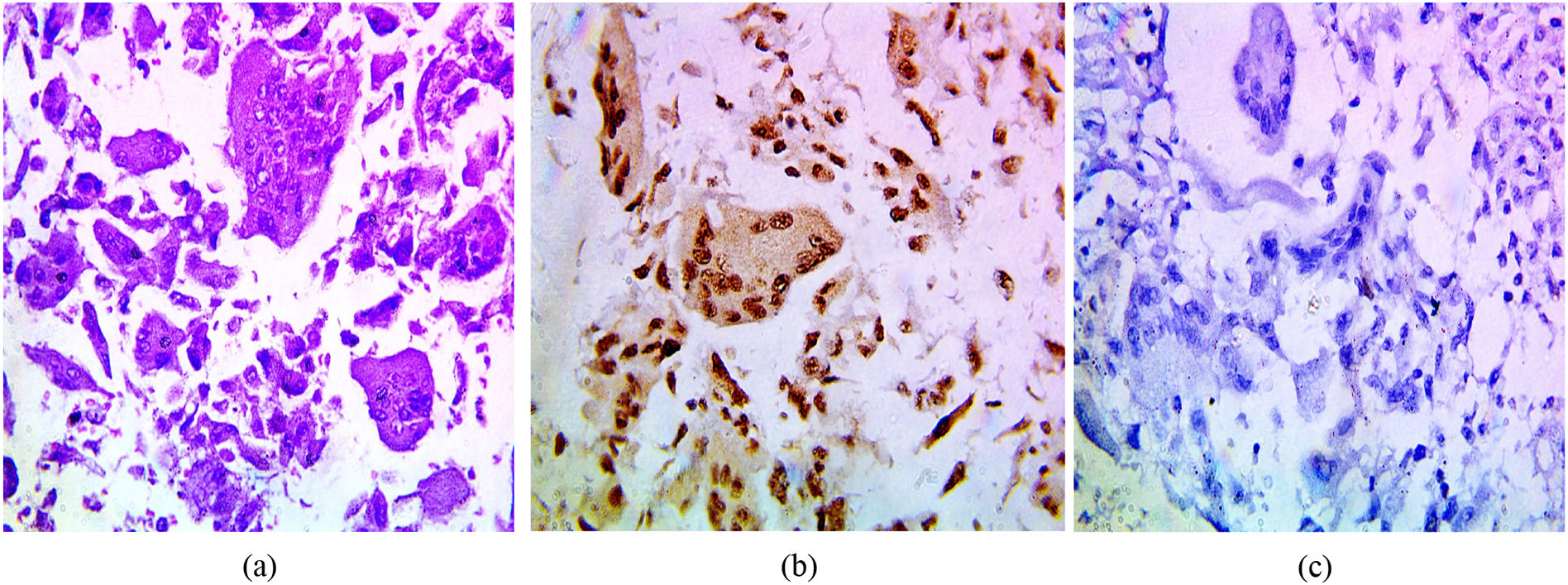
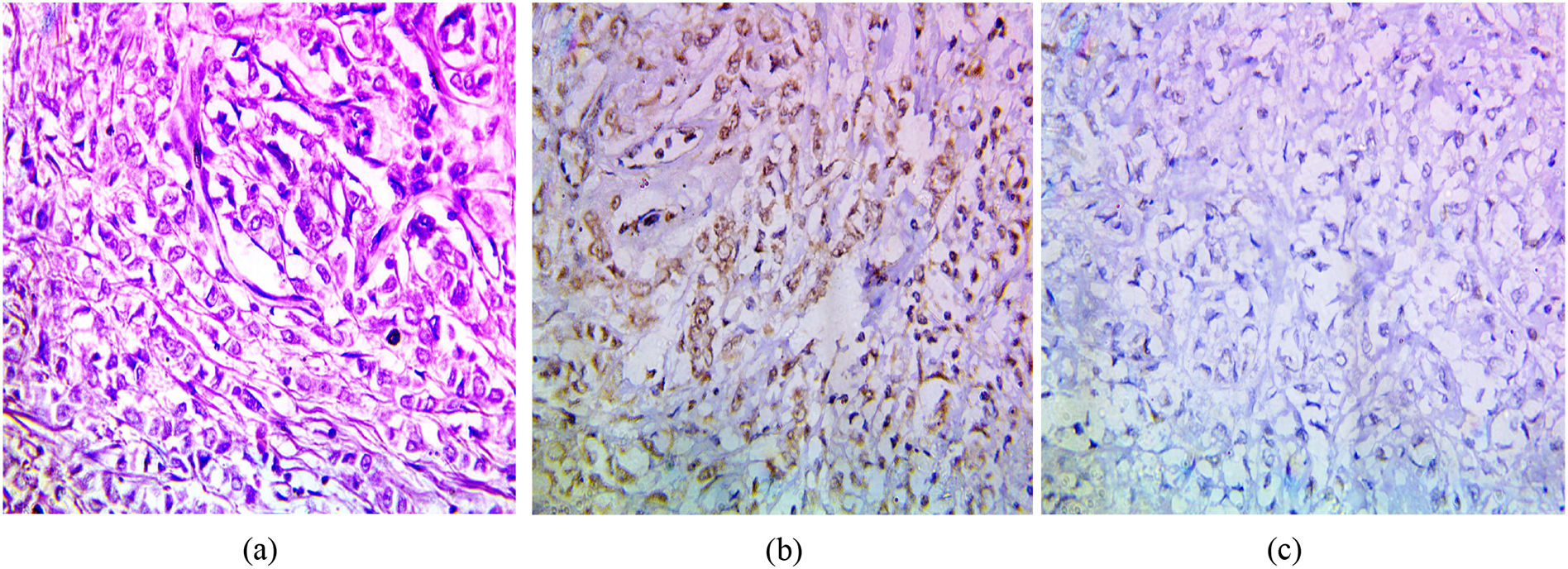
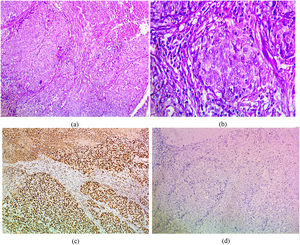
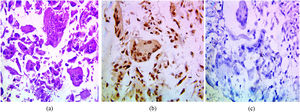
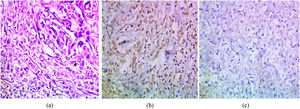
The immunohistochemical expression of PELP1 in tumor cells was positive in 42 out of 45 cases (93.3%). Instead of GATA3 expression, 25 out of 45 cases were positive (55.56%). Moreover, the majority of the PELP1 positive cases showed diffuse strong staining, making observation of the staining easy. In the present study, PELP1 revealed a (96.67%) positive expression rate in primary TNBC and (86.67%) positive in metastatic TNBC as well as PELP1 immunoreactivity is consistently seen in primary TNBC and paired metastasis. In comparison to GATA3 revealed (53.33%) positive expression rate in primary TNBC and (60%) positive in metastatic TNBC and there is difference expression of GATA3 in paired primary and metastatic TNBC in 2 patients (Figs. 1,2,3)(Table 2).
(a) IDC medullary subtype showing syncytial growth pattern (H&E 100×), (b) large and pleomorphic malignant cells with vesicular cytoplasm and surrounded by lymphocytic infiltrates (H&E 400×), (c)IDC medullary subtype showing strong positive nuclear PELP1 expression (IHC 100×), (d) IDC medullary subtype showing negative GATA3 expression (IHC 100×).
Relation between PELP1 IHC and GATA3 IHC expression among the studied cases.
| Variable | PELP1 | GATA3 | ||||||
|---|---|---|---|---|---|---|---|---|
| -ve | +ve | -ve | +ve | |||||
| N | % | N | % | N | % | N | % | |
| All cases: (n = 45) | 3 | 6.67 | 42 | 93.33 | 20 | 44.44 | 25 | 55.56 |
| Primary only: (n = 30) | 1 | 3.33 | 29 | 96.67 | 14 | 46.67 | 16 | 53.33 |
| IDC NST:(n = 27) | 1 | 3.7 | 26 | 96.3 | 12 | 44.44 | 15 | 55.56 |
| Lobular:(n = 1) | 0 | 0 | 1 | 100 | 1 | 100 | 0 | 0 |
| Special:(n = 2) | 0 | 0 | 2 | 100 | 1 | 50 | 1 | 50 |
| Metastasis cases: (n = 15) | 2 | 13.33 | 13 | 86.67 | 6 | 40.00 | 9 | 60 |
Categorical variables were expressed as number (percentage).
Locally recurrent and distant metastatic BC can pose considerable diagnostic and therapeutic challenges. Confirmation of a primary mammary origin in locally recurrent and distant metastatic sites is of significance to decide the line of treatment. Due to lack expression of three main receptors (ER, PR, and HER2), TNBC is an aggressive type of BC that lacks targeted therapeutic approaches such as hormone therapy.17 Therefore, novel diagnostic immunostain with high sensitivity and can detect TNBC in real-time and accurate are urgently needed.
It was agreed upon that young women with BC, when compared to older women, exhibited more advanced stage disease at time of diagnosis, larger tumors, hormone receptor-negative disease, marked pleomorphism, and more positive lymph nodes.18 In the present study, according to clinicopathological parameters, the mean and median ages of patients were 48.31 years and 46.5 years respectively. The majority of cases were grade 3 (60%), stage III (83%). Most cases were lymph node positive (90%). This is also accepted by Dent and college, who found regarding TNBC cases, the mean age at diagnosis was 53 years, had grade3 tumors (66%), and the mean tumor size was 3.0 cm.19
Although earlier research has revealed that PELP1 functions as an oncogene that is deregulated in BC,20,21 PELP1 was associated with poor outcome in luminal cancers20 and in TNBC when combined with Ki67.22 Also Zhang and colleague stated there was no significant associations between PELP1 protein expression and other clinicopathological variables.22 Little is known about its diagnostic value in primary and metastatic TNBC. PELP1's localization in our investigation was solely nuclear. This finding is supported by recent immunohistochemical investigations in a variety of cell types using commercially available PELP1 antibodies,20,23 However, previous IHC research reported PELP1 to have widely distributed in the cytoplasm in a panel of tumor tissues.24 A possible explanation for this discrepancy may lie in the different antibodies against PELP1 used in this research.
In the current research, PELP1 revealed (96.67%) positive expression rate in primary TNBC and (86.67%) positive in metastatic TNBC. These finding near to Dang and colleague who reported (96%) of studied primary TNBC and (100%) of metastatic TNBC positive PELP1 expression.7
Interestingly, PELP1 protein overexpression does not occur exclusively in TNBC as shown in this study and others but also in ER-positive breast cancer (luminal-like subtype).20 Its expression shows a slightly higher frequency in TNBC (96.7%) in our study than in ER-positive breast carcinoma in a previous study (81%).20 Wang and colleague studied PELP1 expression among 1063 BC cases, a significant differential distribution was found, with diffuse staining mostly in luminal B (76.4%), followed by basal like BC (72.7%), HER2 (68.9%), luminal A BC (63.7%).9 Other study reported that PELP1 expression in the luminal B breast cancer subtype was significantly higher than in TNBC.22 These differences might be due to racial differences or the different methods utilized.
IHC for GATA3 is commonly utilized in surgical pathology to support urothelial or breast origin in a cancer of uncertain origin. In this situation, GATA3 is sensitive but not completely specific. Although GATA3 labeling is more prominent in ER-positive BC, it also identifies ER-negative BC, making it particularly useful in the diagnosis of TNBC.7,13 Data regarding GATA3 expression in TNBC are somewhat limited.
Defining a threshold of 5% tumor cell staining as positive, we demonstrate GATA3 expression in (53.33%) of primary TNBC and (60%) of metastatic TNBC. This result is near to two previous studies which are specific to TNBC who reported 43% and 48% positivity cases of TNBC, respectively.16,25
In contrast to other studies that have explicitly assessed the expression of GATA3 in TNBC, Yang and Nonaka26 found expression in 5% of cases, and Albergaria and colleagues27 found expression in 16% of cases.
Different methodologies, antibody clone and dilution, specimen type, or tissue source, as well as different cutoffs employed to identify GATA3 positive in prior research, might explain the variable degrees of expression observed. The nuclear labeling cutoffs used to identify GATA3 positive in the literature have ranged from larger than 1%,26 5%,16 and 30%.27
GATA3 expression is known to be related to ER signaling and is correlated with ER positivity in breast cancer.13 The expression of GATA3 in ER-negative breast cancers, suggests an ER-independent mechanism of GATA3 regulation in these tumors. This may help us understand the role of this transcription factor in mammary development and tumorigenesis.
PELP1 is more useful than GATA3 in the detection of TNBC, according to our findings. Despite the limitation of the small sample size used in this study, our study included different histopathologic types of TNBC (IDC NST, IDC medullary subtype, pleomorphic invasive lobular BC, and metaplastic BC). PELP1 showed positive immunoreactivity in medullary, metaplastic, and even pleomorphic lobular carcinomas in our study, which are GATA3-negative. There have been no previous researches that have established the relevance of PELP1 immunoreactivity in relation to BC histopathologic subtypes. In contrast to Cimino-Mathews and colleagues, who observed more than half of metaplastic carcinomas labeled GATA3.16 In addition, GATA3 positivity has been ranged 33%–83% of medullary carcinomas and 17%–56% of metaplastic breast carcinomas.28,29 Lu and colleague30 who study cases which are double negative for CK7 and GATA3 found only 30% positive expression of metaplastic carcinoma but all cases of lobular are positive.
In the metastatic situation, immunohistochemical markers that support the original breast origin are required for correct diagnosis. Such indicators, on the other hand, may be effective in identifying the primary versus uncommon metastatic origin of poorly differentiated TNBC due to loss of biomarker expression and especially in needle biopsies that do not have an in situ component. Ultimately, only history and follow-up can definitively confirm the breast origin in some cases.
In our study, GATA3 labels 60% of metastatic TNBC. It has also been documented that GATA-3 labeled 56% of metastatic TNBC.16 One should be cautious that there could be a likelihood of change in GATA3 status in metastasis if changes of hormonal receptor status were also observed in the metastatic diseases.
Also, the high frequency of diffuse and strong PELP1 nuclear staining is more than GATA3 in TNBC suggests that PELP1 may have potential diagnostic utility for metastatic TNBC in non-breast organs in an appropriate clinical context, such as history of primary TNBC. PELP1 has a low expression rate (20%) in colorectal cancer, gastric cancer, and renal cell carcinoma, but is greater in lung cancer (49.1%) and ovarian cancers (42.3%), according to Wang and colleague.9
The mechanism/molecular pathway for PELP1 protein overexpression in TNBC might be questioned, as well as whether PELP1 can be exploited as a molecular target for the treatment of TNBCs for which there is presently no targeted therapy. These concerns have remained unanswered due to the scarcity of published data on PELP1 in TNBC.
ConclusionDespite the limitation of a small sample size used in this study, our findings indicate that considering PELP1 as a sensitive lineage marker for both primary and metastatic TNBC and labels approximately 97% of cases. We conclude that using both GATA3 and PELP1 is recommended for confirming a breast site of origin in putative breast cancer metastases that lack ER, PR, and HER2 expression.
LimitationsThere were a few flaws in our research. Because this was a retrospective study, the database did not include treatment information such as adjuvant and neoadjuvant chemotherapy, making it unable to assess the impact of chemotherapy directly. In addition, data on additional molecular characteristics of TNBC was lacking, preventing further research into the relationship between biological characteristics and prognosis. Furthermore, the limited sample size of our study might be an impediment to obtaining more powerful results.
FundingThis research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical considerationsThe study protocol was carried out at the Pathology department and was approved by the Institutional Review Board (IRB) of Zagazig University's Faculty of Medicine.