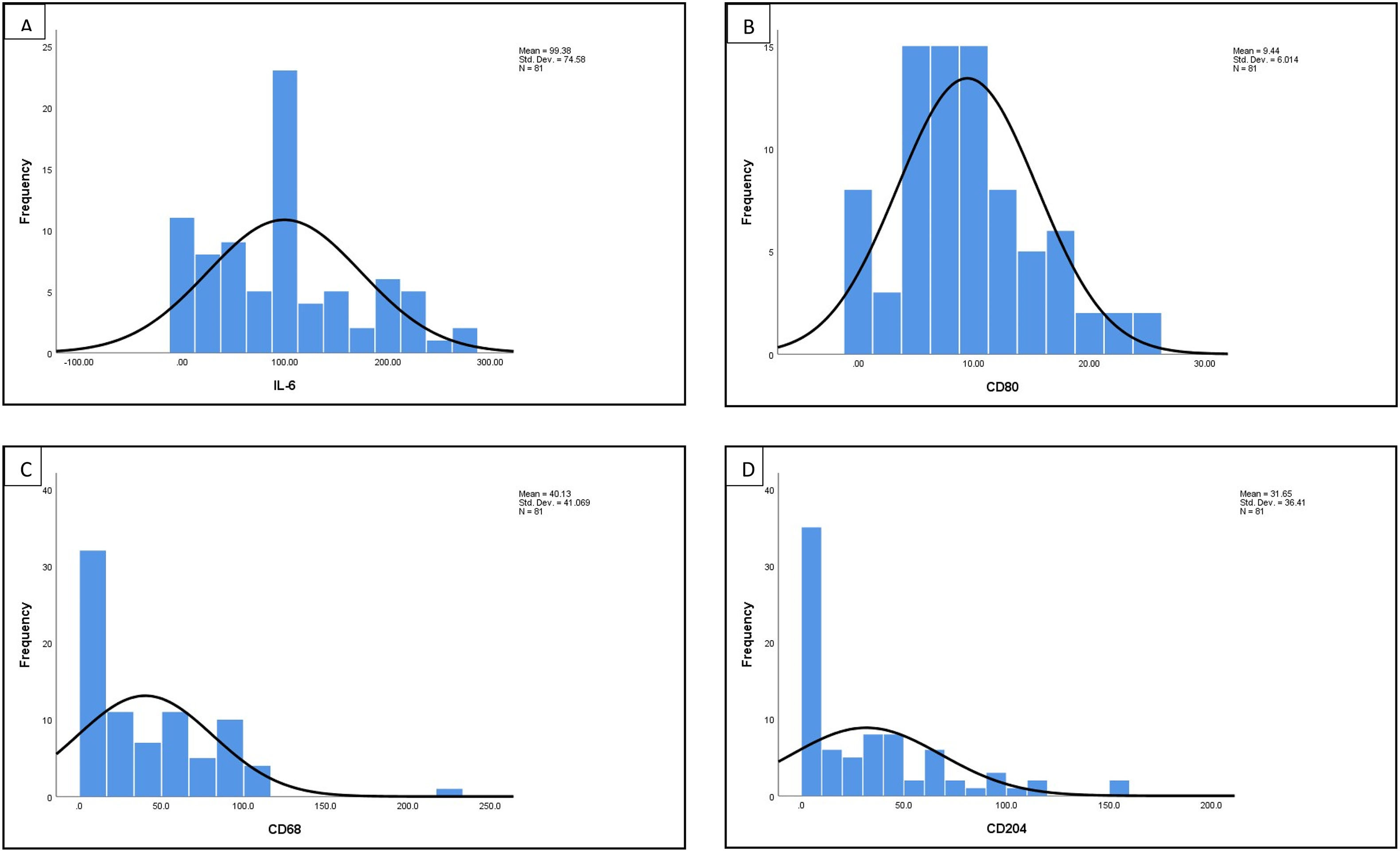
Breast cancer metastasis accounts for the majority of deaths from breast cancer. The knowledge on IL-6 affecting cancer cell metastatic behaviour need to be studied.
ObjectivesThis study aim to examine the association of macrophage polarisation status and IL-6 with clinicopathological criteria and lymphovascular invasion (LVI) of breast carcinoma.
Material & Method81 cases of FFPE breast carcinoma samples were stained with IL6, CD80 (M1 macrophage), CD204 and CD163 (M2 macrophage) and CD68 (pan-macrophage marker). The macrophages count were evaluated based on three hotspots of positively stained cells. IL-6 scoring was done using the H-score method.
ResultSignificant association was observed between CD68 marker with blood vessel invasion (p-value = 0.014), lymphatic vessel invasion (p-value = 0.005), and metastasis (p-value =0.028). CD68 was also significantly associated with CD204 (p = 0.027). CD80 biomarker also showing significant association with patient tumour grade (p-value = 0.054), ER (0.028) and PR (0.010) in patient clinical data and CD204 is significantly associated with ER (0.053) and PR (0.054) patient clinical data. Meanwhile, there is no significant association of IL-6 with the patient clinical data.
ConclusionThere is no significant association of IL-6 with the patient clinicopathological data obtained in this study while CD68 showed significant correlation with M2 macrophage biomarker and LVI indicating the influence of M1 and M2 macrophage in breast cancer metastatic pathway through blood and lymphatic vessel invasion.
La metástasis del cáncer de mama representa la mayoría de las muertes por cáncer de mama. Es necesario estudiar el conocimiento sobre la IL-6 que afecta el comportamiento metastásico de las células cancerosas.
ObjetivosEste estudio tiene como objetivo examinar la asociación del estado de polarización de los macrófagos y la IL-6 con los criterios clínico-patológicos y la invasión linfovascular (LVI) del carcinoma de mama.
Material y método81 casos de muestras de carcinoma de mama FFPE se tiñeron con IL6, CD80 (macrófago M1), CD204 y CD163 (macrófago M2) y CD68 (marcador pan-macrófago). El recuento de macrófagos se evaluó en base a 3 hotspots de células teñidas positivamente. La puntuación de IL-6 se realizó mediante el método de puntuación H.
ResultadoSe observó una asociación significativa entre el marcador CD68 con invasión de vasos sanguíneos (valour de p = 0,014), invasión de vasos linfáticos (valour de p = 0,005) y metástasis (valour de p = 0,028). CD68 también se asoció significativamente con CD204 (p = 0.027). El biomarcador CD80 también muestra una asociación significativa con el grado del tumour del paciente (valour p = 0,054), ER (0,028) y PR (0,010) en los datos clínicos del paciente y el CD204 se asocia significativamente con los datos clínicos del paciente ER (0,053) y PR (0,054). Mientras tanto, no existe una asociación significativa de IL-6 con los datos clínicos del paciente.
ConclusiónNo existe una asociación significativa de IL-6 con los datos clínico-patológicos del paciente obtenidos en este estudio, mientras que CD68 mostró una correlación significativa con el biomarcador de macrófagos M2 y LVI que indica la influencia de los macrófagos M1 y M2 en la vía metastásica del cáncer de mama a través de la invasión de vasos sanguíneos y linfáticos.
Breast cancer has a good prognosis when detected early, and could lead to mortality when discovered too late. Globally, there are 2.3 million women diagnosed with breast cancer with 685,000 deaths due to breast cancer, making it the world's most prevalent cancer.1 Malaysian National Cancer Registry Report in 2007 to 2011 followed up until 2016 reported that 1 in 20 women in Malaysia will develop breast cancer in their lifetime.2,3 However, majority of deaths from breast cancer are not due to the primary tumour itself, but the result of metastasis to other organs in the body. Metastasis starts with the local invasion of surrounding host tissue by cells originating from the primary tumour and continues until the tumour cells invade and intravasate into blood or lymphatic vessels.4,5 A tumour lump in the breast carcinoma can change its surrounding microenvironment and the microenvironment can influence the tumour growth and metastasis.5
Macrophages are the most abundant immune cells present in the tumour microenvironment.6 Macrophage is an important component of innate immunity and plays central roles in inflammation and host defences. Monocyte–macrophage lineage cells are denoted by considerable diversity and plasticity. Two different states of activated macrophages have been proposed by analogy to the Th1/Th2 classification such as classically (M1) and alternatively activated (M2) macrophages.7,19 M1 macrophages produce high level of pro-inflammatory cytokines such as TNF-α, CD80, CD86 and IL-23. M1 macrophages promote Th1 response and enhanced microbicidal and tumouricidal activities. M2 macrophages also known as type 2 or alternatively activated macrophages are polarised via distinct stimuli and can further subdivided into M2a, M2b and M2c macrophages.8,11 M2 macrophages mainly involved in parasite clearance, tissue remodelling, immune modulation and tumour progression due to their expression of scavenger receptor (SR) and mannose receptor (MR). Cluster of differentiation 80 or CD80 also known as B7-1 is differentiated from one of the M1 macrophage lineages. CD80 could be found on the surface of various immune cells including B cell, monocytes and antigen presenting cell (APC) such as dendritic cells. Up-regulation of CD80 may indicate T cell tolerance in the breast tumour microenvironment thus showing the potential to be used as a CD80/CD86–CTLA4 pathway blocking therapy.9 Meanwhile CD68 has been identified as a marker of macrophage in tumour microenvironment as it is highly expressed by cell in the monocyte lineage, by circulating macrophages and also by the tissue macrophages such as Kupffer cell or microglia.10 CD68 is recognised as pan-macrophage marker which is now generally utilised to identify TAMs in diagnostic biopsy samples.10 Research has reported that CD68 marker is not specific to M2 macrophage but recognises both M1 and M2 macrophages.11 CD204 or also known as Macrophage Scavenging Receptor 1(MSR1) is a protein which is encoded by the MSR1 gene. MSR has been shown to participate in multiple macrophage metabolic processes, including adhesion, phagocytosis, production of reactive oxygen species and host defence.12 The macrophage scavenging receptor 1 (CD204) is used as a specific M2 macrophage marker.13 CD163 and CD204 have been noted to be used as markers of M2 macrophages in several studies.14,22,23 Meanwhile, Intercellular Adhesion Molecule-1 (ICAM-1) is a protein which is typically expressed on endothelial cells and cells of the immune system. ICAM-1 can be induced by interleukin-1 (IL-1) and tumour necrosis factor (TNF) which the expressed by the vascular endothelium, macrophages, and lymphocytes. ICAM-1 is a ligand for LFA-1 (integrin), a receptor found on leukocytes.22 When activated, leukocytes bind to endothelial cells via ICAM-1/LFA-1 and then transmigrate into tissues. Apart from increasing inflammation and stimulating the immune system, macrophages also play an important anti-inflammatory role and can decrease immune reactions through the release of cytokines.15,22,24 IL-6 is a pleiotropic cytokine that plays a significant role in the growth and differentiation, two opposing mechanisms, of cells. Several studies have addressed the role of IL-6 in tumour cell growth in vitro, but its exact role remains varied and unclear. Concerning the biology of in vitro breast cancer cells, IL-6 seems to be a double-edged sword16 There are several reports indicating IL-6 to be both a tumour-promoting and a tumour-counteracting cytokine. In the invasive breast tumours, the percentage of cases showing immunoreactivity for IL-6, gp130, and IL-6Ra was much higher than in non-malignant lesions, and the intensity of expression was 2–3 times higher.16,17 The breast tumour cells not only produce more IL-6 than normal breast epithelial cells, but also the response on the tumour cells to this interleukin is greater. Furthermore, high expression of IL-6 and its receptors in breast tumours might be related to the enhanced cell proliferation in breast cancer.13,14 IL-6 was also showed to be involved in metastasis through its role in promoting breast cancer cell motility. IL-6 decreased cell adhesion of three breast cancer cell lines and this was associated with a decrease in E-cadherin expression.15 The finding that the oestrogen receptor represses IL-6 expression in breast cancer cell lines, suggests that up-regulation of the cytokine may be involved in the high invasiveness and metastatic capability of oestrogen receptor-negative tumours.16
Currently, the traditional prognostic markers are only able to confidently identify the group of approximately 30% of patients, who are most likely to have either a very favourable or a very poor outcome for cancer metastasis. For the remaining 70% patients, approximately 30% will still develop metastases.17,26,28 Hence, the aim of this study is to examine the association of macrophage polarisation status and IL-6 with clinicopathological criteria and invasion of breast carcinoma through immunohistochemistry staining.
Material and methodsPatients and samplesThis study was conducted on random sampling of 130 cases of FFPE breast carcinoma samples (year 2005 until 2015) obtained from Hospital Universiti Sains Malaysia (HUSM). A total of 31 cases were excluded due to poor sample fixation and another 18 sample were excluded due to lack of sample block. Therefore, a total of 81 cases were proceeded with this study. Ethical approval for this study was granted by the Human Research Ethics Committee (HREC), Universiti Sains Malaysia (JEPeM code: USM/JEPeM/15100367).
IHC stainingFour consecutive sections of breast carcinoma from each block were proceeded with polymer-based IHC staining. Polymer-based detection method increased sensitivity and involved fewer steps compared to avidin-biotin-enzyme (ABC) and labelled streptavidin-biotin (LSAB) methods. Slides were first fixed, de-paraffinised and rehydrated prior to treatment with citrate buffer pH 6.0 antigen retrieval solution (DAKO, Germany). One litre of pH 9.0 citrate buffer was pre-heated using automated PT Link (DAKO, Germany) machine. Slides were lifted from the PT Link machine and immediately dipped into distilled water. After that, the slides were arranged in Shandon Sequenza. The tissue sections were then treated with 100 μl of 3% hydrogen peroxide for 5 min to block endogenous hydrogen peroxidase activities. The slides were washed with distilled water followed by tris-buffered saline–tween 20 (TBST). Next, 100 μl of primary antibodies (1:200 for IL-6, 1:100 for CD80, 1:500 for CD68 and 1:1000 for CD204 to stain the cytokine, M1 macrophage, pan-macrophage and M2 macrophage respectively) were dropped onto each slide and incubated overnight at 4 °C or 1 h at room temperature according to the antibody used. The unbound primary antibodies were washed with TBST twice, followed by the addition of horseradish peroxidase (HRP) rabbit/mouse (DAKO, Germany) for 30 min at room temperature. The unbound HRP enzymes were washed with TBST twice. Next, the slides were taken out from the Shandon Sequenza. The slides were treated with diaminobenzidine (DAKO REAL DAB+ Chromogen 50x) (DAKO, Germany) for 5 min which then developed immunohistochemical reactions.
Next, the slides were washed under running tap water and then the slides were dipped into filtered haematoxylin for 3–5 times to counterstain the tissue sections. Excess counterstain was removed by washing the slides under running tap water for 5 min. After that, the slides were treated with a series of ascending ethanol concentrations (70%, 80%, 90% and 100% ethanol x 2 min at each concentration) for dehydration. Next, the slides were fixed in two changes of xylene for 2 min each. The slides were mounted using DPX and coverslips and left to dry overnight before observation under the light microscope. Tonsil and breast carcinoma tissue sections were used as both positive and negative controls for each run of staining was conducted. Positive controls were treated following the same procedure as above whereas for the negative controls the primary antibody was excluded.
Microscopic analysis and assessment of macrophages countHotspot methodologyMacrophage count was evaluated on intra-tumoural and peri-tumoural area of IL-6, CD80, CD68 and CD204 stained slides. Macrophage density was assessed in the three most positively dense area following a brief scan of the entire section at low power. For each area, an eyepiece-mounted 25-point Chalkley array graticule was used to assess the positive staining. The Chalkley point array was rotated so that the maximum number of points coincided with stained macrophages, and a count was taken. The median of the three Chalkley counts was used for the subsequent statistical analysis.
H-score methodFor Interleukin-6, H-score method was applied which was divided by percentage into no staining (0), weakly stained (1), moderately stained (2) and strongly stained (3) macrophages from overall of 100%. The following formula was applied to calculate the overall score of IL6 staining.
0 = No staining.
1 = Weak staining.
2 = Moderate staining.
3 = Strong staining.
The slides were assessed by at least two independent assessor. Both assessor viewed the slide and independently decide on an intensity score. Later, the evaluation by both of the assessors was discussed to obtain consensus.
Statistic analysisDescriptive analysis was initially carried out for all IL-6,CD80, CD68, CD204, and M1/M2 macrophage ratio to obtain median values and distribution histogram. The results of descriptive analysis written as median ± standard error of the median (SEM). Median values were used to categorise both markers as high when macrophage count/IL6 score is greater than median value and low when macrophage count/IL6 score is equal or lower than median value. The relationship between M1/M2 macrophages ratio, IL-6 and all the biomarkers versus clinicopathological data, and LVI parameters were carried out using Pearson Chi Square test of association or (Fisher's Exact test if expected count <5). The correlation between M1/M2 macrophages ratio, IL-6, and all biomarker were carried out using Spearman's correlation with p-value <0.05 was considered significant (α) value. All statistical analysis were carried out using Statiscal Package for the Social Sciences (SPSS) (IBM SPSS Statistical version 24.0).
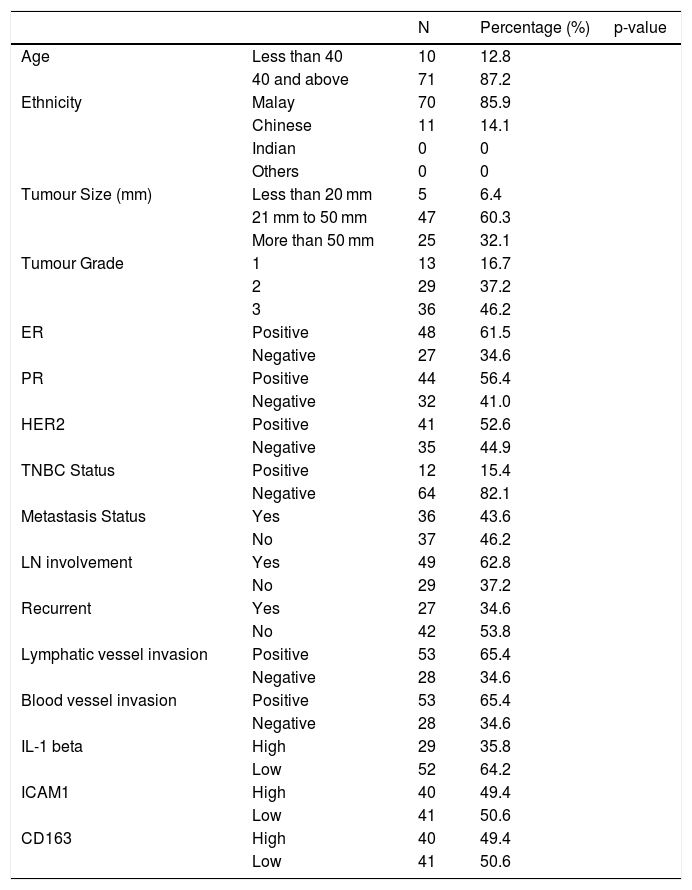
ResultTable 1 show the clinical data of the patients involved. Majority of the patient's age were 40 and above with 71 patients out of 81. Malays ethnicity with 70 out of 81 patients dominating the ethnicity category among all patients. The tumour size detected was between 20 mm to 50 mm, 47 out of 81 patients with majority of the tumour grade is at grade 3. Lymph nodes are also playing a critical involvement in breast cancer metastasis with 49 out of 81 patients is positive. While for the hormone receptor, all of ER (48 out of 81 patients), PR (44 out of 81 patients), and HER2 (41 out of 81 patients) receptor leaning to the positive side. TNBC negative status (82.1%) was higher than the positive status (12 out of 81 patients was positive and 64 out of 81 was negative). 27 out of 81 patients were positive with breast cancer recurrent. 53 out of 81 patients were positive for lymphatic vessel invasion and 28 out of 81 patients were negative. 53 out 81 patients were positive for blood vessel invasion and 28 out of 81 patients were negative. Apart from that, 29 patients shown high IL-1beta 40 patients with high ICAM-1 intensity and 40 patients with high CD163 expression
The clinical data of patients (N = 81).
| N | Percentage (%) | p-value | ||
|---|---|---|---|---|
| Age | Less than 40 | 10 | 12.8 | |
| 40 and above | 71 | 87.2 | ||
| Ethnicity | Malay | 70 | 85.9 | |
| Chinese | 11 | 14.1 | ||
| Indian | 0 | 0 | ||
| Others | 0 | 0 | ||
| Tumour Size (mm) | Less than 20 mm | 5 | 6.4 | |
| 21 mm to 50 mm | 47 | 60.3 | ||
| More than 50 mm | 25 | 32.1 | ||
| Tumour Grade | 1 | 13 | 16.7 | |
| 2 | 29 | 37.2 | ||
| 3 | 36 | 46.2 | ||
| ER | Positive | 48 | 61.5 | |
| Negative | 27 | 34.6 | ||
| PR | Positive | 44 | 56.4 | |
| Negative | 32 | 41.0 | ||
| HER2 | Positive | 41 | 52.6 | |
| Negative | 35 | 44.9 | ||
| TNBC Status | Positive | 12 | 15.4 | |
| Negative | 64 | 82.1 | ||
| Metastasis Status | Yes | 36 | 43.6 | |
| No | 37 | 46.2 | ||
| LN involvement | Yes | 49 | 62.8 | |
| No | 29 | 37.2 | ||
| Recurrent | Yes | 27 | 34.6 | |
| No | 42 | 53.8 | ||
| Lymphatic vessel invasion | Positive | 53 | 65.4 | |
| Negative | 28 | 34.6 | ||
| Blood vessel invasion | Positive | 53 | 65.4 | |
| Negative | 28 | 34.6 | ||
| IL-1 beta | High | 29 | 35.8 | |
| Low | 52 | 64.2 | ||
| ICAM1 | High | 40 | 49.4 | |
| Low | 41 | 50.6 | ||
| CD163 | High | 40 | 49.4 | |
| Low | 41 | 50.6 |
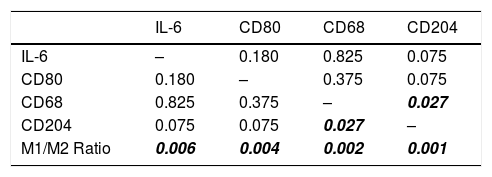
The distribution histogram of each biomarker and cytokine is shown in Fig. 1. The median value for IL-6 is 100.00 ± 8.28 and the median value for macrophages biomarker CD80 is 8.50 ± 0.66, CD68 is 39.33 ± 39.79, CD204 is 35.02 ± 35.09, while the median for M1/M2 ratio is 0.400 ± 0.463. There are significant association between CD68 and CD204 (p = 0.027). There is also significant correlation between M1/M2 ratio with IL-6 (p = 0.006) (Table 2).
Association between M1/M2 Ratio, IL-6, CD80, CD68, and CD204 (p-value) with CD68, CD204, and M1/M2 macrophage ratio showing a significant value of association.
| IL-6 | CD80 | CD68 | CD204 | |
|---|---|---|---|---|
| IL-6 | – | 0.180 | 0.825 | 0.075 |
| CD80 | 0.180 | – | 0.375 | 0.075 |
| CD68 | 0.825 | 0.375 | – | 0.027 |
| CD204 | 0.075 | 0.075 | 0.027 | – |
| M1/M2 Ratio | 0.006 | 0.004 | 0.002 | 0.001 |
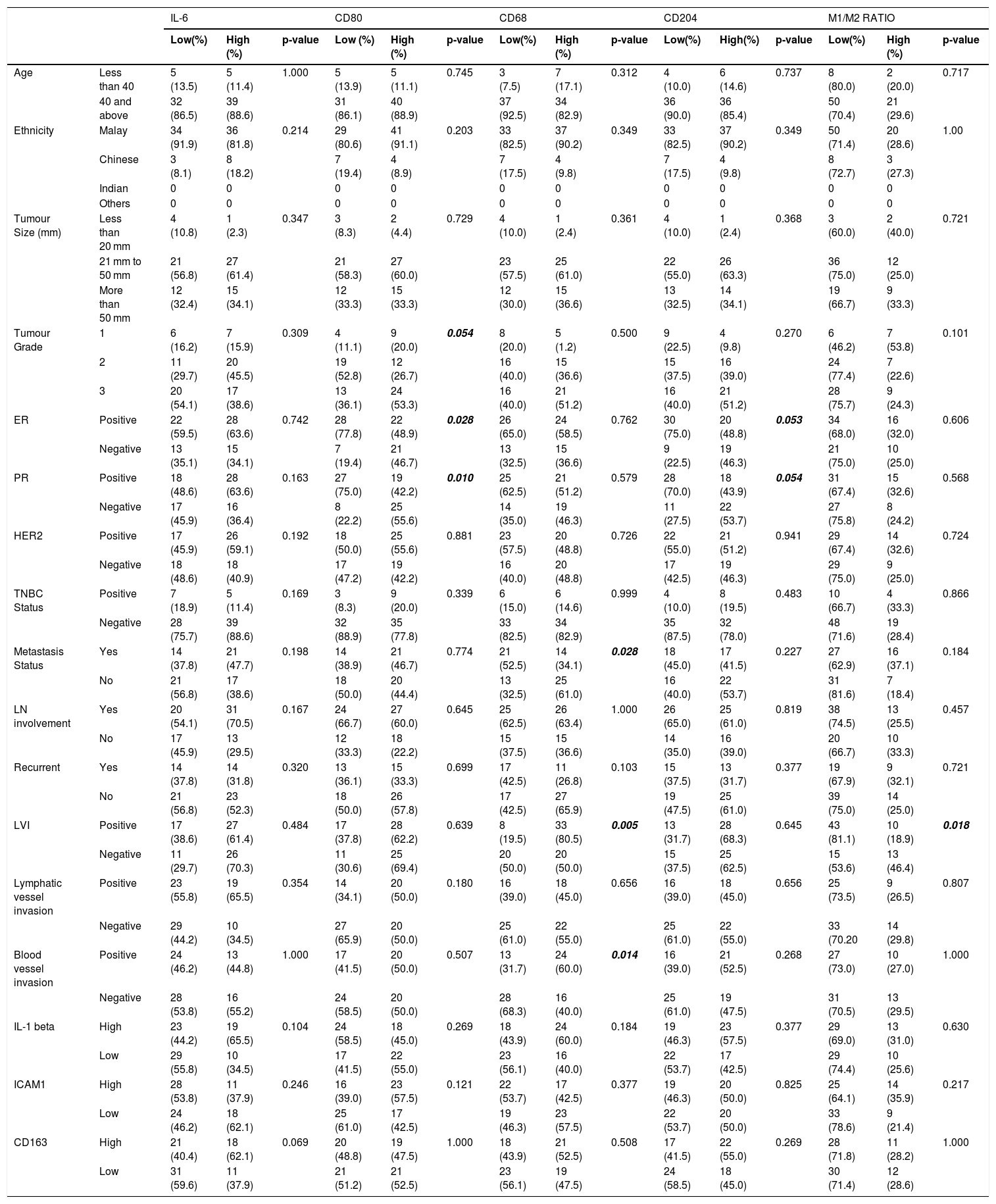
Table 3 shows the association of IL-6 score with patient clinicopathological data. There are no significant association of IL-6 biomarker with any of the patient's clinicopathological data but there are significant correlation of IL-6 with M1/M2 ratio (p = 0.006). Besides, M1/M2 ratio also shows a significant association with LVI (lymphovascular invasion) (p = 0.018) in the patient clinicopathological data. There are significant association of CD80 with the patient tumour grade (p = 0.054), ER (p = 0.028), and PR (p = 0.010) (Table 3) receptor. In CD68 biomarker, there is significant association with patient metastasis status (p = 0.028), LVI (p = 0.005) and blood vessel invasion (p = 0.014) (Table 3). Meanwhile, CD204 biomarker showed significant association only with ER (p = 0.053) and PR (p = 0.054) (Table 3) receptor.
shows the association of cytokine and biomarker with the patient clinicopathological data.
| IL-6 | CD80 | CD68 | CD204 | M1/M2 RATIO | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low(%) | High (%) | p-value | Low (%) | High (%) | p-value | Low(%) | High (%) | p-value | Low(%) | High(%) | p-value | Low(%) | High (%) | p-value | ||
| Age | Less than 40 | 5 (13.5) | 5 (11.4) | 1.000 | 5 (13.9) | 5 (11.1) | 0.745 | 3 (7.5) | 7 (17.1) | 0.312 | 4 (10.0) | 6 (14.6) | 0.737 | 8 (80.0) | 2 (20.0) | 0.717 |
| 40 and above | 32 (86.5) | 39 (88.6) | 31 (86.1) | 40 (88.9) | 37 (92.5) | 34 (82.9) | 36 (90.0) | 36 (85.4) | 50 (70.4) | 21 (29.6) | ||||||
| Ethnicity | Malay | 34 (91.9) | 36 (81.8) | 0.214 | 29 (80.6) | 41 (91.1) | 0.203 | 33 (82.5) | 37 (90.2) | 0.349 | 33 (82.5) | 37 (90.2) | 0.349 | 50 (71.4) | 20 (28.6) | 1.00 |
| Chinese | 3 (8.1) | 8 (18.2) | 7 (19.4) | 4 (8.9) | 7 (17.5) | 4 (9.8) | 7 (17.5) | 4 (9.8) | 8 (72.7) | 3 (27.3) | ||||||
| Indian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Others | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Tumour Size (mm) | Less than 20 mm | 4 (10.8) | 1 (2.3) | 0.347 | 3 (8.3) | 2 (4.4) | 0.729 | 4 (10.0) | 1 (2.4) | 0.361 | 4 (10.0) | 1 (2.4) | 0.368 | 3 (60.0) | 2 (40.0) | 0.721 |
| 21 mm to 50 mm | 21 (56.8) | 27 (61.4) | 21 (58.3) | 27 (60.0) | 23 (57.5) | 25 (61.0) | 22 (55.0) | 26 (63.3) | 36 (75.0) | 12 (25.0) | ||||||
| More than 50 mm | 12 (32.4) | 15 (34.1) | 12 (33.3) | 15 (33.3) | 12 (30.0) | 15 (36.6) | 13 (32.5) | 14 (34.1) | 19 (66.7) | 9 (33.3) | ||||||
| Tumour Grade | 1 | 6 (16.2) | 7 (15.9) | 0.309 | 4 (11.1) | 9 (20.0) | 0.054 | 8 (20.0) | 5 (1.2) | 0.500 | 9 (22.5) | 4 (9.8) | 0.270 | 6 (46.2) | 7 (53.8) | 0.101 |
| 2 | 11 (29.7) | 20 (45.5) | 19 (52.8) | 12 (26.7) | 16 (40.0) | 15 (36.6) | 15 (37.5) | 16 (39.0) | 24 (77.4) | 7 (22.6) | ||||||
| 3 | 20 (54.1) | 17 (38.6) | 13 (36.1) | 24 (53.3) | 16 (40.0) | 21 (51.2) | 16 (40.0) | 21 (51.2) | 28 (75.7) | 9 (24.3) | ||||||
| ER | Positive | 22 (59.5) | 28 (63.6) | 0.742 | 28 (77.8) | 22 (48.9) | 0.028 | 26 (65.0) | 24 (58.5) | 0.762 | 30 (75.0) | 20 (48.8) | 0.053 | 34 (68.0) | 16 (32.0) | 0.606 |
| Negative | 13 (35.1) | 15 (34.1) | 7 (19.4) | 21 (46.7) | 13 (32.5) | 15 (36.6) | 9 (22.5) | 19 (46.3) | 21 (75.0) | 10 (25.0) | ||||||
| PR | Positive | 18 (48.6) | 28 (63.6) | 0.163 | 27 (75.0) | 19 (42.2) | 0.010 | 25 (62.5) | 21 (51.2) | 0.579 | 28 (70.0) | 18 (43.9) | 0.054 | 31 (67.4) | 15 (32.6) | 0.568 |
| Negative | 17 (45.9) | 16 (36.4) | 8 (22.2) | 25 (55.6) | 14 (35.0) | 19 (46.3) | 11 (27.5) | 22 (53.7) | 27 (75.8) | 8 (24.2) | ||||||
| HER2 | Positive | 17 (45.9) | 26 (59.1) | 0.192 | 18 (50.0) | 25 (55.6) | 0.881 | 23 (57.5) | 20 (48.8) | 0.726 | 22 (55.0) | 21 (51.2) | 0.941 | 29 (67.4) | 14 (32.6) | 0.724 |
| Negative | 18 (48.6) | 18 (40.9) | 17 (47.2) | 19 (42.2) | 16 (40.0) | 20 (48.8) | 17 (42.5) | 19 (46.3) | 29 (75.0) | 9 (25.0) | ||||||
| TNBC Status | Positive | 7 (18.9) | 5 (11.4) | 0.169 | 3 (8.3) | 9 (20.0) | 0.339 | 6 (15.0) | 6 (14.6) | 0.999 | 4 (10.0) | 8 (19.5) | 0.483 | 10 (66.7) | 4 (33.3) | 0.866 |
| Negative | 28 (75.7) | 39 (88.6) | 32 (88.9) | 35 (77.8) | 33 (82.5) | 34 (82.9) | 35 (87.5) | 32 (78.0) | 48 (71.6) | 19 (28.4) | ||||||
| Metastasis Status | Yes | 14 (37.8) | 21 (47.7) | 0.198 | 14 (38.9) | 21 (46.7) | 0.774 | 21 (52.5) | 14 (34.1) | 0.028 | 18 (45.0) | 17 (41.5) | 0.227 | 27 (62.9) | 16 (37.1) | 0.184 |
| No | 21 (56.8) | 17 (38.6) | 18 (50.0) | 20 (44.4) | 13 (32.5) | 25 (61.0) | 16 (40.0) | 22 (53.7) | 31 (81.6) | 7 (18.4) | ||||||
| LN involvement | Yes | 20 (54.1) | 31 (70.5) | 0.167 | 24 (66.7) | 27 (60.0) | 0.645 | 25 (62.5) | 26 (63.4) | 1.000 | 26 (65.0) | 25 (61.0) | 0.819 | 38 (74.5) | 13 (25.5) | 0.457 |
| No | 17 (45.9) | 13 (29.5) | 12 (33.3) | 18 (22.2) | 15 (37.5) | 15 (36.6) | 14 (35.0) | 16 (39.0) | 20 (66.7) | 10 (33.3) | ||||||
| Recurrent | Yes | 14 (37.8) | 14 (31.8) | 0.320 | 13 (36.1) | 15 (33.3) | 0.699 | 17 (42.5) | 11 (26.8) | 0.103 | 15 (37.5) | 13 (31.7) | 0.377 | 19 (67.9) | 9 (32.1) | 0.721 |
| No | 21 (56.8) | 23 (52.3) | 18 (50.0) | 26 (57.8) | 17 (42.5) | 27 (65.9) | 19 (47.5) | 25 (61.0) | 39 (75.0) | 14 (25.0) | ||||||
| LVI | Positive | 17 (38.6) | 27 (61.4) | 0.484 | 17 (37.8) | 28 (62.2) | 0.639 | 8 (19.5) | 33 (80.5) | 0.005 | 13 (31.7) | 28 (68.3) | 0.645 | 43 (81.1) | 10 (18.9) | 0.018 |
| Negative | 11 (29.7) | 26 (70.3) | 11 (30.6) | 25 (69.4) | 20 (50.0) | 20 (50.0) | 15 (37.5) | 25 (62.5) | 15 (53.6) | 13 (46.4) | ||||||
| Lymphatic vessel invasion | Positive | 23 (55.8) | 19 (65.5) | 0.354 | 14 (34.1) | 20 (50.0) | 0.180 | 16 (39.0) | 18 (45.0) | 0.656 | 16 (39.0) | 18 (45.0) | 0.656 | 25 (73.5) | 9 (26.5) | 0.807 |
| Negative | 29 (44.2) | 10 (34.5) | 27 (65.9) | 20 (50.0) | 25 (61.0) | 22 (55.0) | 25 (61.0) | 22 (55.0) | 33 (70.20 | 14 (29.8) | ||||||
| Blood vessel invasion | Positive | 24 (46.2) | 13 (44.8) | 1.000 | 17 (41.5) | 20 (50.0) | 0.507 | 13 (31.7) | 24 (60.0) | 0.014 | 16 (39.0) | 21 (52.5) | 0.268 | 27 (73.0) | 10 (27.0) | 1.000 |
| Negative | 28 (53.8) | 16 (55.2) | 24 (58.5) | 20 (50.0) | 28 (68.3) | 16 (40.0) | 25 (61.0) | 19 (47.5) | 31 (70.5) | 13 (29.5) | ||||||
| IL-1 beta | High | 23 (44.2) | 19 (65.5) | 0.104 | 24 (58.5) | 18 (45.0) | 0.269 | 18 (43.9) | 24 (60.0) | 0.184 | 19 (46.3) | 23 (57.5) | 0.377 | 29 (69.0) | 13 (31.0) | 0.630 |
| Low | 29 (55.8) | 10 (34.5) | 17 (41.5) | 22 (55.0) | 23 (56.1) | 16 (40.0) | 22 (53.7) | 17 (42.5) | 29 (74.4) | 10 (25.6) | ||||||
| ICAM1 | High | 28 (53.8) | 11 (37.9) | 0.246 | 16 (39.0) | 23 (57.5) | 0.121 | 22 (53.7) | 17 (42.5) | 0.377 | 19 (46.3) | 20 (50.0) | 0.825 | 25 (64.1) | 14 (35.9) | 0.217 |
| Low | 24 (46.2) | 18 (62.1) | 25 (61.0) | 17 (42.5) | 19 (46.3) | 23 (57.5) | 22 (53.7) | 20 (50.0) | 33 (78.6) | 9 (21.4) | ||||||
| CD163 | High | 21 (40.4) | 18 (62.1) | 0.069 | 20 (48.8) | 19 (47.5) | 1.000 | 18 (43.9) | 21 (52.5) | 0.508 | 17 (41.5) | 22 (55.0) | 0.269 | 28 (71.8) | 11 (28.2) | 1.000 |
| Low | 31 (59.6) | 11 (37.9) | 21 (51.2) | 21 (52.5) | 23 (56.1) | 19 (47.5) | 24 (58.5) | 18 (45.0) | 30 (71.4) | 12 (28.6) | ||||||
The aim of this study is to examine the association of macrophage polarisation status and IL-6 with clinicopathological criteria and invasion of breast carcinoma through immunohistochemistry staining. As there is significant association between CD68 (pan-macrophage biomarker) and CD204 (M2-macrophage biomarker), this shows that both of the biomarker stains a similar subset of M2 macrophage as CD204 is a biomarker for M2 macrophage and CD68 is a pan-macrophage marker which could stain both M1 and M2 macrophages.18,19,25 Besides that, there are also significant association between blood vessel invasion, lymphatic vessel invasion with CD68 biomarker which show that the metastatic pathway used by the cancer cell to migrate to the other site in the body through blood and lymphatic vessel. Even though there is no significant association between IL-6 with CD204 biomarker, there is only a small gap to a significant association (p = 0.075) (Table 2) between the two biomarkers which make it worthwhile to proceed investigating IL-6 in in-vitro study, probably due to small number of sample size. In this study, we analysed patient clinicalpathological data with IL-6, each macrophage biomarker (CD80, CD68, CD204), and M1/M2 ratio. The balance between M1 and M2 polarised macrophages is important due as tumour-associated macrophages are predominantly M2-like macrophages and they are associated with cancer progression. In recent study, increase in M1-like/M2-like macrophage ratio in tumour immune microenvironment or known as remodelling of the imbalance, has been reported to associate with response to cancer chemotherapy.15,16 Tumour associated macrophages are not fixed with irreversible phenotype and M2-like macrophages can be converted and polarised into the M1-like macrophage, even with little change in tumour immune microenvironment by intervention due to its extreme plasticity nature.17,18 These facts led us to analyse the significant of M1/M2 macrophages ratio with IL-6 and patient clinicalpathological data. As there are significant correlation of IL-6 with M1/M2 ratio, as the M1/M2 ratio increased, the higher the level of IL-6 expression. Current approaches to treatment of breast cancer metastasis or cancer immunotherapy using macrophages which involve cytokines such as IL-6 are found to elicit immune responses.18 These approaches have been studied in order to reduce tumour size and angiogenesis by recruiting the immune cells to the tumour site, and prevent the polarisation of macrophages to an M2 phenotype. Besides that, by changing the dynamics of macrophage polarisation in cancer treatment which favour polarised M1 macrophages, we might be able to reduce the tumour growth and generate anti-tumoral response.
In the current literature, there are very few studies conducted in Malaysia to study clinicopathological characteristics with breast carcinoma. Therefore, this result cannot be compared with other studies on association between age and ethnicity in Malaysia. However, a study suggested that high density of CD68 was correlated to younger age.19 This may be due to lean body habitus and recent use of oral contraceptives which was not significant in this study.20,21 This might be due to different cohort of patients used which might affect the observation. In biological classification, for the last decade, tumour size and lymph node status have been used as the main factors for the selection of suitable treatment in breast carcinoma.17,27,29,30 Previous studies showed that positive nodes with increased size of tumour was associated with decreased survival rate.22,23 However, the technique was improved by the alternative option which has been demonstrated at St. Gallen Consensus in 2009, specifically the oestrogen hormone receptors (ER), progesterone hormone receptor (PR), Ki-67, and HER-2 status.24,25 The classification of tumour biological subtypes by the ER, PR, and HER-2 biomarkers in IHC are applicable in routine clinical work and it was proved to be useful for deciding therapeutic strategy in the routine clinical management among breast carcinoma patients.26
TNBC is clinically referred to absence of expression of ER and PR and no overexpression or amplification of HER2. This subgroup of tumours generally marked by more aggressive phenotype that associated with elevated risk of early recurrence with distant metastasis and poor prognosis.27 TNBCs produce more granulocyte colony stimulating factor (G-CSF) compared to other subtypes thus, TNBCs have the ability to prime M1 cells into anti-inflammatory phenotype (M2) to promote tumour growth and proliferation.26,27 Apart from that, in this study we also found that CD68 showed significant association with patient metastasis status while CD204 biomarker showed significant association with only ER and PR receptor. There are several studies showing that CD68 aid in tumour cell metastasis and significantly worsen the relapse-free survival (RFS) and overall survival (OS).19,20,27 CD68 also showed no association with oestrogen receptor (ER) and progesterone receptor (PR). This result support a study that suggested that the level of M2 macrophage infiltration is inversely linked with the expression of ERα as well as PR. Another study stated that high density of CD204 macrophages was associated with poor clinical course including relapse-free survival, distant relapse-free survival and also breast cancer-specific survival in patients with invasive breast cancer.10,17,27
Lymphovascular invasion (LVI) is defined as invasion of tumour emboli into lymphatic vessels, blood vessels or both in the peri-tumoural area.28 As metastasis status is highly associated with lymphovascular invasion, we concluded that CD68 biomarker could contribute to distant metastasis of breast carcinoma. Higher density of CD68-positive macrophages was linked with higher tumour grade and larger tumour size.27,28 High density of CD68-positive macrophages also showed connection with large tumour size and high histological grade.28 Besides that, CD68 biomarker also correlated with worse prognosis in breast cancer patients. Although there is no significant association between IL-6 and LVI but a study shows IL-6 to be a positive prognosticator.28 Normal mammary epithelial cells from healthy women were shown to release IL-6, but the expression of IL-6 was abolished in ductal infiltrating carcinomas. Consequently, it was suggested that the alteration of IL-6 expression is associated with pathogenesis in breast cancer. mRNA specific to IL-6, IL-6R and gp130 were expressed in 57, 53, and 71% of breast carcinoma tissues, respectively. Expression was strongly correlated with earlier stages of breast carcinoma. In univariate analysis, the expression of IL-6 and its receptor subunits proved to be a positive prognostic factor for overall survival (OS) and disease-free survival. In advanced stages, the expression of IL-6 and its receptor subunits predicts better prognosis.29,30
ConclusionThere is no significant association of IL-6 with the patient clinicopathological data obtained in this study while CD68 which is a pan-macrophage marker show correlation with patient metastasis status, lymphovascular invasion and blood vessel invasion. Besides, CD204 marker was correlated with oestrogen receptor (ER) and progesterone receptor (PR) and CD80 marker correlated with the patient tumour grade, ER and PR. We conclude that M1–M2 macrophage polarisation show different outcome in clinicopathological criteria and play different role in breast cancer invasion.
FundingThis work was supported by the Research University (RU) Grant Universiti Sains Malaysia [Grant Number: 1001/PPSK/8012315].
Confidentiality of dataThe authors declare that they have followed the protocols of their center on the publication of patient data.