This article is focused on the differential diagnosis of infantile acute subdural haematoma (SDH). The objective is focus on bringing more diagnostic tools when it comes to differentiate an spontaneous SDH from the traumatic SDH, being this accidental or intentional in the context of a child abuse. A complete state of the art is made from the review of the most recent available literature, collecting in the same paper the most relevant clinical features, radiologic findings and anatomopatological criteria for the diagnosis of infantile SDH. With the aim to simplify and clarify the handling of the patients with SDH, a multidisciplinary diagnostic algorithm has been developed in this article, combining the provided information from several authors and raising for the first time, the possibility of 3 final diagnostic options in the cases of infantile SDH: accidental traumatic SDH, intentional traumatic SDH, and spontaneous SDH.
El presente estudio se centra en el diagnóstico diferencial etiológico del hematoma subdural agudo (HSD) infantil. El principal objetivo es aportar herramientas diagnósticas que faciliten la diferenciación entre el HSD espontáneo y el HSD traumático, ya sea de causa accidental o intencional en el contexto de un maltrato infantil. Con este propósito, se ha realizado un estado del arte completo a través de una revisión de la literatura más reciente, recogiendo en el mismo trabajo los criterios clínicos, radiológicos y anatomopatológicos más relevantes para el diagnóstico de los HSD infantiles. Con el objetivo de simplificar y clarificar el manejo de los pacientes con HSD, se desarrolla un algoritmo diagnóstico que cuenta con un enfoque multidisciplinar, combinando la información aportada por diversos autores y planteando por primera vez tres diagnósticos finales posibles en los casos de HSD infantiles: HSD traumático accidental, HSD traumático intencional y HSD espontáneo.
Acute subdural haematoma (ASH) is defined as an accumulation of blood beneath the dura mater, specifically in the space between the dura mater and the arachnoid. In the paediatric age group, its incidence is 12/100 000 in children under 2 years of age and 24/100 000 in children under 1 year of age, with the highest peak incidence between 0 and 4 months of age.1 Its main origin is traumatic, accounting for 75% of diagnosed cases.2 Within this traumatic aetiology, it is important to differentiate between accidental trauma (more frequent) and intentional trauma (in the context of child abuse).
Classically, ASH has been encompassed as part of the classic triad of child abuse, which is based on the joint association of ASH, retinal haemorrhages (RH) and brain damage.3,4 Child abuse is also the most common cause of lethal brain injury in children under 2 years of age5 and can have a mortality of 12%–30% and neurodevelopmental morbidity of up to 60%–70% in survivors.5–7 Currently, in clinical practice, the first issue in the diagnosis of ASH is whether it was caused by accidental trauma or whether it is the result of intentional trauma. This diagnosis is difficult and highly controversial because of the serious clinical, social and medico-legal implications associated with the diagnosis of child abuse.8,9
Within the area of child abuse, until a few years ago, the entity known as shaken baby syndrome10 stood out. Traditionally, this entity has been linked to ASH. In this syndrome, the child's head is subjected to a mechanism of acceleration and deceleration by sudden and forced flexion–extension when the child is shaken by the trunk. This mechanism is considered, in some cases, to be responsible for triad injuries without the need for external trauma per se.11 However, in recent years, the diagnostic value of the triad associated with child abuse has often been questioned, principally because of the existence of false positives, mainly associated with spontaneous illness or cardiorespiratory arrest recovered in a patient with or without underlying involvement.12–15
Regarding the pathophysiological mechanisms involved in ASH, it has been classically considered that rupture by elongation of the bridging veins is the mechanism responsible for the bleeding.16 However, new neuropathological studies suggest that the actual cause of this haematoma may not be the bridging veins, but veins of much smaller calibre, such as the vessels of the dura mater itself.1,16
Parallel to this new neuropathological approach, there has also been an advance in the biomechanical studies of these lesions, giving rise to a clear discussion as to whether the pure shaking mechanism, without impact, could really be responsible for the triad lesions.17 In this sense, there are authors who defend as more plausible a multifactorial injury mechanism, such as shaking plus impact and sudden deceleration of the head. This is why currently the most accepted terminology is that of intentional head trauma,18 in order to encompass all possible injury mechanisms, including shaken baby syndrome.13,15
Although the main causal differential diagnosis focuses on the intentional or unintentional nature of the traumatic causes, there are up to 25% of ASHs that are not caused by a traumatic mechanism, but are due to spontaneous causes.2 Spontaneous ASH is often forgotten in the algorithm of aetiological diagnostic possibilities in these patients, probably due to the greater causality and implications of traumatic aetiology. Nevertheless, its incidence is not negligible and its diagnostic possibility must always be considered.14
At present, there are no clear algorithms available, nor sufficiently contrasted and rigorous data to enable a systematic and accurate aetiological diagnosis of ASH in children. Moreover, existing algorithms tend to exclude spontaneous causes in their aetiological diagnosis and focus, like most of the literature, on traumatic causes.15 In view of the above, the aim of this paper is 2-fold: on the one hand, to review the most recent literature on the diagnosis of ASH; and on the other hand, as a result of this review, an algorithm is proposed for the aetiological differential diagnosis of ASH, differentiating between accidental, intentional, and spontaneous traumatic causes. The approach to the diagnostic criteria that make up the algorithm is based on a multidisciplinary approach, with a clinical (anamnesis and physical examination), radiological, and anatomopathological approach. Through the proposed differential diagnosis algorithm, the aim is to improve the clinical and medico-legal management of these patients, broadening the range of diagnostic causes, and providing degrees of certainty that facilitate the interpretation of the findings and decision-making.
Material and methodsThis article is a narrative review of the differential diagnosis of ASHs. Due to the lack of common systematics among the studies consulted, it is not possible to perform a meta-analysis of the existing studies. Similarly, a systematic review would be difficult due to the broad scope of the question posed in this paper, which covers the entire differential diagnosis of traumatic versus spontaneous ASHs.
To perform the literature review, publications in Pubmed-Medline from the last 15 years were filtered using the keywords "infant subdural haematoma", "spontaneous subdural haematoma," and "abusive head trauma". This search resulted in 368 articles, of which 92 were selected on the basis of their title and abstract. Articles that dealt with topics other than differential diagnosis, which were not available in English or Spanish, or of which the full text was not available were initially discarded.
Of the 92 articles initially selected, 33 papers corresponding to case reports were rejected. The remaining 59 papers were supplemented by 11 articles that were manually searched through references in other articles. Finally, after reading the full article, a total of 41 papers were selected for this study.
ResultsThis section focuses primarily on the first objective of this study and presents the most important findings of the review on ASH. The findings are divided into the 3 main approaches to the diagnosis of ASH: the clinical approach, the radiological approach, and finally the pathological approach. The concepts discussed below are summarised in Tables 1 and 2.
Clinical diagnostic criteria for the different types of acute subdural haematoma.
| Intentional traumatic ASH | Accidental traumatic ASH | Spontaneous ASH | |
|---|---|---|---|
| Anamnesis | Inconsistent historyFalls from low height5 | Explicable trauma and observed by third parties8 | No evidence of trauma |
| Risk factors | Children < 6 mYoung parentsMultiple childrenPovertyChildren with special needs5,4 | Children > 3 years19males8 | Hereditary diseasesConsanguinity20 |
| Associated injuries | Rib and long bone fracturesOlder fracturesBlunt injuries in various locations generally "non-traumatising | Ecchymoses confined to the area of trauma | No other injuries |
| Retinal haemorrhages (RH) | Bilateral, multiple ad peripheral RH5 | Unilateral, single RH5 | Unilateral, single and posterior pole RH15 |
| Severity (apnoea and level of consciousness) | +++5 | + | + |
| Sequelae | +++5 | +/- | +/- |
| Underlyikng disease | No | No | Benign external hydrocephalus +/- macrocraniaMetabolopathies (glutaric aciduria type 1)Coagulopathies (haemophilia A, B and Von Willebrand factor)Neonatal IBDIntracranial vascular malformations (aneurysms and venous sinus thrombosis)Infections (meningitis, sepsis)Other rare genetic diseases |
Summary of clinical criteria for the main causes of acute spontaneous subdural haematoma.
| ASH probability | RH | Neuroimage | Diagnostic test | |
|---|---|---|---|---|
| Haemophilia A | 1/1.1 million | Infrequent, single and posterior pole aneurysms | No specific changes | Haemogram and PT |
| Haemophilia B | ¼.8 million | |||
| vW factor | 1/59,000 | |||
| Metabolopathies | 20%–30% | Very infrequent and in the posterior pole | Cortical frontotemporal atrophy, alterations in basal ganglia and widening of temporal opercular area | Neonatral screening and urine analysis |
| Aneurysms | 4%–4.6% | Severe and highly extensive | Visualisation of aneurysm and subarachnoid haemorrhage | CT |
The clinical picture of a subdural haematoma is often non-specific and with a highly broad spectrum of severity, which complicates its diagnosis in the emergency department. In most cases, the symptoms appear immediately after the injury, whether intentional or accidental.21 Some of the most characteristic symptoms are: drowsiness, low interaction, irritation, and decreased appetite.5,14 In severe cases, there may be severe alteration of the level of consciousness with seizures and respiratory distress or apnoea.21,22 The clinical diagnosis of ASH always requires a thorough physical examination and a multidisciplinary approach.5 Despite its non-specific symptomatology, worst prognostic criteria such as epileptic seizures and apnoea are significantly more associated with traumatic causes, in particular intentional trauma,23,24 with positive-predictive values (PPV) for intentional trauma of 66% and 93% respectively25 (Table 1).
Anamnesis and physical examinationIt is important to carry out a correct anamnesis of the circumstances surrounding the onset of the clinical condition. This essentially involves the account of the parents or legal guardians, as in cases of child abuse there are often inconsistencies between the testimony of the facts and the severity of the injuries presented.26 It is also important to take into account the child's age and stage of psychomotor development to establish the plausibility of the events, as well as to pay special attention to the description of minor blows, falls from a low height and unwitnessed falls.5 Low falls do not usually result in serious injury in most children, as only 1/1 000 000 of these falls can result in a fatal injury.5,26
Another element to take into account is the obstetric history, in this sense, it has been described that up to 50% of newborns may have small foci of ASH5 even in normal vaginal delivery and, in particular, in instrumented deliveries (vaccum and fórceps).8 These bleedings are usually asymptomatic, mild, and remit on their own by 4 weeks.8 In these patients and in half of the cases (52%), they may be accompanied by RH but most remit within 10 days.5 It has been questioned whether these birth bleeds can have medium- or long-term repercussions, causing spontaneous rebleeding or in the face of minor trauma, which greatly complicates the medico-legal assessment of the case. At present, there is no consensus on this issue, but it is strongly recommended that obstetric history be taken into account.12,14,27
Together with anamnesis, physical examination is another essential element for the correct aetiological diagnosis of ASHs. The examination allows us to detect possible injuries associated with child abuse, such as blunt injuries in generally "non-traumatisable" locations, as well as suspected bone fractures. In addition, a thorough examination allows an assessment of the child's general condition, as well as the recognition of risk factors for child abuse. Some of the main risk factors that have been described are: children younger than 6 months, young parents, multiple children, poverty, and children with special needs.4,5
Assessment of retinal haemorrhagesFunduscopy is essential for the correct diagnosis of ASHs.5,6,28 Morphologically, multiple, peripheral retinal and/or bilateral RH are highly suggestive of child abuse.27 On the other hand, less severe, unilateral, single, and predominantly posterior pole RH have been described in cases of spontaneous ASH associated with pathological processes such as meningitis, cerebral hypoxia, coagulopathies, and severe metabolopathies.9,12,28 Despite these general differentiating features, it should be noted that there is no pathognomonic sign in the morphology of the RH for the aetiological diagnosis, particularly in cases of intentional trauma.29
Other examinationsAlthough less common, there are numerous non-traumatic causes of infantile ASHs8 (Table 1). These causes include intracranial malformations, neonatal hypoxic–ischaemic encephalopathy, haematological, and coagulation disorders, brain infections, metabolic disorders, and a multitude of rare genetic diseases. Of these, the only coagulopathies associated with spontaneous ASHs are haemophilia A and B, and von Willebrand factor deficiency, with a frequency of spontaneous ASHs of 1/1.1 million, 1/4.8 million and 1/59 000, respectively30 (Table 2). These coagulopathies, since they can easily cause ecchymosis, bruising, and RH,8 are among the most likely to be mistaken for child abuse. A basic blood test with coagulation study allows a first orientation of the possible predisposing factors to bleeding.6 Among rare metabolic diseases, the most important is glutaric aciduria type I, which has a prevalence of 1/100 000 newborns and it is estimated that 20%–30% may present with ASH. Currently, this disease is part of neonatal screening in many countries31,20 (Table 2). If the suspicion of these rare diseases is high, and basic laboratory tests do not help in their diagnosis, it is recommended to request a specific study for these diseases.
Radiological diagnosisTable 3 contains a summarised form of radiological tests and the most significant findings associated with the aetiological ASH diagnosis.
Radiological–pathological diagnostic criteria for different types of acute subdural haematoma
| Intentional traumatic ASH | Accidental traumatic ASH | Spontaneous ASH | |
|---|---|---|---|
| ASH (computed tomography) | Multiple ASHs with mixed densities or single ASH of mixed density21 | Homogeneous or mixed hyperintense ASH within 48 h11 | Homogeneous or mixed ASHs |
| Skull fracture (computed tomography) | Simple linear parietal fractureComplex fractures32 (comminuted, diastatic, depressed)Brain contusions without fracture (shaken baby syndrome)8 | Simple linear parietal fracture19High-energy trauma: comminuted and depressed fractures | No fracture |
| Brain parenchymal lesions (MRI) | Cortical contusionParenchymal lacerationsDiffuse axonal injury and IBD | Localised cortical contusion | No contusionPosssible IBD |
| Plain X-ray of long bones | Rib, long bone and metaphysary fractures | No other fractures/fractures explained by the fall or blow | No fractures |
| Bone scintigraphy | Old fractures | No old fractures | No old fractures |
Computed tomography (CT) of the skull is the test of choice in cases of traumatic brain injury and is useful to observe cranial fractures and acute bleedings.11
Neuroimaging is particularly useful to establish the age of ASHs, especially when using a combination of CT and magnetic resonance imaging (MRI).21 Haemoglobin metabolism can distinguish acute (<10 days) from sub-acute (2–3 weeks) and chronic (>3 weeks) ASHs. Acute ASHs, typically associated with accidental trauma, will manifest as hyperintense and homogeneous lesions. In contrast, sub-acute and chronic ASHs will manifest as isointense and hypointense lesions, respectively.21
According to Adamsbaum etal.,21 in more than 50% of cases, the perpetrators of the abuse have admitted that it was a recurrent event. In these cases, multiple haemorrhagic lesions, in different foci and with mixed densities, are likely to be found. The presence of this finding indicates the existence of lesions of different ages, a fact highly suggestive of a repeated intentional mechanism, where the child has been exposed to successive episodes of trauma and/or shaking.11,33 However, it should also be noted that this finding should not be considered pathognomonic of child abuse, as they can appear during the first 48 h of an accidental or spontaneous bleeding, due to the inflammatory changes in the brain.21
An important element related to the aetiology of ASHs is the location of the ASHs. No consensus has yet been reached on whether there is a specific location of ASHs in child abuse,33 but it can be affirmed that in accidental, intentional, and birth-related cases, the most common locations are: supratentorial, in the frontoparietotemporal convexities, along the posterior interhemispheric fissure, and in the posterior fossa.8,16,33,34
Another valuable tomographic finding is the pattern of cranial fractures.8 The literature shows that the single linear parietal fracture is the most frequent in both intentional and accidental injuries.16,20,33 On the other hand, multiple, bilateral fractures that cross cranial sutures, comminuted, diastases, and depressed fractures should raise suspicion of intentional injury,32 however, there is not enough scientific evidence to be able to state this association with certainty.8,16,32In addition to bone fractures, there may be lacerations or contusions of the brain parenchyma. The existence of these contusions, without evidence of external trauma, is highly suspicious of an intentional mechanism due to shaking,8 and the presence of multiple foci of cerebral contusion is also suspicious of intentionality and repetitiveness of the trauma, especially when associated with ASH with different signal intensity (evolution).14,32
With regard to spontaneous ASH (Table 2), the diagnosis of certainty by means of neuroimaging is complex, as it is a diagnosis of exclusion, and must be made on the basis of the overall results. However, there are some conditions such as benign external hydrocephalus (BED) with or without macrocrania, which have been recognised as positive signs for the diagnosis of spontaneous ASH.8,14
External hydrocephalus is considered a benign entity caused by haemodynamic imbalance of an immature brain, and manifests on CT scan as isointense dilatation of the subarachnoid spaces, subaracnoideos.35 It is more common in boys, should stabilise by 1 year of life and may result in weakness of the dura mater. For this reason, it is suggested that these patients may present with ASH more easily, and in some cases even without trauma or minor trauma.8,36
Bleeds attributed to a BEH are usually asymptomatic or manifest primarily as a macrocrania (head circumference > p 95 or > 2 SD). On CT, they are evident as moderately hyperintensive bleeding.8 According to a cohort,35 only 5.6% of patients with BEH and macrocrania presented with ASH, so they point out that, although they may be slightly predisposed to these bleedings, a study of childhood abuse is necessary.37
The relationship of isolated macrocrania with ASH is not yet sufficiently studied, but given that it may be associated with old bleeds accompanied by inflammation and brain injury,12,16,34 it is very important to systematically screen for child abuse.
Magnetic resonanceAlthough MRI is not the test of choice in acute traumatic brain injury, it provides a higher resolution to observe the brain parenchyma and diagnose small bleedings,8 in this sense, several authors defend its combined use when there is a high suspicion of child abuse.5,18
The most frequent parenchymal lesions in cases of child abuse are diffuse axonal injury,38 secondary to trauma or shaking, and hypoxic–ischaemic encephalopathy (HIE), which is believed to be multifactorial in cause.18 Encephalopathy resulting from these lesions can be seen on MRI as cerebral oedema and vascular congestion.14,18,33 Other lesions such as bridging vein thrombosis21 have been associated with shaken baby syndrome, and can be seen as rounded clots at the cerebral vertex.In cases of spontaneous subdural ASH due to glutaric aciduria type 1, MRI plays a major role as there are disease-specific parenchymal abnormalities such as cortical frontotemporal atrophy, basal ganglia alterations, and widening of the temporal opercular area (Sylvian fissure), for which MRI offers high diagnostic value.20
Lesions in the brain parenchyma appear to have less weight in the differential diagnosis than HSAs, but are of great prognostic value. The presence of diffuse brain inflammation either secondary to IBD or diffuse axonal injury due to trauma is associated with worse outcomes as it may involve the cardiorespiratory centre.18,39
Plain radiography and scintigraphyThe presence of fractures in the posterior rib arches, metaphyseal, or diaphyseal fractures in the long bones is highly suggestive of child abuse. Their diagnosis can be made perfectly well by plain radiography. On the other hand, finding old bone fractures or fractures in different stages of development on scintigraphy is also highly suggestive of child abuse.8,21,25
Anatomopathological diagnosisViolent or suspicious deaths are a criterion for medico-legal autopsy.17 Mortality due to child abuse is estimated to be as high as 15%–38%.14,15 Post-mortem analysis provides findings analogous to imaging tests. In fact, these autopsies are often guided by neuroimaging findings, as well as clinical and care data in order to complement, expand, and ultimately clarify the circumstances of death.
The most frequent findings in cases of intentional trauma in the context of abuse are peridural haematomas, IBD, cerebral oedema, subcortical lacerations, soft tissue bleeds adjacent to the spinal cord, RH, and haemorrhages of the optic nerve sheath, contusions both on the scalp and the rest of the body, as well as the presence of fractures (skull, ribs, long bones) and visceral lacerations as a consequence of polytrauma40 (Table 3).
From a medico-legal point of view, the diagnosis of traumatic ASH in the context of child abuse has serious criminal consequences that make this diagnosis one of the greatest challenges for the forensic physician. Consequently, reliable diagnostic algorithms are required to provide elements of certainty in decision-making. Although there is no data from post-mortem analysis that can be considered specific to child abuse, there are some findings that can help the professional to make a diagnosis. In this sense, haemorrhage of the optic nerve sheath17 or evidence of rupture of the bridging veins,41 accompanied by the appropriate context such as the clinical triad, an inconsistent history and above all the association of other fractures, especially of long bones or ribs, are findings that are highly suspicious, if not indicative, of child abuse.17
DiscussionThe aetiological diagnosis of ASH in children is a clinical challenge for the different specialists involved in the management of the patient. These haematomas are frequently of traumatic aetiology. However, differential diagnosis with spontaneous haematomas is raised and, within traumatic haematomas, the main diagnostic challenge is to differentiate between accidental trauma and intentional trauma in the context of child abuse. The important medico-legal consequences of the diagnosis of intentional traumatic haematoma mean that solid elements of diagnostic certainty are necessary for all professionals involved in the diagnostic assessment of the patient. Analysis of the most recent literature shows that, in order to solve the challenge of the differential diagnosis of ASH, most authors focus on comparing the clinical features of the 2 most frequent and potentially serious causes: child abuse and accidental trauma. In contrast, it is a difficult task to find literature related to the spontaneous causes of ASH, with few articles on its pathogenesis, most of them being isolated cases reported by different authors. In this sense, the definition of spontaneous causes of childhood ASH is unclear in the literature, although it is clear that it is a diagnosis of exclusion. Although it is true that spontaneous causes represent a minority, we propose that the differential diagnosis of ASH should be individualised in each case and, if possible, based on well-defined criteria of suspicion or probability.
The literature review shows that there is currently no algorithm that clearly defines the aetiological diagnosis of ASH. This probably explains why up to one-third of patients with an intentional injury receive an initial misdiagnosis of spontaneous or accidental injury, and 40% may suffer a delay of more than 1 week in their diagnosis.5 On the other hand, as Gabaeff9 has shown, there are also cases diagnosed as child abuse when in fact there was a spontaneous cause as an explanation. In order to try to avoid these false positives and negatives, we believe it is important to individualise the diagnosis, taking into account both traumatic and spontaneous causes.
Some of the most relevant contributions on this differential diagnosis are those made by Vinchon etal.12 and Maguire et al.,13 who establish a positive-predictive value for the diagnosis of child abuse through a set of clinical criteria. Vinchon et al. established 3 criteria: ASH, severe RH, and absence of impact, with a positive-predictive value of .685, .961, and .830, respectively. Of the 3 findings, the authors note that only severe RH without ocular trauma is specific for child abuse. When all 3 criteria are present simultaneously, specificity increases to 100%, but with only 24.4% sensitivity.
More recently Maguire et al. conducted a study with independent data on 1053 patients participating in 6 different studies, all of whom were under 3 years of age and of whom 348 were diagnosed with child abuse. These authors extended the criteria of Vinchon et al. by defining 6 clinical criteria: apnoea, RH, fractures of the skull, ribs and long bones, epileptic seizures, and finally cranial or vertebral haematomas. According to the association of these 6 criteria, they established positive predictive values for the diagnosis of abuse. When rib fractures or RH are present with any of the other criteria the PPV rises to > 85%. Likewise, any combination of 3 or more of the above criteria also amounts to a PPV of > 85%. This classification offers for the first time the option to support the clinical diagnosis with estimated probability values. However, the same authors conclude that this model is a first approximation, which should be further developed through a large prospective study.
It is interesting to note that, as these studies show, RH is the clinical data with the strongest association with child abuse. In a recent meta-analysis, it was found that 78% of children with intentional trauma have RH and 83% of them are bilateral. In contrast, only 5% of children with accidental trauma have been reported to have RH and only 8.3% bilateral.5,27
Another criticism that can be made based on the results obtained is that the studies discussed so far do not include the possible contributions of neuroimaging in their diagnostic criteria. Radiology, particularly CT, is of great value in the differential diagnosis of ASH, revealing data on the mechanism of injury, associated lesions, and the timing of haematomas. Hyperintense and homogeneous ASHs are present in 74% of accidental trauma cases, and in only 33% of cases of intentional trauma.11 ASHs with mixed densities are more associated with cases of child abuse, but can also appear in the first 48 h of an ASH due to accidental or spontaneous trauma.8,14,33 Other authors suggest that the most specific finding in repetitive child abuse is the presence of multiple ASHs in different locations and with different densities.8,21,28,34 Finally, with respect to MRI and compared to CT, its high sensitivity for detecting and analysing parenchymal lesions gives it greater prognostic than diagnostic weight, although for this reason it is also very useful for the diagnosis of spontaneous ASH and as a combined test with CT when there is a high suspicion of abuse.
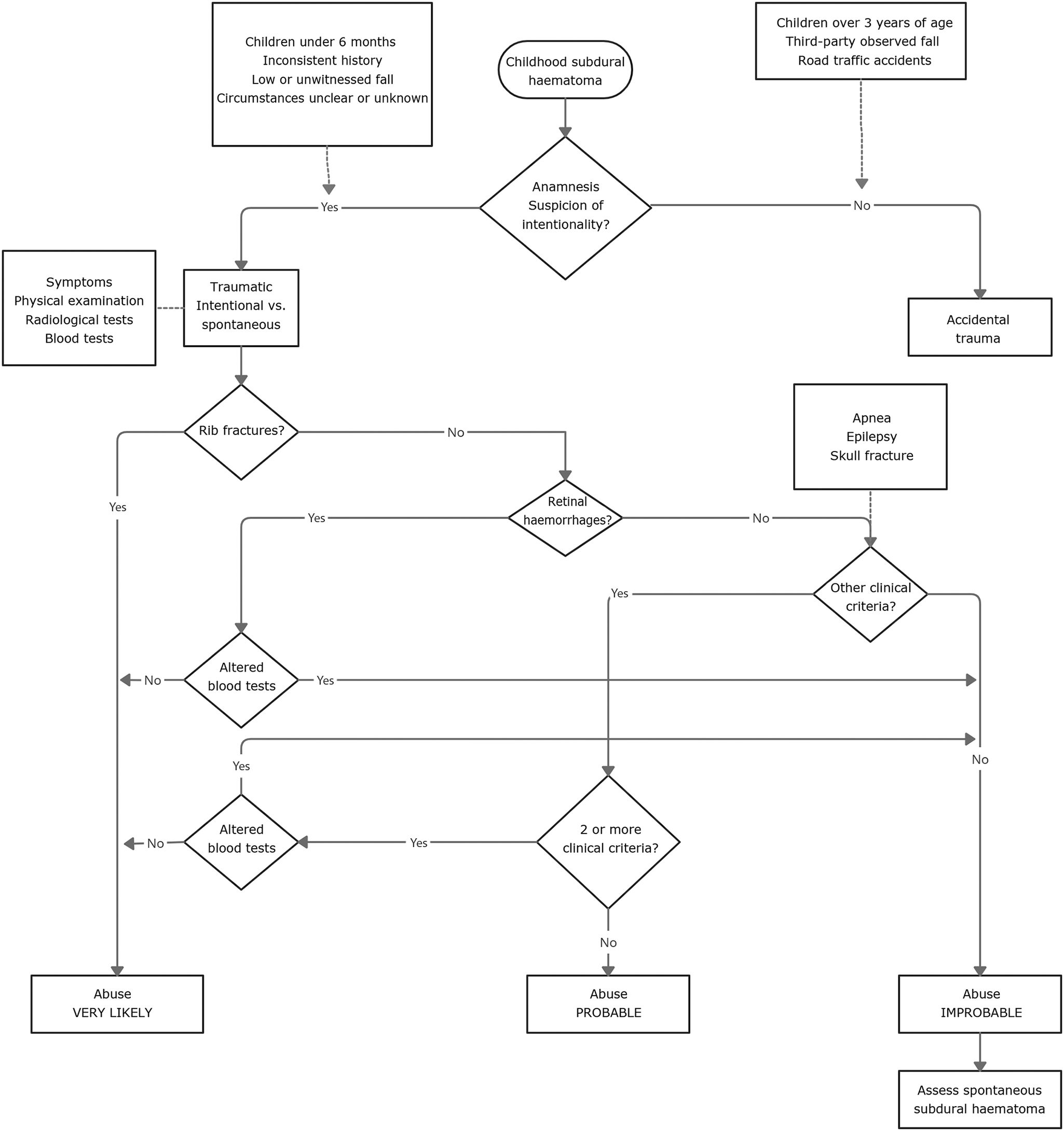
Considering the main diagnostic elements collected in the literature reviewed and outlined in the previous section, it is possible to draw up a proposed aetiological diagnostic algorithm for childhood ASHs (Fig. 1). The differential diagnosis should always be based on a thorough anamnesis. The anamnesis should be considered suspicious of intentionality when the parents'/guardians' account of the events is inconsistent or inconsistent with the injuries presented.26 In the case of an anamnesis suspicious of intent, the aim should be to rule out intentional trauma in the context of abuse. On the other hand, accidental traumatic ASH is diagnosed directly in the case of trauma that is certainly witnessed by a third party or in the case of traffic accidents.
Based on the updated criteria of Maguire etal.13 (apnoea, RH, fractures of the skull, ribs and long bones, epileptic seizures, and HSAs), a diagnostic sequence for intentional traumatic HSA can be established (Fig. 1). We propose adding to this protocol the systematic performance of a blood test aimed at detecting possible predisposing factors for spontaneous bleeding, i.e., the presence of different systemic diseases, mainly coagulopathies and metabolopathies.6 It is important to include this test in the protocols for the study of paediatric HSAs, as it can avoid false positives and help to diagnose a spontaneous cause in the first diagnostic steps of the case.
Regarding the different clinical and radiological–pathological findings associated with intentional traumatic HSA, the presence of rib fractures, which are highly prevalent in cases of abuse, can be considered as the first criterion for screening a possible case of this aetiology. This criterion is followed by the presence of RH and, depending on these, the presence of more or less 2 extra clinical criteria (apnoea, epilepsy, and skull fracture) until the final diagnosis is reached. Finally, we suggest concluding the diagnostic algorithm with 3 possible levels of probability for intentional traumatic HSA in the context of abuse: very probable, probable, and unlikely (Fig. 1).
ConclusionsThis paper has focused on the aetiological differential diagnosis of childhood ASH. The review carried out shows, firstly, the need to continue to deepen our knowledge of the pathophysiology of traumatic ASH, especially in cases of abuse by shaking and, secondly, the lack of consensus and systematisation when it comes to approaching the differential diagnosis of infantile ASH. The consequence of this lack of evidence is the high error rate and diagnostic delay in these patients. The lack of systematisation also limits the creation of protocols for action and diagnostic algorithms, as it prevents the availability of databases large enough to perform a meta-analysis and increase the level of scientific evidence.
Based on the main findings and the diagnostic criteria described in the most recent literature, a basic differential diagnostic algorithm is proposed. As a future line of work, it would be appropriate to use a retrospective cohort with a blinded observer, using as study variables the same 6 multidisciplinary criteria included in this algorithm. Finally, in order to reduce the subjectivity inherent in several of the diagnostic criteria, we propose to approach the final diagnosis of childhood ASH using fuzzy logic as a tool that would allow modelling the knowledge of one or several experts, and gain objectivity in diagnostic decision-making.
Please cite this article as: Cabrera Borotau G, Galtés I. Diagnóstico diferencial etiológico del hematoma subdural agudo en la edad pediátrica. Revista Española de Medicina Legal. 2022. https://doi.org/10.1016/j.reml.2021.12.001