The study of the post-mortem interval is an important element in the investigation of criminal acts. It is also one of the most difficult variables to quantify and establish. Various cadaveric phenomena are used to assess it, some of which are ocular, such as intraocular pressure. There are currently no reliable indicators of its behaviour over time.
Material and methodsProspective, longitudinal, descriptive, and analytical study. Ante-mortem and post-mortem intraocular pressure was measured in seriously ill patients with progression to death, performing descriptive analysis of variables and application of the student’s t test, χ2 and ANOVA.
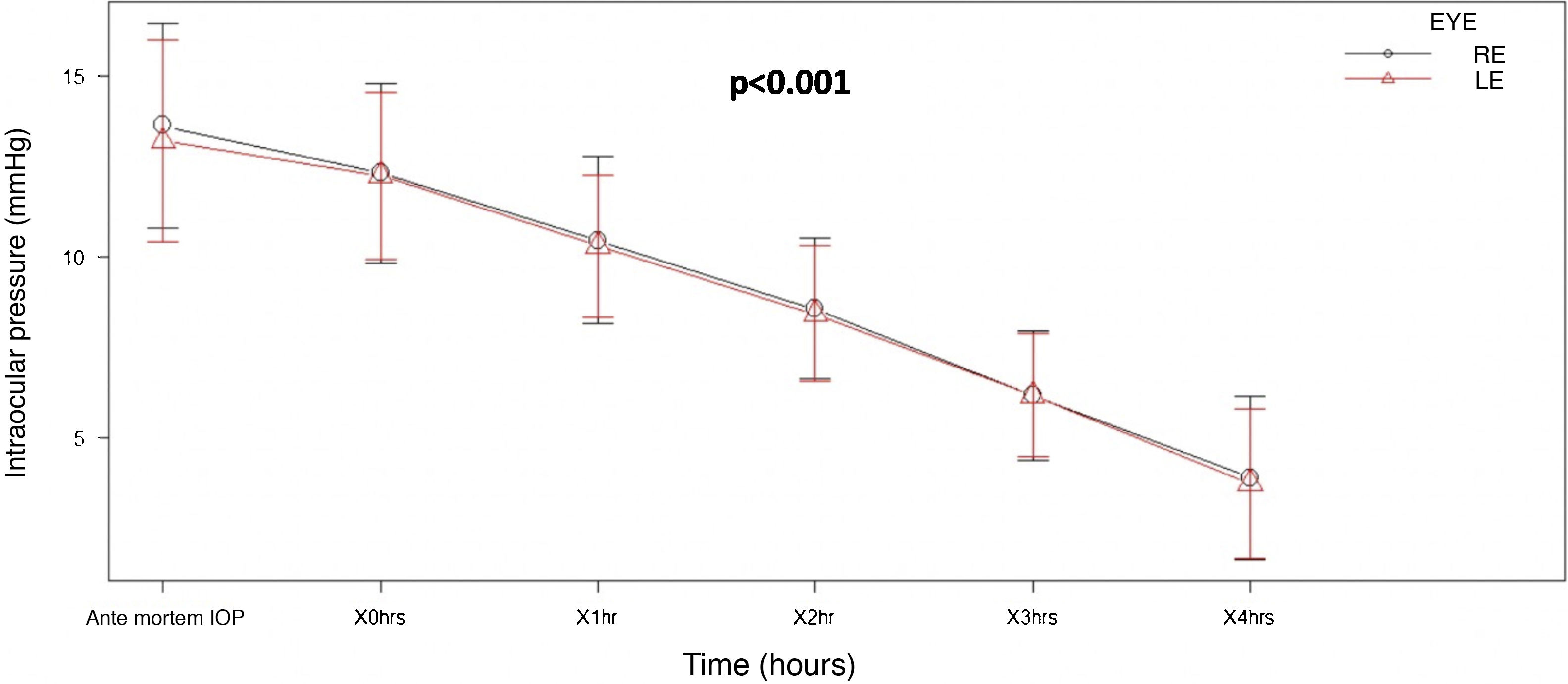
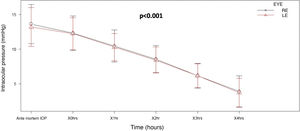
ResultsNinety-five eyes were studied, obtaining a mean ante-mortem intraocular pressure of 13.4mmHg, which progressively decreased during the four hours post-mortem, 10.4mmHg per hour, 8.48mmHg at 2h, 6.17mmHg at 3h, and 3.81mmHg at 4h. In the repeated measures ANOVA test, a clinically significant value of P<.001 was obtained.
ConclusionsIn this study, an inverse relationship was observed between post-mortem intraocular pressure and post-mortem interval, with a linear decrease over time of 2mmHg per hour. Therefore, the measurement of intraocular pressure is a fast and reproducible method, which can be used as an additional element to estimate the post- mortem interval.
El estudio del intervalo post mortem es un elemento importante en la investigación de hechos delictivos, además, es una de las variables más difíciles de cuantificar y establecer. Para su valoración, se utilizan diversos fenómenos cadavéricos, algunos de ellos oculares, como la presión intraocular, sin existir actualmente indicadores confiables de su comportamiento a través del tiempo.
Material y métodosEstudio prospectivo, longitudinal, descriptivo y analítico. Se midió la presión intraocular ante mortem y post mortem de pacientes gravemente enfermos con evolución a muerte, realizándose análisis descriptivo de variables y aplicación de las pruebas t de Student, χ2 y ANOVA.
ResultadosSe analizaron 95 ojos, obteniendo una presión intraocular media ante mortem de 13,4mmHg, la cual disminuyó progresivamente durante las 4horas de seguimiento post mortem: la presión intraocular media fue de 12,3mmHg a la hora cero post mortem, 10,4mmHg a la hora, 8,48mmHg a las 2horas, 6,17mmHg a las 3horas y 3,81mmHg a las 4horas. En la prueba de ANOVA de medidas repetidas se obtuvo un valor clínicamente significativo de p<0,001.
ConclusionesEn este estudió se observó una relación inversa entre la presión intraocular post mortem y el intervalo postmortem, con una disminución lineal con el tiempo de 2mmHg por hora, por lo que la medición de la presión intraocular es un método rápido y reproducible, que puede ser utilizado como elemento adicional para estimar el intervalo.
Determination of the post-mortem interval (PMI) is equivalent to the time that has passed between death and the moment the cadaver was discovered. This is a critical step in the investigation of homicides, although the determination of this time is still one of the major challenges in medical-legal investigation; it is also one of the hardest values to quantify and establish1, due to the large number of variables which it is not possible to control, and which influence changes during this time.2 These factors include previous pathologies, atmospheric characteristics at the time of death and factors associated with the scene of death. Moreover, the majority of investigations which seek to establish a method for estimating the PMI do so under controlled circumstances, in a similar environment and with similar diagnoses of death, thereby making such methods unsuitable for use in real situations.
Currently, cadaveric phenomena such as changes in the skin, temperature, cadaveric rigidity and more sophisticated methods such as metabolic changes3 are used to estimate the PMI; ocular cadaveric phenomena too take place in this time interval, such as the fall in intraocular pressure (IOP)4–7. However, none of these estimation methods analyse its relationship with the immediate PMI in a practical way8–10.
IOP derives its balance from the production and reabsorption of aqueous humour11. Few studies have been conducted to evaluate the evolution in changes in IOP in cadavers and their association with the PMI. Balci et al. established that IOP falls quickly after death, identifying an IOP of 20–25mmHg after 30 at 120min. PMI, 15–20mmHg at 120–240min. PMI, 10–15mmHg at 240–360min. PMI, 5–10mmHg at 360–480min. PMI and less than 5mmHg after 480min. PMI; however, they did not evaluate IOP ante mortem, the exact PMI and the cause of death, all of which are highly important variables12.
Material and methodsA prospective, longitudinal, descriptive, analytical and observational study was performed. It analysed IOP behaviour ante mortem (AM) and post mortem (PM), in patients of both sexes and with no age limit, in the Hospital Central Dr. Ignacio Morones Prieto in the City of San Luis Potosí, Mexico. The patients were in a critical condition and hospitalised in the adult or paediatric intensive care units, the emergency department, internal medicine and surgical wards.
The sample was selected in a non-probabilistic way by convenience, and its size was calculated using a logistic model, including the following variables; PM IOP Final hour 4∼AM IOP+PM IOP hour 0+PM IOP hour 1+PM IOP hour 2+PM IOP hour 3. The result was 5° of liberty and a sample of 50–100 patients. This research was approved by the Ethics Committee and prior to commencing the informed consent of the responsible family member was obtained for each one of the patients included.
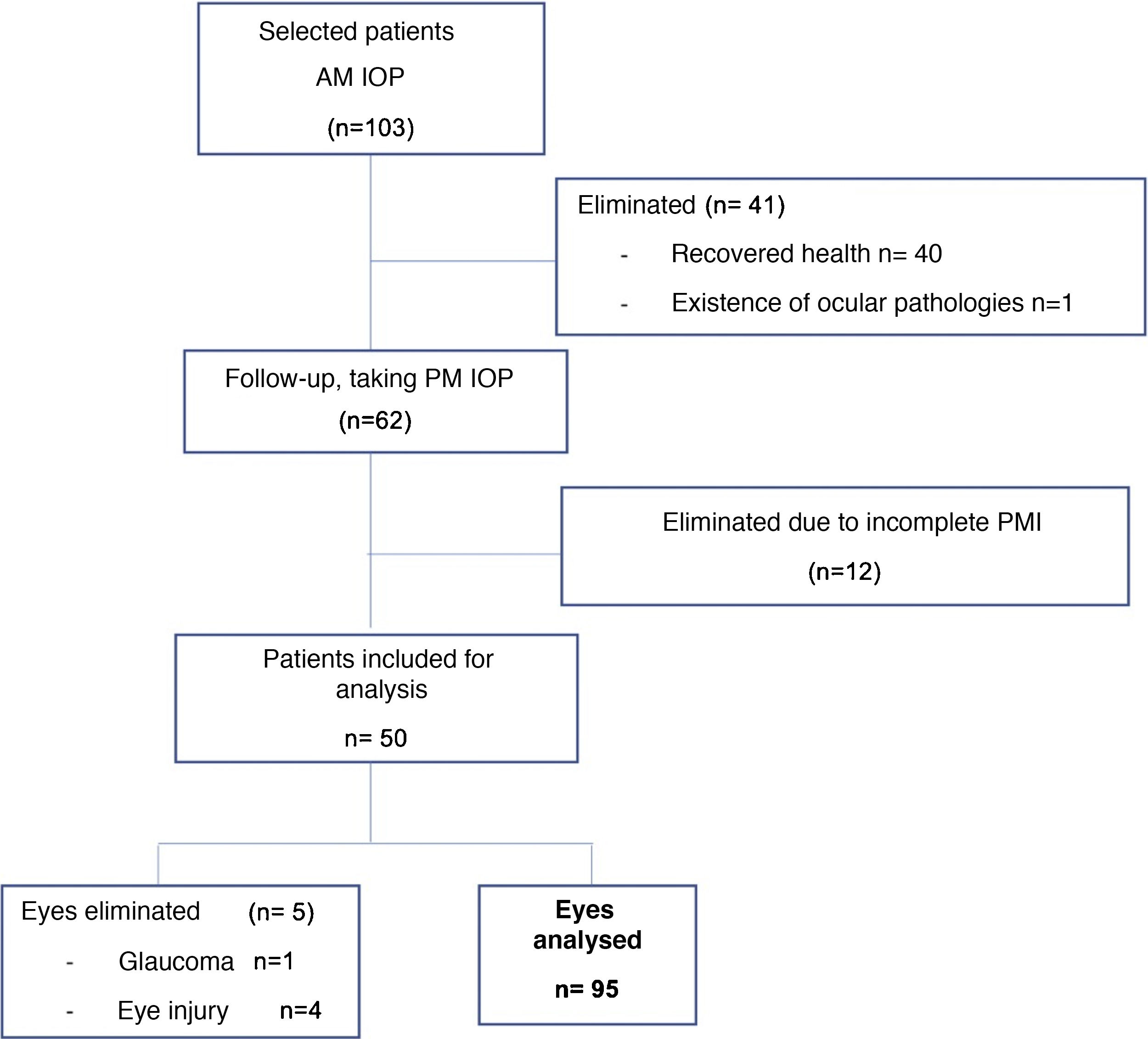
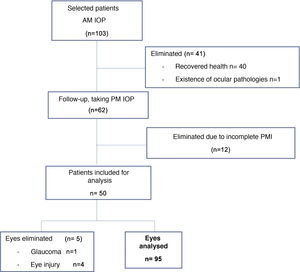
Patients in a critical state with a prognosis of death were included. Patients with a history of ocular pathology were excluded, including: glaucoma, ocular hypertension, uveitis, retinal detachment, traumatic eye injuries, a history of eye surgery in at least 2 years, patients with an unknown time and cause of death and those whose health recovered (Fig. 1).
The level of interobserver agreement on IOP measurements was checked beforehand using the intraclass correlation coefficient, giving a value of 0.94 CI [0.86–0.98]. A portable applanation tonometer was used to measure the IOP (TONO-PEN® XL). One measurement was obtained AM and other 4h PM; the AM IOP was taken at the patient’s bed where they were hospitalised, and the subsequent PM IOP was taken in the cadaver reception area.
The results were analysed by means of descriptive statistics of the variables using version R 3.5.3 of Rcmdr 2.6–0 software, with a confidence level of 95%13. Central tendency measures were used and continuous variables were expressed as an average (±standard deviation) or median [IQ range] depending on how the variables were distributed, and categorical variables were expressed as percentages.
Bivariate analysis using the Student t-test and the Kruskal–Wallis test were used for the inferential statistics of continuous variables, depending on how the variables were distributed; the Chi-squared test or Fisher’s exact test were used for the categorical variables.
An ANOVA test of repeated measurements was subsequently performed to obtain the behaviour of the IOP in mmHg, with the time that had passed after death. A value of P<.05 was considered to be significant.
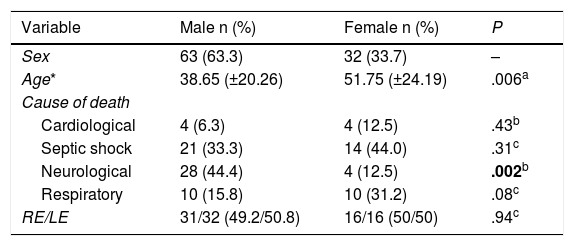
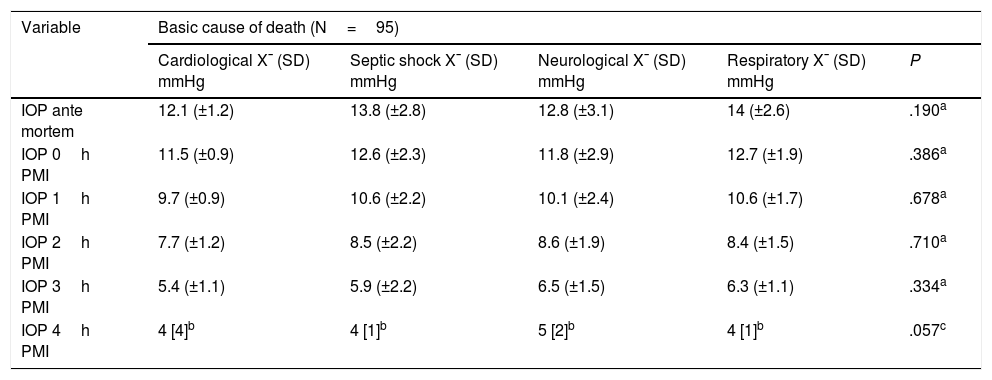
ResultsFifty patients were included and 5 eyes were excluded due to identified ocular pathologies; a total of 95 eyes were analysed (Fig. 1). 63 of the eyes analysed were male (64.9%) and 32 were female (33.0%), 47 were right eyes (49.5%) and 48 were left eyes (50.5%). The average age was 43.2 years, with a SF of ±22.4; the causes of death cited on the death certificates were neurological in origin in 32 cases (33.7%), septic shock in 35 cases (36.8%), cardiogenic shock in 8 cases (8.4%) and respiratory processes in 20 cases (21.1%) (Table 1).
Basal characteristics of the population studied.
| Variable | Male n (%) | Female n (%) | P |
|---|---|---|---|
| Sex | 63 (63.3) | 32 (33.7) | – |
| Age* | 38.65 (±20.26) | 51.75 (±24.19) | .006a |
| Cause of death | |||
| Cardiological | 4 (6.3) | 4 (12.5) | .43b |
| Septic shock | 21 (33.3) | 14 (44.0) | .31c |
| Neurological | 28 (44.4) | 4 (12.5) | .002b |
| Respiratory | 10 (15.8) | 10 (31.2) | .08c |
| RE/LE | 31/32 (49.2/50.8) | 16/16 (50/50) | .94c |
RE: right eye; LE: left eye.
Statistically significant figures are in bold type.
The average AM IOP obtained was 13.4mmHg [SD±2.80], 12.3mmHg immediately PM, 10.4mmHg at one hour PMI, 8.48mmHg at 2h PMI, 6.17mmHg at 3h PMI and 3.81mmHg at 4h PMI.
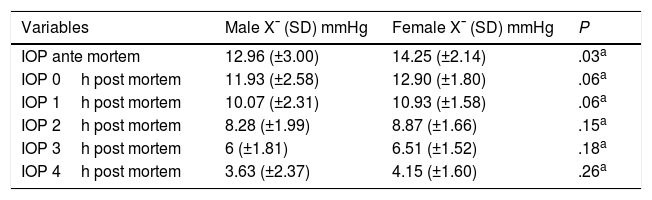
Bivariate analysis of IOP identified a higher AM IOP in women than in men, with a statistically significant differences (P=.03); as well as a statistically significant differences between IOP in men and women when the cause of death involved neurological processes, as it was higher in men (P=.002). The other measurements were not found to be statistically significant (Table 2).
AM IOP and how it changes PM according to sex.
| Variables | Male X¯ (SD) mmHg | Female X¯ (SD) mmHg | P |
|---|---|---|---|
| IOP ante mortem | 12.96 (±3.00) | 14.25 (±2.14) | .03a |
| IOP 0h post mortem | 11.93 (±2.58) | 12.90 (±1.80) | .06a |
| IOP 1h post mortem | 10.07 (±2.31) | 10.93 (±1.58) | .06a |
| IOP 2h post mortem | 8.28 (±1.99) | 8.87 (±1.66) | .15a |
| IOP 3h post mortem | 6 (±1.81) | 6.51 (±1.52) | .18a |
| IOP 4h post mortem | 3.63 (±2.37) | 4.15 (±1.60) | .26a |
AM: ante mortem; IOP: intraocular pressure; PM: post mortem; SD: standard deviation; X¯: average.
The continuous variables were analysed using the Student t-test, and a statistically significant difference in pressure was found between the right and left eyes. Although no significant difference was found when IOP and its relationship with the causes of death was analysed, it should be pointed out that those patients who died due to a cardiological cause had lower IOPs than the patients who died from other causes (Table 3).
AM IOP and how it changes PM according to cause of death.
| Variable | Basic cause of death (N=95) | ||||
|---|---|---|---|---|---|
| Cardiological X¯ (SD) mmHg | Septic shock X¯ (SD) mmHg | Neurological X¯ (SD) mmHg | Respiratory X¯ (SD) mmHg | P | |
| IOP ante mortem | 12.1 (±1.2) | 13.8 (±2.8) | 12.8 (±3.1) | 14 (±2.6) | .190a |
| IOP 0h PMI | 11.5 (±0.9) | 12.6 (±2.3) | 11.8 (±2.9) | 12.7 (±1.9) | .386a |
| IOP 1h PMI | 9.7 (±0.9) | 10.6 (±2.2) | 10.1 (±2.4) | 10.6 (±1.7) | .678a |
| IOP 2h PMI | 7.7 (±1.2) | 8.5 (±2.2) | 8.6 (±1.9) | 8.4 (±1.5) | .710a |
| IOP 3h PMI | 5.4 (±1.1) | 5.9 (±2.2) | 6.5 (±1.5) | 6.3 (±1.1) | .334a |
| IOP 4h PMI | 4 [4]b | 4 [1]b | 5 [2]b | 4 [1]b | .057c |
AM: ante mortem; IOP: intraocular pressure; PM: post mortem; PMI: post mortem interval; SD: standard deviation; X¯: average.
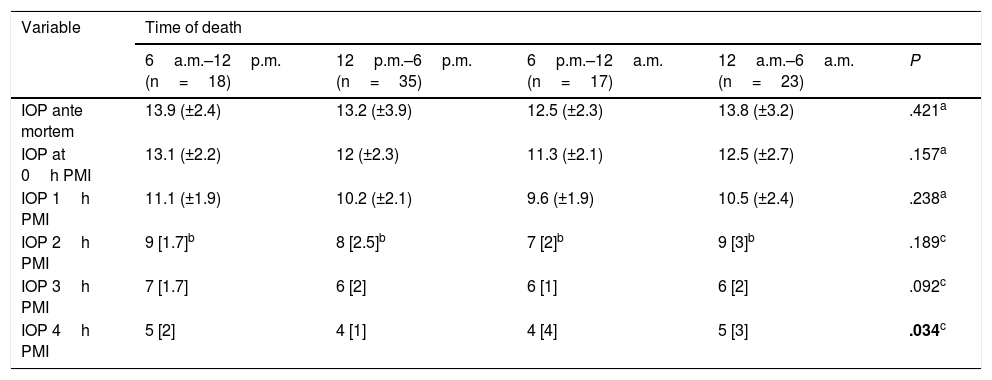
When patients were grouped according to time of death, a statistically significant differences was found when IOP was taken 4h after death, with higher measured IOP in patients who died in the early morning and morning (12a.m.–12p.m.) (Table 4).
AM IOP and how it changes PM according to time of death.
| Variable | Time of death | ||||
|---|---|---|---|---|---|
| 6a.m.–12p.m. (n=18) | 12p.m.–6p.m. (n=35) | 6p.m.–12a.m. (n=17) | 12a.m.–6a.m. (n=23) | P | |
| IOP ante mortem | 13.9 (±2.4) | 13.2 (±3.9) | 12.5 (±2.3) | 13.8 (±3.2) | .421a |
| IOP at 0h PMI | 13.1 (±2.2) | 12 (±2.3) | 11.3 (±2.1) | 12.5 (±2.7) | .157a |
| IOP 1h PMI | 11.1 (±1.9) | 10.2 (±2.1) | 9.6 (±1.9) | 10.5 (±2.4) | .238a |
| IOP 2h PMI | 9 [1.7]b | 8 [2.5]b | 7 [2]b | 9 [3]b | .189c |
| IOP 3h PMI | 7 [1.7] | 6 [2] | 6 [1] | 6 [2] | .092c |
| IOP 4h PMI | 5 [2] | 4 [1] | 4 [4] | 5 [3] | .034c |
AM: ante mortem; IOP: intraocular pressure; PM: post mortem; PMI: post mortem interval; X¯: average.
Statistically significant data in bold.
A lineal fall in IOP was observed with increasing time after death. This fall amounted to 2mmHg per hour, and it was very similar in both eyes. ANOVA analysis of repeated measurements found a statistically significant difference (P<.001), when all of the IOP measurements taken were compared (Fig. 2).
DiscussionEstimation of the PMI is a highly important datum when investigating a death without witnesses. One of the phenomena observed after death is the process of cadaveric dehydration. This manifests at ocular level in the form of a fall in IOP, so that the results of this study of IOP behaviour and its PM follow-up show that when AM IOP is analysed, there is no statistically significant difference respecting cause of death and the time it occurred. It is only when AM IOP is compared in both sexes that the Student t-test identifies the existence of P=.03.
Although higher levels of IOP were found when death occurred during the early morning and morning, no statistically significant difference could be found here; nevertheless, this phenomenon was already known, as in living patients the secretion of cortisol increases IOP, which peaks at 6a.m.14,15, making it possible to explain our observation.
With respect to the evolution and behaviour of PM IOP, no statistically significant difference was found between the hours of follow-up except at a PMI of 4h; when this was analysed, depending on the time of death it had a P=.0342, so that the time of death may be a modifying variable after 4h PMI.
In our study the differences in IOP, as the PMI increased, were found to be statistically significant in the ANOVA analysis of repeated measurements, showing a lineal fall until 4h. This agrees with the findings and analysis of Balci et al. in 2010, who performed 100 measurements of IOP; however, the IOP obtained by them at 2–4h was the same or even higher than the average IOP in a living patient (15–20mmHg). This disagrees with the results of our study and with the ocular dehydration that is expected to occur hours after death; moreover, they did not examine whether any differences arose depending on the cause and time of death, sex or age. Nor do they mention the measuring instrument or instruments they used12.
As Riaz et al.1 describe, PMI indicators are essential in the inclusion of suspects in a trial, and the more indicators we have, the surer will be the sentence. There are many other markers, chiefly biochemical ones, which can help us to obtain the PMI with greater exactitude10,16–19. Nevertheless, it is necessary to take a tissue sample for this, as well as to use specialised laboratory instruments. This leads to a major increase in the cost of analysis, as well as delaying decision-making.
We followed-up IOP in our study for 4h PMI, although it is recommendable to do so over a longer time, with the aim of identifying the moment at which IOP becomes undetectable. This would enable the establishment of a highly valuable criterion for those who investigate crimes, so that they could estimate the PMI. Additionally, it should be pointed out that the cadavers were kept in a hospital environment where it would be difficult to include some of the variables which may modify IOP and which could arise in a judicial scenario; however, when IOP behaviour immediately post mortem is observed, this is able to allow us to make an estimation of the time that has passed since the moment of death.
New protocols can be introduced to include the modifying variables20–24. and to prepare corrective calculations for different scenarios. In the future it will be possible to obtain a model and corrective factors to calculate the PMI, including ocular tonometry.
ConclusionsThis study finds an inverse relationship between the fall in the PM IOP and the PMI. The longer the PMI, the lower the IOP, with a lineal fall over time at a rate of 2mmHg, with an average IOP of 12.3mmHg immediately PM, 10.4mmHg one hour PM, 8.48mmHg at 2h, 6.17mmHg at 3h and 3.81mmHg at 4h. The measurement of IOP by applanation tonometry is therefore simple, swift and economical, as well as easy to perform. Although it cannot be used as an exact method to determine the PMI, it is practical to use as an additional element, apart from the other cadaveric phenomena which are currently taken into account.
The limitations of this study were identified as the above-mentioned modifying variables of the IOP, as well as the impossibility of knowing the AM IOP or whether there are ocular pathologies which modify the IOP, the impossibility of using the technique if there are direct injuries to the eyes, and the limited time of follow-up which was analysed in this study.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the interns of all of the different specialities (internal medicine, paediatrics, surgery and pathology) who contributed to the recruitment of patients for this study.
We would also like to thank the administrative staff of Central Dr. Ignacio Morones Prieto Hospital, who permitted entry so that patients could be evaluated in the cadaver reception area.
We would also like to thank the family members of the patients and the patients studied, for their contribution to science.
Please cite this article as: Ramirez Ojeda SV, Rangel Charqueño MG, Hernandez Mier C. Tonometría ocular en pacientes críticos y su comportamiento. Rev Esp Med Legal. 2021;47:150–156.