People with schizophrenia and predominant negative symptoms (PNS) present a different clinical and functional profile from those without such symptomatology. Few studies have examined the risk factors and the incidence of PNS in first-episode schizophrenia patients (FES) and differentiating by sex. This study aims to assess prevalence, demographic and clinical characteristics related to PNS from early stages and to study if there are sex-specific features in terms of developing PNS.
MethodsIn a sample of 121 FES patients derived from a multicentre and naturalistic study, those who developed PNS at 12-months were identified. Environmental, clinical, functional, and cognitive ratings were examined longitudinally. Binary logistic regressions were applied to detect baseline risk factors for developing PNS at one-year follow-up.
ResultsIn the present FES cohort, 24.8% of the patients (n=30) developed PNS (20% of the women, 27.6% of the men). Compared to non-PNS (75.2%, n=91), at baseline, PNS group had more negative (t=−6.347; p<0.001) and depressive symptoms (t=−5.026; p<0.001), poorer premorbid adjustment (t=−2.791; p=0.006) and functional outcome (t=−2.649; p<0.001), more amotivation (t=−7.333; p<0.001), more expressivity alterations (t=−4.417; p<0.001), worse cognitive reserve (t=2.581; p<0.011), a lower estimated intelligent quotient (t=2.417; p=0.017), worse verbal memory (t=2.608; p=0.011), and worse fluency (t=2.614; p=0.010). Regressions showed that the premorbid adjustment was the main predictor of PNS in females (p=0.007; Exp(B)=1.106) while in males were a worse verbal memory performance (p=0.031; Exp(B)=0.989) and more alterations in the motivation domain (p=0.001; Exp(B)=1.607).
ConclusionsA different baseline clinical profile and notable risk factors differences in the development of PNS between males and females were found. Results suggest that sex may be an important confounder in studies comparing schizophrenia patients with predominant and non-predominant negative symptomatology.
Schizophrenia is a complex and multi-dimensional disorder and one of the most incapacitating conditions worldwide.1 The course of the disorder can be marked by psychotic episodes with positive symptoms, negative symptomatology, and cognitive impairment, which results in functional outcome impairment.2 Schizophrenia and first-episode psychosis are characterized by heterogeneity of symptoms, illness course, and psychosocial and pharmacological treatment response.
This variability in the clinical presentation at onset may be sex-related, as it has been well demonstrated that sex differences with respect to schizophrenia spectrum disorders are an important factor in the understanding of the heterogeneity manifestation and development of the illness).3,4 In this context, already from the first stages of the illness, men and women show different symptomatology and different levels of social functioning.5 It has been suggested than men tend to present more negative symptoms than women, whereas women show higher levels of depressive symptomatology. In addition, it has also been reported than females present more functioning unmet needs for care, but higher levels of insight into illness in comparison to males.5,6 A recent review and meta-analysis including thirty-five studies has also reported that men experienced more negative symptomatology than women, whereas women experienced more depressive symptomatology, had a lower prevalence of substance abuse and had higher functioning.7 Notwithstanding, prior literature on sex/gender differences in symptoms of schizophrenia have been inconclusive, due to the difficulties to differentiate between sex and gender in the different studies; that is, whether differences in clinical profiles are due to biologic sex differences or if gender may also play a role in these profiles.7 In this line, given the stability and validity of its definition, the study of the sex variable may help explain at least part of these phenotypical differences, a comprehensive summary of evidence on the early course of illness is necessary.3
Negative symptoms are a heterogeneous clinical construct that constitutes an independent core psychopathological domain of schizophrenia. This symptomatology can be stable or transient and is divided into primary, when they are intrinsic to the illness, or secondary, originated from confounding conditions (medication side effects, positive, affective symptoms, and/or environmental deprivation).8 Patients with the presence of primary and enduring negative symptoms (lasting>1 year) are said to meet the criteria for the deficit syndrome (DS) of schizophrenia, while those patients without these symptoms have non-deficit schizophrenia.9 Besides the DS, other concepts have been raised for the assessment of negative symptomatology, as predominant negative symptoms (PNS). This symptomatology is characterized by the presence of moderate-to-sever negative symptomatology that are more severe than co-occurring positive symptomatology.9 Nonetheless, general negative symptomatology is known to predict disease burden better than positive symptoms and worsened quality of life target.10,11 In this line, the study of risk factors associated to primary and persistent negative symptomatology has gained special relevance.12,13
Moreover, negative symptomatology is multidimensional, comprising five subdomains (anhedonia, avolition, asociality, blunted affect, alogia) with two higher-order dimensions: expression (EXP), and motivation and pleasure (MAP), both of which have more basic subordinate domains (MAP=anhedonia, avolition, asociality; EXP=blunted affect, alogia). Both factors represent separable treatment targets with distinct pathophysiology and different impacts on functional outcomes.9
Although most first-episode of schizophrenia (FES) patients may improve their symptomatology after antipsychotic treatment, many may continue to have long-term impairments in functioning and persistence of some symptomatology.9 The early identification of clinical, cognitive and socio-demographic features may be important in identifying subsets of patients with similar characteristics, facilitating personalized treatment approaches.14 Thus, identifying specific dimensions and risk factors that underlie the development of PNS from early stages of schizophrenia could improve the understanding and treatment of such invalidating symptomatology and the prevention of DS diagnose at long-term.11–13
A subtyping strategy according to meeting criteria for predominant negative symptomatology and according to the sex of the patients has been utilized to identify homogeneous clinical profiles from the first stages after the FES. We analyzed a cohort of FES patients to examine the potential risk factors that may play a role in the development of predominant negative symptoms in later stages. Our aims were (1) to analyze those potential clinical and socio-demographic predictors of PNS, and (2) to determine whether there were sex-differences for socio-demographic, clinical, and cognitive variables between PNS and non-PNS groups. We hypothesized that (1) there will be specific risk factors that predict the development of PNS, and (2) there will be distinct clinical risk factors between females and males in terms of developing PNS after one-year of evolution.
MethodsSubjectsData was collected from the naturalistic, multicentered, and coordinated project “Clinical and neurobiological determinants of second episodes of schizophrenia, a longitudinal study of first-episode of psychosis”, known as the ‘2EPs Project’, that arose to identify those clinical, environmental, and biological factors that predict a relapse within the first years after a first episode. Under the umbrella of CIBERSAM, 15 participating centers invited those patients with a suitable profile for the study. All the information about the 2EPs Project can be found elsewhere.14,15
Inclusion criteria for the 2EPs Project were: (a) have presented a FEP in the last 5 years and are currently in remission according to Andreasen criteria.16 Remission is achieved when the patient's Positive and Negative Symptom Scale (PANSS) score is 3 or less in 8 items (mild severity). Severity symptoms must be maintained for a minimum of 6 months and the patient must not have relapsed after the episode; (b) met diagnostic criteria according to DSM-IV for schizophrenia or schizophreniform disorder17; (c) aged between 16 and 40 years at the first evaluation; (d) ability to speak Spanish correctly; (e) signed informed consent.
Exclusion criteria were: (a) having experienced a traumatic brain injury with loss of consciousness; (b) an Intelligence Quotient (IQ)<70 and with significant difficulties/malfunctioning with adaptive processes, and/or (c) presenting somatic pathology with mental repercussion.
From the initial 222 patients recruited in the 2EPs, 121 (54.5%) adult patients completed the 12-month follow-up visits and were included in the analyses. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. All participants provided written informed consent before inclusion.
AssessmentsAt baseline, patients performed a complete evaluation that included: socio-demographic and substance use determination, clinical, functional, and premorbid adjustment scales, and pharmacological treatment records. At 12-month follow-up, clinical, functional, pharmacological treatment records and substance use determinations were collected again. Scales were administered by trained/expert clinical staff except for the self-administered scales.
Socio-demographic, clinical and substance use assessmentDemographic data were collected through semi-structured interviews. Diagnoses were determined according to the DSM-IV-TR.17 Sex, age, and age at onset of the illness were collected along with the duration of untreated psychosis (DUP). The DUP was defined as the number of days elapsed between the onset of positive psychotic symptoms and the initiation of the first appropriate treatment for psychosis; it was estimated using the Symptom Onset in Schizophrenia (SOS) inventory. Parental socioeconomic status (SES) was also determined. The diagnosis was confirmed using the Structured Clinical Interview for DSM (SCID-I).18 A psychopathological assessment was carried out with the Spanish versions of the following scales: depressive symptom severity was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS)19; and positive, negative, and general symptoms, with the Positive and Negative Syndrome Scale (PANSS).20 We have also used the PANSS-Marder Negative Symptom Factor Score (NSFS), as it is more restrictive than the negative subscale from the PANSS to assess negative symptomatology.21 The sum of the following items of the PANSS were used to calculate the NSFS: N1, N2, N3, N4, N6, G7, G16. In all the scales mentioned, higher scores indicate greater severity.
As previously mentioned, the literature revealed the existence of two factors within the construct of negative symptoms: MAP (motivation and pleasure) and EXP (expressivity/diminished expression). Following a previous study, EXP-factor was calculated summing the following PANSS items: N1, N3, N6, G7. The MAP factor, summing N2, N4, G16.22
The present FES sample was classified according to those patients who met predominant negative symptomatology (PNS) and non-predominant negative symptomatology (non-PNS) criteria at 12-months after inclusion in the study. Patients classified in the PNS group met the following criteria: a score of at least 20 on the PANSS negative-subscale, and this score had to be ≥6 points over the positive-subscale (to be considered predominant over the positive symptomatology).23 To ensure that these predominant negative symptoms were primary and not better classified as secondary, if patients met the following criteria, they were not classified in the PNS group: a PANSS-positive subscale score of ≥19 (or with a score of ≥4 on two or more items, or a score of ≥5 in at least one item from this subscale); presence of severe depressive symptomatology (MADRS total score>19.23
Antipsychotic mean doses were collected and converted to chlorpromazine equivalents (CPZ) based on international consensus.24 Tobacco and cannabis consumption at the moment of both visits (baseline and 12-month follow-up) was assessed using the adaptation of the multidimensional assessment tool European Addiction Severity-Index (EuropAsi). Samples for urine drug test detection were also collected.
Cognitive assessmentThe neuropsychological battery measured the following cognitive domains: (a) Estimated IQ from Block-Design and Vocabulary of the Wechsler Adult Intelligence Scale (WAIS-III); (b) Verbal learning and memory, evaluated with the España-Complutense Verbal-Learning Test-CVLT; (c) Sustained attention, assessed with the Continuous Performance Test-II (CPT-II);(d) Processing speed, with the Trail-Making Test (TMT-A) and Digit-Symbol (WAIS-III); (e) Executive functioning and specific impairments of planning were evaluated using the Tower of London (TOL); (f) Working memory was based on the Digit-Span Subtest and the Letter-Number Sequencing Subtest, WAIS-III; (g) Visual memory, with the Brief-visuospatial memory test-revised (BVMT-R); (h) Verbal fluency was evaluated using semantic fluency (animals) and phonological (F-A-S tests); and (i) Emotional intelligence was evaluated with the Mayer-Salovey-Caruso Emotional-Intelligence-Test (MSCEIT). Higher scores correspond to better performance in all the cognitive domains.
A Principal Component Analysis (PCA) was performed between neuropsychological battery tests to reduce the dimensionality of a data set consisting of many variables correlated with each other. Seven domains were identified: verbal memory, visual memory, executive function, sustained attention, working memory, verbal fluency, and processing speed (Supplementary Table 1).
Functional assessmentThe overall functional outcome was assessed by the Functioning Assessment Short Test (FAST) and the Global Assessment of Functioning Scale (GAF). Higher scores in the FAST represent a higher disability; higher scores in the GAF correspond to better functioning.
Premorbid adjustment and cognitive reservePremorbid adjustment, namely levels of functioning before the onset of psychosis, was assessed with the Premorbid Adjustment Scale (PAS). Sociability, school adaptation, socio-affective and sexual relationships, school performance, occupation, and behavior before the onset of psychosis are assessed. Only childhood and early adolescence life periods were considered since they were the two periods for which all the participants’ answers were available. Higher scores indicate worse premorbid adjustment.
To create the ‘Cognitive reserve score’ at baseline, the three most proposed proxy indicators of cognitive reserve (CR) were used.25 The following evaluation was carried out to measure CR at baseline: (1) The estimated premorbid IQ was calculated with the vocabulary subtest of the WAIS-III; (2) Education was assessed taking into account the number of years of compulsory education that subjects had completed as well as lifetime school performance; (3) Lifetime participation in leisure, social and physical activities was assessed by the PAS scale (scholastic performance) and by the FAST, which allows us to assess specific life-domains such as interpersonal relationships and leisure time. Higher scores in this proxy corresponds to better performance. When patients were assessed, they had already experienced a FES; thus, we could only estimate premorbid variables.
Statistical analysisDemographic, clinical, functional, and neurocognitive differences between groups (female and male; with PNS- and non-PNS) were examined using t-tests/chi-square. The differences on continuous variables with normal distributions were assessed using a two-tailed t-test. A two-tailed nonparametric Man–Whitney U test was used in two situations: when continuous variables did not meet the assumption of normality and when comparative samples were less than n=20, as it is the case of the women PNS and non-PNS groups. To explore which of these basal factors could predict PNS at follow-up in the general sample and females and males separately, three steps were undertaken: (1) Candidate exploratory variables were selected carefully taking into account their possible role in the prediction of PNS. Due to sample size differences between males and females, and due to the large number of potential candidate variables to be included in the model, inclusion of these preferred variables in the regression models were based on the following strict criteria26: according to previous theory and literature, as well as experience and clinical knowledge to which candidate variables should be considered for inclusion; grouping and combining similar or related variables based on subject knowledge and statistical techniques to restrict the number of variables. The variables selected were: age, DUP, age at psychosis onset, socioeconomic status, personal and family psychiatric history, premorbid adjustment, cognitive reserve, positive (PANSS-P) and negative (NSFS) symptomatology, depressive symptoms (MADRS), motivational and expressive factors, psychosocial functioning, antipsychotic medication treatment, functioning in verbal memory, executive functions, sustained attention, working memory, visual memory, verbal fluency, processing speed, and cannabis and/or tobacco consumption (all these variables from the baseline visit); (2) A correlation analysis was performed to determine, from the variables mentioned above, those factors that were associated with PNS for general sample and each group separately (female and males); and (3) To explore which of these factors could predict PNS at follow-up, those factors that were significantly correlated with PNS were included in the regression model using stepwise forward. Again, due to the high number of variables and the limited sample size, for the general sample we selected those factors that correlated with PNS with a cut-off of p<0.001 or less; in females group, those with a p<0.002, and in males group, those variables with a p<0.005. See supplementary Table 2 for more details.
Data were analyzed using the Statistical Package for the Social Sciences (SPSSv25). All statistical tests were two-tailed, with an alpha level of significance set at p<0.05.
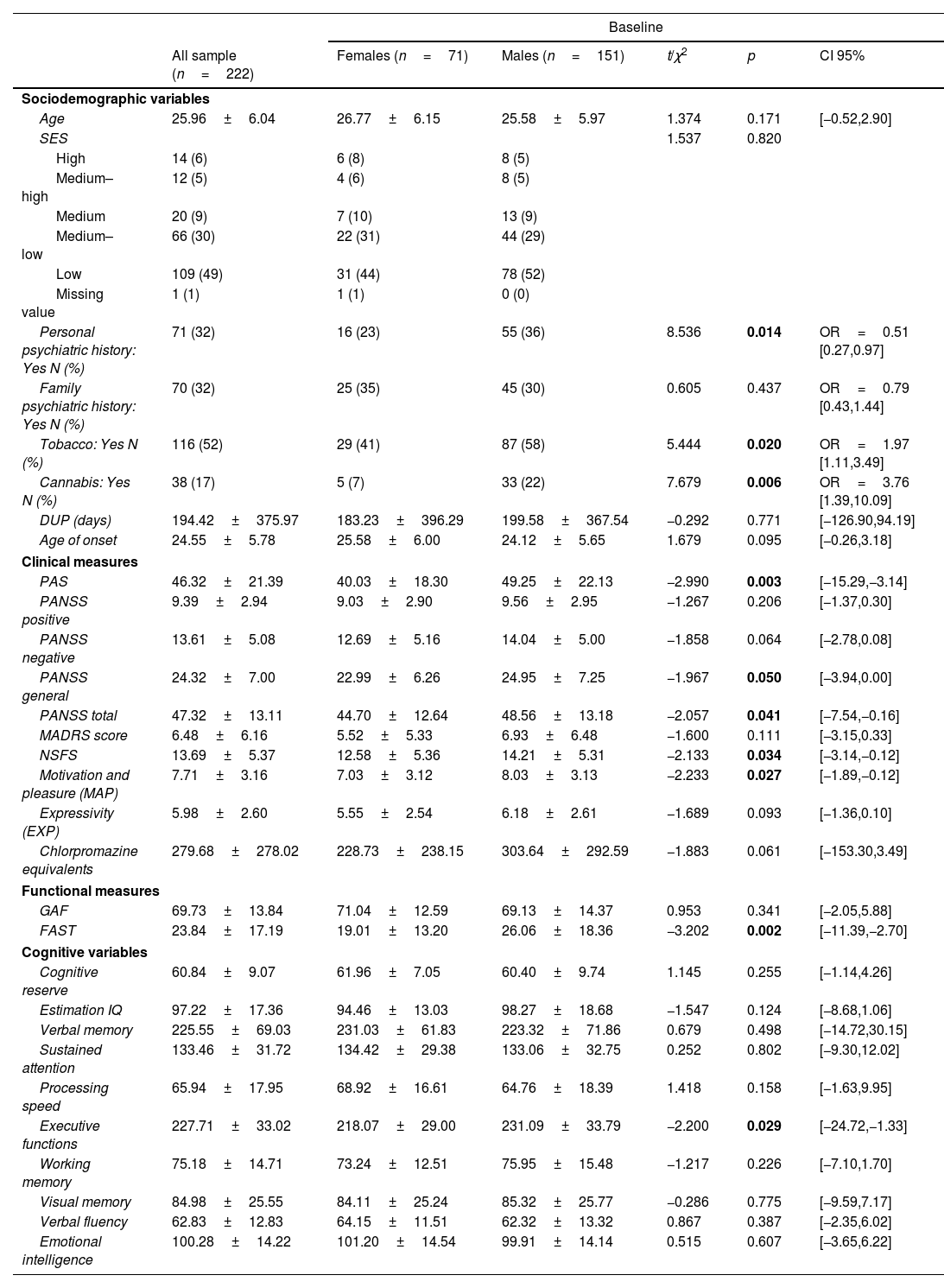
ResultsSex differences in sociodemographic, clinical, and functional characteristics at baselineFrom the initial 222 patients recruited in the 2EPs Project, 121 (54.5%) completed the 12-month follow-up visit and were included in the analyses; 45 (37.19%) were females. As it is shown in Table 1, taking into consideration the whole sample at baseline, male patients had a higher presence of personal psychiatric history, poorer premorbid adjustment, and more males reported tobacco and cannabis use than females. Males also presented higher general and total PANSS scores, worse psychosocial functioning, greater severity of negative symptoms, and more severe amotivation, but not diminished expressivity, from the negative symptomatology dimension. Females presented worse neurocognitive performance in executive functions.
Sex differences in sociodemographic, clinical, functional and neurocognitive characteristics from the general 2EPs sample at baseline (n=222).
| Baseline | ||||||
|---|---|---|---|---|---|---|
| All sample (n=222) | Females (n=71) | Males (n=151) | t/χ2 | p | CI 95% | |
| Sociodemographic variables | ||||||
| Age | 25.96±6.04 | 26.77±6.15 | 25.58±5.97 | 1.374 | 0.171 | [−0.52,2.90] |
| SES | 1.537 | 0.820 | ||||
| High | 14 (6) | 6 (8) | 8 (5) | |||
| Medium–high | 12 (5) | 4 (6) | 8 (5) | |||
| Medium | 20 (9) | 7 (10) | 13 (9) | |||
| Medium–low | 66 (30) | 22 (31) | 44 (29) | |||
| Low | 109 (49) | 31 (44) | 78 (52) | |||
| Missing value | 1 (1) | 1 (1) | 0 (0) | |||
| Personal psychiatric history: Yes N (%) | 71 (32) | 16 (23) | 55 (36) | 8.536 | 0.014 | OR=0.51 [0.27,0.97] |
| Family psychiatric history: Yes N (%) | 70 (32) | 25 (35) | 45 (30) | 0.605 | 0.437 | OR=0.79 [0.43,1.44] |
| Tobacco: Yes N (%) | 116 (52) | 29 (41) | 87 (58) | 5.444 | 0.020 | OR=1.97 [1.11,3.49] |
| Cannabis: Yes N (%) | 38 (17) | 5 (7) | 33 (22) | 7.679 | 0.006 | OR=3.76 [1.39,10.09] |
| DUP (days) | 194.42±375.97 | 183.23±396.29 | 199.58±367.54 | −0.292 | 0.771 | [−126.90,94.19] |
| Age of onset | 24.55±5.78 | 25.58±6.00 | 24.12±5.65 | 1.679 | 0.095 | [−0.26,3.18] |
| Clinical measures | ||||||
| PAS | 46.32±21.39 | 40.03±18.30 | 49.25±22.13 | −2.990 | 0.003 | [−15.29,−3.14] |
| PANSS positive | 9.39±2.94 | 9.03±2.90 | 9.56±2.95 | −1.267 | 0.206 | [−1.37,0.30] |
| PANSS negative | 13.61±5.08 | 12.69±5.16 | 14.04±5.00 | −1.858 | 0.064 | [−2.78,0.08] |
| PANSS general | 24.32±7.00 | 22.99±6.26 | 24.95±7.25 | −1.967 | 0.050 | [−3.94,0.00] |
| PANSS total | 47.32±13.11 | 44.70±12.64 | 48.56±13.18 | −2.057 | 0.041 | [−7.54,−0.16] |
| MADRS score | 6.48±6.16 | 5.52±5.33 | 6.93±6.48 | −1.600 | 0.111 | [−3.15,0.33] |
| NSFS | 13.69±5.37 | 12.58±5.36 | 14.21±5.31 | −2.133 | 0.034 | [−3.14,−0.12] |
| Motivation and pleasure (MAP) | 7.71±3.16 | 7.03±3.12 | 8.03±3.13 | −2.233 | 0.027 | [−1.89,−0.12] |
| Expressivity (EXP) | 5.98±2.60 | 5.55±2.54 | 6.18±2.61 | −1.689 | 0.093 | [−1.36,0.10] |
| Chlorpromazine equivalents | 279.68±278.02 | 228.73±238.15 | 303.64±292.59 | −1.883 | 0.061 | [−153.30,3.49] |
| Functional measures | ||||||
| GAF | 69.73±13.84 | 71.04±12.59 | 69.13±14.37 | 0.953 | 0.341 | [−2.05,5.88] |
| FAST | 23.84±17.19 | 19.01±13.20 | 26.06±18.36 | −3.202 | 0.002 | [−11.39,−2.70] |
| Cognitive variables | ||||||
| Cognitive reserve | 60.84±9.07 | 61.96±7.05 | 60.40±9.74 | 1.145 | 0.255 | [−1.14,4.26] |
| Estimation IQ | 97.22±17.36 | 94.46±13.03 | 98.27±18.68 | −1.547 | 0.124 | [−8.68,1.06] |
| Verbal memory | 225.55±69.03 | 231.03±61.83 | 223.32±71.86 | 0.679 | 0.498 | [−14.72,30.15] |
| Sustained attention | 133.46±31.72 | 134.42±29.38 | 133.06±32.75 | 0.252 | 0.802 | [−9.30,12.02] |
| Processing speed | 65.94±17.95 | 68.92±16.61 | 64.76±18.39 | 1.418 | 0.158 | [−1.63,9.95] |
| Executive functions | 227.71±33.02 | 218.07±29.00 | 231.09±33.79 | −2.200 | 0.029 | [−24.72,−1.33] |
| Working memory | 75.18±14.71 | 73.24±12.51 | 75.95±15.48 | −1.217 | 0.226 | [−7.10,1.70] |
| Visual memory | 84.98±25.55 | 84.11±25.24 | 85.32±25.77 | −0.286 | 0.775 | [−9.59,7.17] |
| Verbal fluency | 62.83±12.83 | 64.15±11.51 | 62.32±13.32 | 0.867 | 0.387 | [−2.35,6.02] |
| Emotional intelligence | 100.28±14.22 | 101.20±14.54 | 99.91±14.14 | 0.515 | 0.607 | [−3.65,6.22] |
Abbreviations: SES=socioeconomic status; DUP=Duration of Untreated Psychosis; PAS=Premorbid Adjustment Scale; PANSS=Positive and Negative Symptom Scale; NSFS=Negative Symptoms Factor Score of the PANSS; MADRS=Montgomery-Asberg Depression Rating Scale; GAF=Global Assessment of Functioning; FAST=Functioning Assessment Short Test; IQ=Intelligence Quotient. Significant differences (p<0.05) marked in bold.
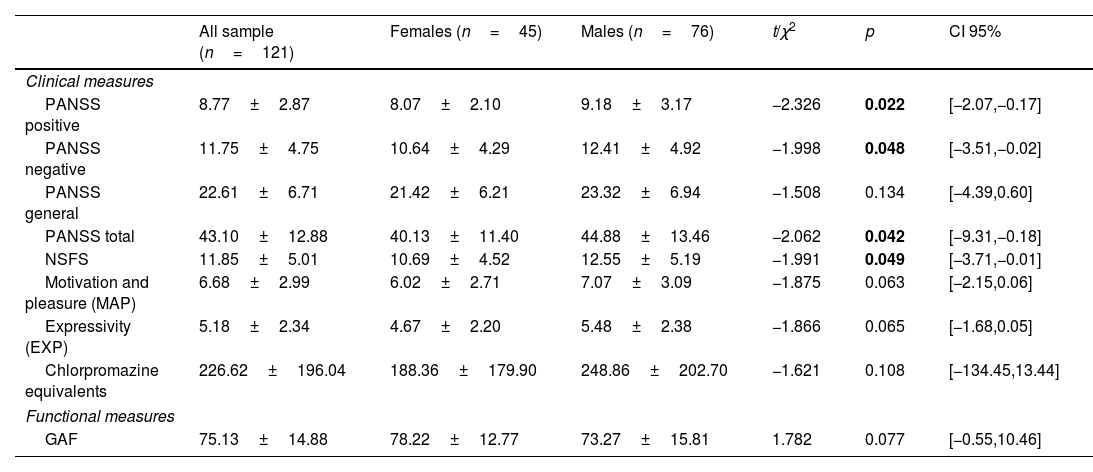
At twelve-months of follow-up, in comparison with females, males presented more positive symptomatology, more negative symptoms (assessed with from the PANSS and with the NSFS), and more severe general symptoms. There were not significant differences in amotivation and expressivity domains at follow-up, but these symptoms presented a trend toward significance (see Table 2 for more details).
Sex differences in sociodemographic, clinical, functional, and neurocognitive characteristics from the general 2EPs sample at one-year follow-up (n=121):.
| All sample (n=121) | Females (n=45) | Males (n=76) | t/χ2 | p | CI 95% | |
|---|---|---|---|---|---|---|
| Clinical measures | ||||||
| PANSS positive | 8.77±2.87 | 8.07±2.10 | 9.18±3.17 | −2.326 | 0.022 | [−2.07,−0.17] |
| PANSS negative | 11.75±4.75 | 10.64±4.29 | 12.41±4.92 | −1.998 | 0.048 | [−3.51,−0.02] |
| PANSS general | 22.61±6.71 | 21.42±6.21 | 23.32±6.94 | −1.508 | 0.134 | [−4.39,0.60] |
| PANSS total | 43.10±12.88 | 40.13±11.40 | 44.88±13.46 | −2.062 | 0.042 | [−9.31,−0.18] |
| NSFS | 11.85±5.01 | 10.69±4.52 | 12.55±5.19 | −1.991 | 0.049 | [−3.71,−0.01] |
| Motivation and pleasure (MAP) | 6.68±2.99 | 6.02±2.71 | 7.07±3.09 | −1.875 | 0.063 | [−2.15,0.06] |
| Expressivity (EXP) | 5.18±2.34 | 4.67±2.20 | 5.48±2.38 | −1.866 | 0.065 | [−1.68,0.05] |
| Chlorpromazine equivalents | 226.62±196.04 | 188.36±179.90 | 248.86±202.70 | −1.621 | 0.108 | [−134.45,13.44] |
| Functional measures | ||||||
| GAF | 75.13±14.88 | 78.22±12.77 | 73.27±15.81 | 1.782 | 0.077 | [−0.55,10.46] |
Abbreviations: PANSS=Positive and Negative Symptom Scale; NSFS=Negative Symptoms Factor Score of the PANSS; GAF=Global Assessment of Functioning. Significant differences (p<0.05) marked in bold.
Those patients assessed at follow-up (n=121) were indistinguishable from those who were not (n=101) in terms of clinical and functional status. However, these two groups differed in terms of CPZ equivalences (t=5.186; p=0.024) and in terms of attention (t=−1.950; p=0.005), with those assessed only at baseline showing a worse performance and being more medicated rather than those patients that completed the follow-up assessments (Supplementary Table 3 for more sample and follow-up details differentiating by sex).
Sociodemographic and clinical details of PNS and non-PNS schizophreniaFrom those FES patients that completed the 12-months follow-up, 30 (24.8%) met PNS criteria. Taking into consideration the whole sample (n=121), in comparison with non-PNS group, PNS group shown more severe negative symptomatology at baseline (from the PANSS and from NSFS), more depressive symptoms, poorer premorbid adjustment, poorer functional outcome (from FAST and from GAF), more amotivation, and alterations in expressivity. In the cognitive area, PNS patients presented a worse CR, a lower estimated IQ, worse verbal memory, and worse fluency (see Supplementary Table 4 for more details).
Differentiating by sex, as shown in Table 3, males with PNS, compared with non-PNS, at baseline had poorer premorbid adjustment, higher severity of negative symptoms (NSFS and PANSS), diminished motivation and expressivity, more depressive symptomatology, poorer functional outcome (FAST and GAF), poorer CR, worse verbal memory performance, poorer visual memory, slower processing speed, and worse verbal fluency. Males with PNS also presented differences with non-PNS group at 12-months in terms of greater negative symptomatology, more amotivation and more diminished expression, more total PANSS symptoms, and lower functioning.
Baseline and follow-up (FUP, at 12 months) demographic, clinical, functional, and neurocognitive characteristics of PNS and non-PNS schizophrenia in female and male groups that completed both visits (n=121):.
| Females (n=45) | Males (n=76) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-PNS (n=36) | PNS (n=9) | U-Mann–Whitney/χ2 | p | CI 95% | Non-PNS (n=55) | PNS (n=21) | t/χ2 | p | CI 95% | |
| Sociodemographic variables | ||||||||||
| Age | 27.11±6.51 | 25.67±6.28 | 141.500 | 0.495 | [−3.42,6.31] | 26.31±5.76 | 24.52±5.22 | 1.239 | 0.219 | [−1.09,4.66] |
| SES | 2.115 | 0.715 | 5.910 | 0.206 | ||||||
| High | 4 (11) | 1 (11) | 3 (6) | 0 (0) | ||||||
| Medium–high | 2 (6) | 0 (0) | 4 (7) | 1 (5) | ||||||
| Medium | 4 (11) | 0 (0) | 7 (13) | 0 (0) | ||||||
| Medium–low | 10 (29) | 4 (44) | 13 (24) | 9 (43) | ||||||
| Low | 15 (43) | 4 (44) | 28 (51) | 11 (53) | ||||||
| Missing value | 1 (1) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Personal psychiatric history: Yes N (%) | 7 (19) | 3 (33) | 0.884 | 0.347 | OR=0.47 [0.09,2.34] | 21 (38) | 9 (43) | 0.139 | 0.709 | OR=0.82 [0.29,2.29] |
| Family psychiatric history: Yes N (%) | 10 (28) | 4 (44) | 1.037 | 0.308 | OR=2.16 [0.48,9.69] | 20 (36) | 8 (38) | 0.020 | 0.889 | OR=1.08 [0.38,3.04] |
| Tobacco: Yes N (%) | 16 (44) | 3 (33) | 0.293 | 0.588 | OR=0.66 [0.14,3.03] | 31 (56) | 10 (48) | 0.468 | 0.494 | OR=0.70 [0.26,1.93] |
| Cannabis: Yes N (%) | 5 (14) | 0 (0) | 1.365 | 0.243 | OR=0.87 [0.76,0.98] | 8 (15) | 3 (15) | 0.003 | 0.954 | OR=0.96 [0.23,4.02] |
| Tobacco FUP: Yes N (%) | 17 (47) | 4 (44) | 0.20 | 0.887 | OR=1.12 [0.24,5.18] | 29 (55) | 13 (62) | 0.317 | 0.574 | OR=1.35 [0.48,3.78] |
| Cannabis FUP: Yes N (%) | 0 (0) | 1 (11) | 4.605 | 0.032 | OR=1.14 [0.88,1.49] | 8 (15) | 2 (10) | 0.399 | 0.527 | OR=0.59 [0.12,3.05] |
| DUP (days) | 63.15±109.40 | 273.50±239.72 | 221.500 | 0.005 | [−411.90,−8.81] | 163.92±278.13 | 266.65±499.89 | −0.868 | 0.394 | [−347.16,141.70] |
| Age of onset | 26.00±6.40 | 24.57±6.21 | 94.00 | 0.530 | [−3.95,6.81] | 24.62±5.50 | 23.29±5.20 | 0.957 | 0.342 | [−1.45,4.12] |
| Clinical measures | ||||||||||
| PAS | 33.52±14.02 | 57.89±18.84 | 252.00 | 0.002 | [−35.86,−12.89] | 45.45±20.90 | 58.59±24.73 | −2.168 | 0.034 | [−25.22,−1.05] |
| PANSS positive | 8.19±1.94 | 9.33±2.60 | 201.500 | 0.338 | [−2.70,0.42] | 9.20±3.00 | 10.19±2.91 | −1.297 | 0.199 | [−2.51,0.53] |
| PANSS negative | 11.03±4.56 | 17.56±4.28 | 283.00 | 0.001 | [−9.92,−3.14] | 12.09±4.78 | 17.71±3.33 | −5.787 | <0.001 | [−7.57,−3.67] |
| PANSS general | 20.67±4.06 | 26.33±6.12 | 256.00 | 0.012 | [−10.47,−0.86] | 23.36±6.47 | 28.38±6.79 | −2.983 | 0.004 | [−8.37,−1.67] |
| PANSS total | 39.89±8.73 | 53.22±11.24 | 265.00 | 0.005 | [−20.29,−6.38] | 44.65±12.65 | 56.29±10.38 | −3.754 | <0.001 | [−17.80,−5.46] |
| MADRS score | 4.50±4.56 | 9.11±6.03 | 247.00 | 0.025 | [−8.27,−0.95] | 5.04±4.51 | 11.52±9.21 | −3.089 | 0.005 | [−10.82,−2.15] |
| NSFS | 11.03±4.81 | 17.67±4.47 | 283.00 | 0.001 | [−10.21,−3.07] | 12.38±4.98 | 18.43±3.41 | −6.029 | <0.001 | [−8.06,−4.03] |
| Motivation and pleasure (MAP) | 5.92±2.67 | 10.11±2.52 | 285.50 | <0.001 | [−6.18,−2.21] | 6.85±2.82 | 10.86±2.17 | −6.584 | <0.001 | [−5.23,−2.78] |
| Expressivity (EXP) | 5.11±2.52 | 7.56±2.19 | 264.00 | 0.006 | [−4.29,−0.60] | 5.53±2.52 | 7.57±1.75 | −3.999 | <0.001 | [−3.07,−1.02] |
| Chlorpromazine equivalents | 205.99±194.19 | 219.38±130.45 | 199.50 | 0.367 | [−151.68,124.91] | 293.16±263.01 | 339.72±197.39 | −0.735 | 0.465 | [−172.80,79.69] |
| PANSS positive FUP | 7.89±2.05 | 8.78±2.28 | 209.500 | 0.181 | [−2.47,0.69] | 9.09±3.38 | 9.43±2.60 | −0.412 | 0.681 | [−1.97,1.29] |
| PANSS negative FUP | 9.11±2.94 | 16.78±3.23 | 310.50 | <0.001 | [−9.92,−5.42] | 10.15±3.51 | 18.33±2.54 | −9.748 | <0.001 | [−9.86,−6.51] |
| PANSS general FUP | 20.47±6.07 | 25.22±5.52 | 255.00 | 0.07 | [−9.24,−0.26] | 21.53±6.36 | 28.25±6.14 | −4.085 | <0.001 | [−10.00,−3.44] |
| PANSS total FUP | 37.47±10.34 | 50.78±9.32 | 279.50 | <0.001 | [−20.94,−5.67] | 40.76±12.34 | 56.20±9.41 | −5.074 | <0.001 | [−21.50,−9.37] |
| NSFS FUP | 9.11±3.20 | 17.00±3.39 | 304.50 | <0.001 | [−10.32,−5.46] | 10.13±3.80 | 18.76±2.23 | −9.757 | <0.001 | [−10.40,−6.87] |
| Motivation and pleasure (MAP) FUP | 5.14±1.97 | 9.56±2.40 | 299.00 | <0.001 | [−5.97,−2.87] | 5.70±2.28 | 10.57±1.91 | −8.664 | <0.001 | [−5.99,−3.75] |
| Expressivity (EXP) FUP | 3.97±1.54 | 7.44±2.30 | 283.00 | <0.001 | [−4.75,−2.19] | 4.43±1.80 | 8.19±1.29 | −8.747 | <0.001 | [−4.62,−2.91] |
| Chlorpromazine equivalents FUP | 80.89±11.77 | 67.56±11.39 | 193.50 | 0.120 | [4.54,22.13] | 240.76±212.10 | 282.77±173.44 | −0.791 | 0.432 | [−147.96,63.95] |
| Functional measures | ||||||||||
| GAF | 74.86±11.43 | 60.00±16.44 | 62.00 | 0.011 | [5.02,24.69] | 73.76±14.01 | 60.24±14.52 | 3.726 | <0.001 | [6.29,20.76] |
| FAST | 14.79±11.33 | 20.88±13.40 | 174.50 | 0.288 | [−15.39,3.23] | 23.30±19.83 | 33.62±15.76 | −2.135 | 0.036 | [−19.96,−0.69] |
| GAF FUP | 80.89±11.77 | 67.56±11.39 | 62.00 | 0.003 | [4.54,22.13] | 76.81±16.10 | 64.14±10.82 | 3.320 | 0.001 | [5.07,20.28] |
| Cognitive variables | ||||||||||
| Cognitive reserve | 62.23±6.89 | 56.49±7.85 | 46.00 | 0.131 | [−0.79,12.27] | 62.53±10.64 | 55.95±9.23 | 2.042 | 0.045 | [0.14,13.02] |
| Estimation IQ | 94.89±13.14 | 85.50±12.36 | 67.500 | 0.091 | [−1.19,19.97] | 102.49±19.18 | 92.13±19.69 | 1.883 | 0.064 | [−0.62,21.35] |
| Verbal memory | 223.99±62.40 | 214.04±61.78 | 112.00 | 0.899 | [−40.53,60.44] | 250.26±63.21 | 192.49±77.11 | 3.014 | 0.004 | [19.48,96.06] |
| Attention | 133.72±30.78 | 124.09±14.67 | 84.00 | 0.522 | [−13.54,32.80] | 127.68±23.29 | 130.18±23.30 | −0.388 | 0.699 | [−15.36,10.36] |
| Processing speed | 69.63±15.54 | 66.53±14.07 | 100.00 | 0.773 | [−9.37,15.59] | 70.36±16.51 | 58.38±20.51 | 2.435 | 0.018 | [2.16,21.80] |
| Executive functions | 220.24±30.13 | 206.68±28.19 | 64.00 | 0.349 | [−11.71,38.82] | 233.47±34.14 | 231.52±38.81 | 0.192 | 0.848 | [−18.42,22.34] |
| Working memory | 75.02±14.20 | 68.88±10.64 | 84.500 | 0.302 | [−4.89,17.17] | 78.64±15.90 | 72.16±15.72 | 1.392 | 0.169 | [−2.82,15.79] |
| Visual memory | 85.00±24.44 | 83.05±34.25 | 112.50 | 0.899 | [−19.69,23.59] | 94.79±26.69 | 77.47±25.61 | 2.334 | 0.023 | [2.50,32.14] |
| Verbal fluency | 64.82±11.84 | 62.93±11.72 | 111.00 | 0.871 | [−7.70,11.47] | 65.62±13.86 | 54.25±13.28 | 2.896 | 0.005 | [3.53,19.21] |
| Emotional intelligence | 103.35±16.64 | 103.71±9.69 | 77.500 | 0.886 | [−14.00,13.27] | 100.29±14.78 | 96.31±12.76 | 0.963 | 0.339 | [−4.28,12.24] |
Abbreviations: SES=Socioeconomic status; DUP=Duration of Untreated Psychosis; PAS=Premorbid Adjustment; PANSS=Positive and Negative Symptom Scale; NSFS=Negative Symptoms Factor Score of the PANSS; MADRS=Montgomery-Asberg Depression Rating Scale; FUP=Follow-up; GAF=Global Assessment of Functioning; FAST=Functioning Assessment Short Test; IQ=Intelligence Quotient. Significant differences (p<0.05) marked in bold.
As it is also shown in Table 3, at baseline, females with PNS showed poorer premorbid adjustment, more DUP, higher negative symptoms (NSF and PANSS), more amotivation and diminished expressivity, and severe depressive symptoms rather than non-PNS group. They also presented poorer functionality based on the GAF scale. In relation to cannabis use at follow-up, PNS females presented more consume; however, due to the small sample size, this result could not be representative. Similar to males, females with PNS also presented differences with non-PNS group at 12-months in terms of negative symptomatology (assessed with the PANSS and the NSFS), more amotivation and alterations in expressivity, more total PANSS symptoms, and lower functioning. At follow-up, females with PNS were also less medicated than non-PNS females. Contrary to males, in females, no differences between PNS and non-PNS were observed regarding remaining variables such as positive symptoms scores, CR and cognitive functioning.
Baseline predictors for PNS and non-PNS schizophrenia at 12-month follow-upAfter performing a binary logistic regression to assess the predictive power of statistically significant socio-demographic, clinical and neurocognitive variables for PNS in the general sample (p<0.001: negative symptomatology, depressive symptomatology, functioning, amotivation, and expressivity), we obtain that the model can explain 27.9% (Cox and Snell R square) and 41.8% (Nagelkerke R square) of the variance and correctly classified 81.7% of the cases. The significant variable in the model was the amotivation dimension (p=0.001; Exp(B)=1.666).
Differentiating by sex, in males, from the variables included in the regression model (p<0.005: negative symptomatology, amotivation, functioning, expressivity and verbal memory), the significant variable in the model was the amotivation domain (p=0.001; Exp(B)=1.607) and verbal memory (p=0.031; Exp(B)=0.989). In females, from the statistically significant variables included (p<0.002: premorbid adjustment and amotivation domain), it was exclusively the premorbid adjustment (p=0.007; Exp(B)=1.106) the main predictor of predominant negative symptomatology development (see Table 4 for more details).
Binary logistic regression to assess the predictive power of sociodemographic, clinical, functional and neurocognitive variables for meeting PNS criteria at twelve-month follow-up in FES schizophrenia patients.
| B | S.E. | Wald | gl | Sig. | Exp(B) | Cox and Snell R2 | Nagelkerke R2 | Correctly classified | |
|---|---|---|---|---|---|---|---|---|---|
| All the sample (n=121) | |||||||||
| Motivation and pleasure (MAP) | 0.511 | 0.103 | 24.691 | 1 | <0.001 | 1.666 | 27.9% | 41.8% | 81.7% |
| Constant | −5.573 | 1.023 | 29.689 | 1 | <0.001 | 0.004 | |||
| Male (n=76) | |||||||||
| Motivation and pleasure (MAP) | 0.474 | 0.147 | 10.365 | 1 | 0.001 | 1.607 | 29.9% | 44.6% | 81.8% |
| Verbal memory | −0.011 | 0.005 | 4.668 | 1 | 0.031 | 0.989 | |||
| Constant | −2.981 | 1.720 | 3.001 | 1 | 0.083 | 0.051 | |||
| Female (n=45) | |||||||||
| Premorbid adjustment (PAS) | 0.101 | 0.037 | 7.317 | 1 | 0.007 | 1.106 | 27.4% | 42.7% | 86.0% |
| Constant | −5.895 | 1.860 | 10.043 | 1 | 0.002 | 0.003 | |||
Significant differences (p<0.05) marked in bold.
Three main findings emerged from the present study. Firstly, we found a different clinical and cognitive profile between males and females with a first episode of schizophrenia. Secondly, compared to non-PNS, PNS group reported a significantly higher severity of negative symptomatology at baseline, more depressive symptoms, poorer premorbid adjustment, poorer functionality, more amotivation, and more alterations in expressivity. In the cognitive area, PNS patients presented a worse cognitive reserve, lower estimated IQ, worse verbal memory, and worse fluency. Thirdly, a distinct pattern of risk factors for the development of predominant negative symptomatology was found between females and males. In the male group, verbal memory and amotivation, but not diminished expressivity, were the main predictors of PNS. Finally, in females, a poor premorbid adjustment was the main clinical risk factor for developing predominant negative symptomatology at short-term after a first episode of schizophrenia.
Focusing on sex differences at baseline in the present FES sample, our results suggest that in comparison to females, male group presented a poorer premorbid adjustment, greater cannabis and tobacco use, more had presence of personal psychiatric history, a worse psychosocial functioning, and more severe general and negative symptomatology, especially more amotivation alterations instead of expressivity deficits. In this line, several clinical studies have observed similar sex-outcome differences in first-episode and schizophrenia patients.3,4,7 These results are also in accordance with previous literature that remarks that compared to females, who have been reported more affective symptoms and alterations in alogia and avolition-apathy dimensions,27 males show a higher incidence of the disorder, an earlier age of onset, more negative symptomatology (specially alterations in social withdrawal and blunted affect), a poorer premorbid adjustment, worse functional outcome, and a more severe progression of the illness.3,5,6 Related to cognitive functioning, the present results suggested few differences in cognitive performance between males and females, only suggesting a worse performance in executive functions in the female group. These lack of significant differences found in the present study between both sexes in the cognitive area are in line with previous studies in first-episode and schizophrenia samples.28,29 Notwithstanding, in first-episode schizophrenia patients, mixed results have been found; one study found few gender differences in a sample of unmedicated first-episode schizophrenia patients,30 while other studies found gender and sex differences in relation with clinical profiles.31 Thus, sex differences in neurocognitive performance are a controversial issue as results remain inconclusive. The discrepancy in these findings could be explained by the heterogeneity in cognitive assessments and in the duration of illness of the samples.28 These controversies make further studies with such samples of particularly clinical interest and, specifically in samples with predominance of negative symptomatology at early stages of the illness.31
As potentially expected according to previous literature that reports that up to one-third of patients with a schizophrenia diagnosis might have idiopathic and stable (primary) negative symptoms,9 in the present study, around 25% of the patients met PNS criteria at one-year follow-up. Given that males have been reported to be more likely to present greater severity in negative symptomatology, to be more resistant to treatments, and to present more severe illness courses than females, an association between PNS -and potential deficit syndrome development at long-term- and being male was expected in the present study. More specifically, in the present FES sample almost thirty per cent of the males developed predominant negative symptoms versus twenty per cent of the females. It is worthy to mention that men group had also more antipsychotic doses, which could be related with the presence of more negative symptomatology secondary to the medication.9 Focusing on the baseline differences between PNS and non-PNS groups, and taking the whole sample into consideration (without differentiating by sex), results also suggested clinical and socio-demographical variances between groups that are also in accordance with previous studies.8,12 In the present study, patients that developed PNS at one year had more negative and depressive symptoms at baseline, less general functioning, a poorer premorbid adjustment, more amotivation, alterations in expressivity, worse cognitive reserve, lower estimated IQ, and presented a worse performance in verbal memory and fluency. In accordance with these results, literature has reported that all these parameters, that is, premorbid adjustment, general functioning, verbal fluency and verbal memory, baseline negative symptomatology, and cognitive reserve, have been extensively associated with long-term negative symptomatology persistence.11,25,32
Focusing on sex differences in PNS and non-PNS groups, FES females who developed PNS had a poorer premorbid adjustment, higher negative and depressive symptomatology at baseline, more amotivation, more severe diminished expressivity, and a worse functional outcome. In comparison, males with PNS also presented more baseline negative symptoms, more amotivation and expressivity, more severe depressive symptomatology, poorer premorbid adjustment, and worse general functioning than non-PNS group. However, in contrast to females, males with PNS also presented alterations in verbal memory performance, and verbal fluency. The association between males and primary negative symptomatology could reflect sex-related factors influencing severity without being etiologic per se. Subsequently, the presence of a deficit syndrome would be an indicator of greater severity of the illness. Such a plausible sex-related factor could be estrogens, which have been proposed to play a protective role by decreasing the risk for schizophrenia.33
In this line, given the potential confounding effect of sex, it seems to be essential to control for sex-differences in studies comparing deficit and non-deficit syndrome on variables on which males and females could differ (e.g. severity of symptoms, age of onset, premorbid adjustment, cognitive functioning, etc.). Contrary to our expectations, age of onset, family psychiatric history, and/or medication dosage were not related to PNS development. Related with the DUP, only females showed statistical differences between those who meet PNS criteria and those who not, presenting more DUP the female PNS group. Focusing on cognitive reserve, as prophesied, male PNS group showed poorer reserve than non-PNS. Against what we expected, this difference between groups were not found in females. Notwithstanding, these results must be taken into consideration with caution as it would be of clinical interest to follow these patients in larger samples and in the long-term to study the stability of this symptomatology and the maintenance of PNS criteria at long-term.
Additionally, it has been described a positive relationship between the negative symptomatology and cognitive alterations.31,34 Some studies have proposed that negative symptomatology mediates the relationship between neurocognitive performance and functional. Specifically, the association between verbal memory impairments and negative symptomatology has been well-documented during the early stages of psychosis, and both have been linked to functional outcomes.32,34 In a previous study, negative symptomatology has already been related to verbal recall and verbal fluency in male samples, whereas no similar association emerged in the female group.35 In our study, we have also found these differences in this domain between males and females and the role that it plays in the development of predominant negative symptomatology in the short- and potential long-term for the male group. In this line, given that negative symptoms are difficult to treat, our results highlight verbal memory deficits in males as treatment target with enhanced therapeutic potential.32 In this way, these findings suggest that potential sex-personalized treatments, as cognitive remediation, metacognitive training psychological interventions – specially in females–, functional remediation, cognitive reserve enhancement, and preventing relapse programs are evidence-based interventions that should be included consistently into clinical guidelines for the treatment of individuals with schizophrenia from its early stages25,36–38 and even in high-risk psychosis populations.39
It is worthy to mention that, while deficit syndrome only applies to schizophrenia spectrum disorder, the current study demonstrates that prominent and predominant negative symptoms can be present from the first stages of the illness. Accordingly, this may suggest that this subgroup of potential primary negative symptoms is not only relevant for patients with a long-standing diagnosis of schizophrenia.40 Adding more evidence and focusing on negative symptom dimensions, affective flattening and alogia (EXP-factor) have been suggested to represent the “core negative symptoms” contributing to poorer functional outcomes.8 Thus, affective flattening has been shown to be significantly more severe in deficit syndrome when compared to non-deficit syndrome patients.9 However, in first-episode, blunted affect and alogia have not always been the most prominent negative symptoms.40 In a previous study, low levels of the ‘diminished expression’ subdomain were found in a cohort of patients with persistent negative symptoms. Hence, it was plausible that these low levels of affective flattening in FES greatly impacted the prevalence of deficit syndrome.40 Contrary to these results, the EXP domain was not associated with PNS in our study. By contrast, in males, the MAP domain was associated with PNS, but not in females. Again, these results underline the relevance of treating negative symptoms separately and by domains and not merely as a single construct.
This study has some limitations which must be considered. Firstly, no specific scale was used to assess negative symptomatology and the use of a transversal proxy/cut-off to stablish predominant negative symptom criteria. Moreover, although the PANSS is widely used for the assessment of negative symptoms, we acknowledge that it has several limitations; for instance, it was not designed to evaluate negative symptoms exclusively; the PANSS can measure the two-correlated factor, but it was not designed for this purpose either; third, it does not evaluate the symptom anhedonia.8 Future studies making use of newer and improved negative symptom scales – such as the Brief Negative Symptom Scale (BNSS) or the Clinical Assessment Interview for Negative Symptoms (CAINS) may be more appropriate for the evaluation of negative symptoms, since they capture both manifestations of the symptom, internal motivation and real-world behavior.8 Also, our study was based on previously collected data, and DSM-IV-TR diagnostic criteria, instead of DSM-5, were applied, which we recognize as an additional limitation. Another potential constraint was that after observing the role of the premorbid adjustment on the development of predominant negative symptomatology in females, it would have been of clinical interest to take into consideration not only the total premorbid adjustment functioning, but also to analyze the individual components of this construct separately, such as the academic and social factors. In addition, a limitation present in all cognitive reserve studies undertaken on a psychiatric population is not having used a valid instrument to measure this parameter, so criteria established and replicated in previous studies were followed.25 Finally, due to a high percentage of patients discontinued the study before the follow-up visit (particularly due to they refused the re-evaluation), this resulted in a small sample size of women's group. Because of this, some aspects should have been considered with caution in order to extrapolate the present findings. For example, results suggest the presence of more women with lower functioning and higher positive symptomatology, and men with higher cannabis consumption that discontinued the study and not completed the follow-up. In this line, according to the design and inclusion criteria of this study, it is important to remark that probably the most clinically severe patients, who tend to present a second relapse within the first years after the FEP, are not often well represented in these longitudinal studies, due to they often stop coming voluntarily for follow-up visits, which is a common difficulty in all studies with a longitudinal design. In the present project, to reduce this discontinuity, personal contact was established with patients and their families when arranging visits and outpatient follow-up was conducted by the psychiatrists participating in the study. Nonetheless, as strength points, it is worthy to remark that it is a naturalistic and multicenter study with a representative and well-characterized sample of non-affective FES on a stable clinical phase.
In conclusion, the main findings of this study suggest a different clinical and cognitive profile at baseline between FES patients that will develop predominant negative symptomatology in comparison to those with non-predominant negative symptoms. More specifically, a distinct pattern of risk factors for the development of PNS was found between both sexes. For males, amotivation, but not diminished expressivity, and verbal memory were the main predictors of predominant negative symptomatology development; whereas, for females, the main risk factor was a poorer premorbid adjustment. In the short term, the deficit and non-deficit differences could not be attributed to other clinical and demographic features such as age of onset, a positive family psychiatric history, and/or the severity of hallucinations, delusions, or formal thought disorder. Thus, the present results suggest that sex may be an important confounder in studies comparing patients that develop predominant negative symptoms or potential deficit schizophrenia. In this line, sex-specific and larger longitudinal studies on the development of predominant negative symptomatology and deficit syndrome in first-episode schizophrenia populations are warranted to establish early interventions and to design targeted personalized treatments.
FundingThis work was supported by: Instituto de Salud Carlos III, the Spanish Ministry of Science, Innovation and Universities, the European Regional Development Fund (ERDF/FEDER) (PI08/0208, PI11/00325, PI18/01055 and PI14/00612); CIBERSAM, with CIBERSAM Intramural Projects 2010: Flamm-PEPs study (PI2010-FLAMMPEPs); the CERCA Program/Generalitat de Catalunya, and Secretaria d’Universitats i Recerca del Departament d’Economia I Coneixement (2017SGR1355).
Conflict of interestMBe has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of AB-Biotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Roviand Takeda. EV has received research support from or served as consultant, adviser or speaker for AB-Biotics, Actavis, Allergan, Angelini, AstraZeneca, Bristol-Myers Suibb, Dainippon Sumitomo Pharma, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Telefónica, the Brain and Behavior Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Program (ENBREC), and the Stanley Medical Research Institute. MBi has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of has received honoraria from talks and/or consultancy of Adamed, Angelini, Casen-Recordati, Ferrer, Janssen-Cilag, Lundbeck, Neuraxpharm, Otsuka, Pfizer and Sanofi, and grants from Spanish Ministry of Health, Instituto de Salud Carlos III (PI20/01066).RRJ has been a consultant for, spoken in activities of, or received grants from: Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid Regional Government (S2010/BMD-2422 AGES; S2017/BMD-3740), JanssenCilag, Lundbeck, Otsuka, Pfizer, Ferrer, Juste, Takeda, Exeltis, Casen-Recordati, Angelini.EB has received research support from Esteve and Monteloeder S.L. AI has received research support from or served as speaker or advisor for Janssen-Cilag, Lundbeck and Otsuka. LPC holds grants from Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (PI17/01997, PI20/1342) and has received fees from Rubió. AGP has received grants and served as consultant, advisor or CME speaker for the following entities: Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Sanofi-Aventis, Exeltis, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), and the Basque Government.JMG is partly funded by a grant from Ministerio de Ciencia y Tecnologia – Republica de Colombia (Convocatoria 885/2020) and has been an advisor/speaker for, or received travel support from Janssen, Eurofarma, Servier, Sanofi, Lilly, and Pfizer.
The rest of authors report no biomedical financial interests or potential conflicts of interest.
We are extremely grateful to all participants.
This study is part of a coordinated-multicentre Project, funded by the Ministerio de Economía y Competitividad (PI08/0208; PI11/00325; PI14/00612), Instituto de Salud Carlos III – Fondo Europeo de Desarrollo Regional. Unión Europea. Una manera de hacer Europa, Centro de Investigación Biomédica en Red de salud Mental, CIBERSAM, by the CERCA Programme/Generalitat de Catalunya AND Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017SGR1355). Departament de Salut de la Generalitat de Catalunya, en la convocatòria corresponent a l’any 2017 de concessió de subvencions del Pla Estratègic de Recerca i Innovació en Salut (PERIS) 2016–2020, modalitat Projectes de recerca orientats a l’atenció primària, amb el codi d’expedient SLT006/17/00345. MBe is also grateful for the support of the Institut de Neurociències, Universitat de Barcelona.
SA has been supported by Sara Borrell doctoral programme (CD20/00177) and M-AES mobility fellowship (MV22/00002), from the Instituto de Salud Carlos III (ISCIII), and co-funded by European Social Fund “Investing in your future”.
EV thanks the support of the Spanish Ministry of Science, Innovation and Universities (PI15/00283) integrated into the Plan Nacional de I+D+I y cofinanciado por el ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER); CIBERSAM, Instituto de Salud Carlos III; and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365) and the project SLT006/17/00357, from PERIS 2016–2020 (Departament de Salut). CERCA Programme/Generalitat de Catalunya. AI thanks the support by the Madrid Regional Government (R&D Activities in Biomedicine S2017/BMD3740: AGES-CM 2-CM) and European Union Structural Funds, and the support by CIBERSAM.RRJ was supported by the Instituto de Salud Carlos III (PI19/00766; Fondo de Investigaciones Sanitarias/FEDER) and of Madrid Regional Government (S2017/BMD-3740).
We also would like to thank the authors of the PEPs group who participated in the development of this manuscript. The acronym 2EPs Group responds to the following authors: María Florencia Forte, Jairo M. González-Díaz, Mario de Matteis, Manuel Durán-Cutilla, Edurne García-Corres, Carmen Martin-Requena, Laura Martínez, Alba Toll, Luis Sanchez-Pastor, Aggie Nuñez-Doyle, Edith Pomarol-Clotet, Maria Ángeles García-León, Anna Butjosa, Marta Pardo, María Ribeiro, Jose M. López-Ilundain, Jerónimo Saiz, Enriqueta Ochoa-Mangado, María José Escartí, Fernarndo Contreras, Concepción De-la-Cámara, Miguel Gutierrez Fraile, Teresa Bobes Bascarán.