There are few studies exploring the pathophysiological pathways that may condition differentially the emergence/course of neurodevelopmental disorders (ND) in very preterm and extremely preterm newborns (VPTN/EPTN). Furthermore, there are no established biological markers predictive of ND in this population. The aim of this study is four-fold: in two cohorts of VPTN/EPTN (i) to characterize the emergence/course of ND up to corrected-age 6 years, (ii) to identify those factors (from prenatal stages up to age 6 years) that explain the interindividual differences related to emergence/course of ND, (iii) to identify in the first hours/days of life a urinary metabolomic biomarker profile predictive of ND, and (iv) to determine longitudinally variations in DNA methylation patterns predictive of ND.
MethodsObservational, longitudinal, prospective, six-year follow-up, multicentre collaborative study. Two cohorts are being recruited: the PeriSTRESS-Valencia-cohort (n=26 VPTN, 18 EPTN, and 122 born-at-term controls), and the PremTEA-Madrid-cohort (n=49 EPTN and n=29 controls).
ResultsWe describe the rationale, objectives and design of the PeriSTRESS-PremTEA project and show a description at birth of the recruited samples.
ConclusionsThe PeriSTRESS-PremTEA project could help improve early identification of clinical, environmental and biological variables involved in the physiopathology of ND in VPTN/EPTN. It could also help to improve the early identification of non-invasive ND biomarkers in this population. This may allow early ND detection as well as early and personalised intervention for these children.
It is currently estimated that the mortality and disability rate of mental illness is comparable to the most common chronic diseases (cardiovascular and oncological) and it entails a high rate of disability-adjusted life years lost, given its high prevalence with very early onset, for example, neurodevelopmental disorders (ND),1 disorders which include autism spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD), or intellectual disability (ID).2 Thus, in children under 5 years of age, ASD is the leading cause of disability due to mental illness and the fourth leading cause in those between 5 and 14 years of age.3 Therefore, in mental health promotion strategies, it is essential to identify risk factors early and study the pathophysiological mechanisms underlying the onset and course of these disorders to improve prevention strategies, and enable early detection and personalised early intervention.1
Preterm infants are a particularly vulnerable population at high risk of developing an ND later in life. Of very preterm newborns (VPTN) (born <32 0/0 weeks gestational age [GA]), 40%-60% screen positive for ASD traits at 24 months,4 a risk 4-12 fold that of born-at-term infants of being diagnosed with ASD and 2-3 times higher of being diagnosed with ADHD in infancy.5,6 Extremely preterm newborns (EPTN) (born <28 0/0 weeks’ GA) are the group with the highest prevalence of ND in infancy (up to 50%-70%6), this group has increased by 35% over the last ten years in Spain.7
ND and prematurity: pathophysiological mechanisms involvedThe aetiopathogenesis and pathophysiology of NDs remains largely unknown, suggesting a multifactorial origin. A limitation of the studies conducted to date is that they generally include individuals with high phenotypic heterogeneity, for example, ASD presenting with very diverse symptoms. The clinical heterogeneity of patients diagnosed with ND is becoming increasingly well known. This calls for the systematisation of the clinical differences between phenotypic variants or “clusters” of ND patients, such as ND patients with a history of prematurity, which will enable greater homogeneity in the study of their underlying neurobiological basis and potential pathophysiological mechanisms involved in the different phenotypes observed. Finding these relationships will allow consistency in the existence and description of the different phenotypes and personalised study of the underlying pathophysiology of each, and then the development and implementation of specific therapeutic strategies that are more appropriate for each of these phenotypic variants.8
In the specific case of individuals with an ND with a history of prematurity, it is postulated that factors of early physiological instability such as hypoxaemia or ischaemia,9 added to postnatal adversities such as bacteraemia, sepsis, or necrotising enterocolitis,10 the scarcity of endogenous neurotrophic protective factors provided by the placenta,11 systemic inflammation, (related to maternal fever, chorioamnionitis or early-onset sepsis, causing early brain damage,12 as well as secondary dysfunction or “immunoparalysis” in the infant), could explain some of the early central brain damage present in preterm newborns and subsequent impaired neurodevelopment. This scenario is compounded by adverse postnatal environmental events that further increase early damage, including extrauterine exposure to intense supraphysiological stimuli (pain, light, noise, invasive medical treatments13), inherent to admission to Neonatal Intensive Care Units (NICU) or deprivation of protective environmental stimuli, fundamental for neurodevelopment (skin-to-skin parental contact, breastfeeding14,15). Socio-familial factors such as parental mental health, socio-economic status and parent-child interaction also seem to play a fundamental role in the neurodevelopment of the preterm infant.16
ND and prematurity: biomarkers of early brain damageTo date, there are no biomarkers obtained by non-invasive or minimally invasive techniques that are indicative of early brain damage and potentially predictive of later onset of ND in these children. Urinary metabolomics have been emerging in recent years as a promising source of such biomarkers and as a useful tool for the early diagnosis of neurological diseases such as cerebrovascular disease17 or acquired brain injury.18 Metabolomics includes the determination in urine of metabolite profiles of lipid peroxidation and oxidative damage to proteins and DNA (which are a measure of oxidative stress in vivo), as well as prostaglandins (which account for the degree of inflammation primary and secondary to the brain damage itself). In the specific case of the preterm population, urine metabolomic profiles indicative of oxidative damage have been described in infants who required resuscitation with higher oxygen concentrations at birth,19 as well as profiles predictive of “low” or “high” white matter damage.20 Similarly, lipid peroxidation profiles associated with the severity of hypoxic-ischaemic encephalopathy have been defined in samples such as cord blood.21 However, to date, there are no studies that have analysed urine biomarker profiles in the first hours/days of life of VPTN/EPTN, indicative of early brain damage and potentially predictive of subsequent progression to ND.
ND and prematurity: longitudinal epigenetic modificationsThe gene-environment interaction model is a promising theory to study the aetiopathogenesis and pathophysiological mechanisms involved in the genesis and course of NDs in general, and in the “high-risk” VPTN/EPTN population in particular. According to this model, adverse perinatal events such as those mentioned above would have a negative impact on the neurodevelopment of preterm newborns through epigenetic modification of DNA in specific genes involved in normal or “typical” neurodevelopment.22 Therefore, the study of “risk” and “protective” environmental factors and of the presence and pattern of longitudinal epigenetic modifications related to the onset of ND in VPTN/EPTN, and to its course, is particularly relevant, because many of these factors are detectable, preventable, or modifiable.
A few studies to date have studied methylation patterns in preterm children who develop an ND, finding, in a cross-sectional manner, and by focusing on candidate genes, alterations in methylation in some of the multiple genes associated with ASD23,24 or ADHD,25 without having performed longitudinal follow-up of these patterns.
Objectives and hypothesisThe overall objective of the PeriSTRESS-PremTEA study is to evaluate a cohort of VPTN newborns (≤32 0/0 GA), including EPTN (≤28 0/0 GA), and a cohort of born-at-term healthy controls, matched for sex and date of birth, to: (i) detect the presence of ND red flags, ND traits, and early ND diagnosis, through comprehensive clinical and functional characterisation of these children at different time points, from birth to 6 years of age; (ii) investigate the influence of risk factors for differentially developing a ND in this population, including the risk factor prematurity per se and other environmental insults added at early developmental stages (pre-, peri-, and postnatal); (iii) to investigate the presence of biomarkers of early brain damage in VPTN/EPTN by analysing oxidative stress metabolites and inflammatory activity using minimally invasive procedures (urine samples), and to describe profiles with high predictive value for ND onset and course; and (iv) to analyse longitudinally the presence of epigenetic modifications in preterm infants who develop an ND (compared to those who do not and to born-at-term healthy controls), using high-resolution methylation arrays and whole methylome studies. In this article we present the design of the PeriSTRESS and PremTEA projects and describe the samples recruited to date at birth.
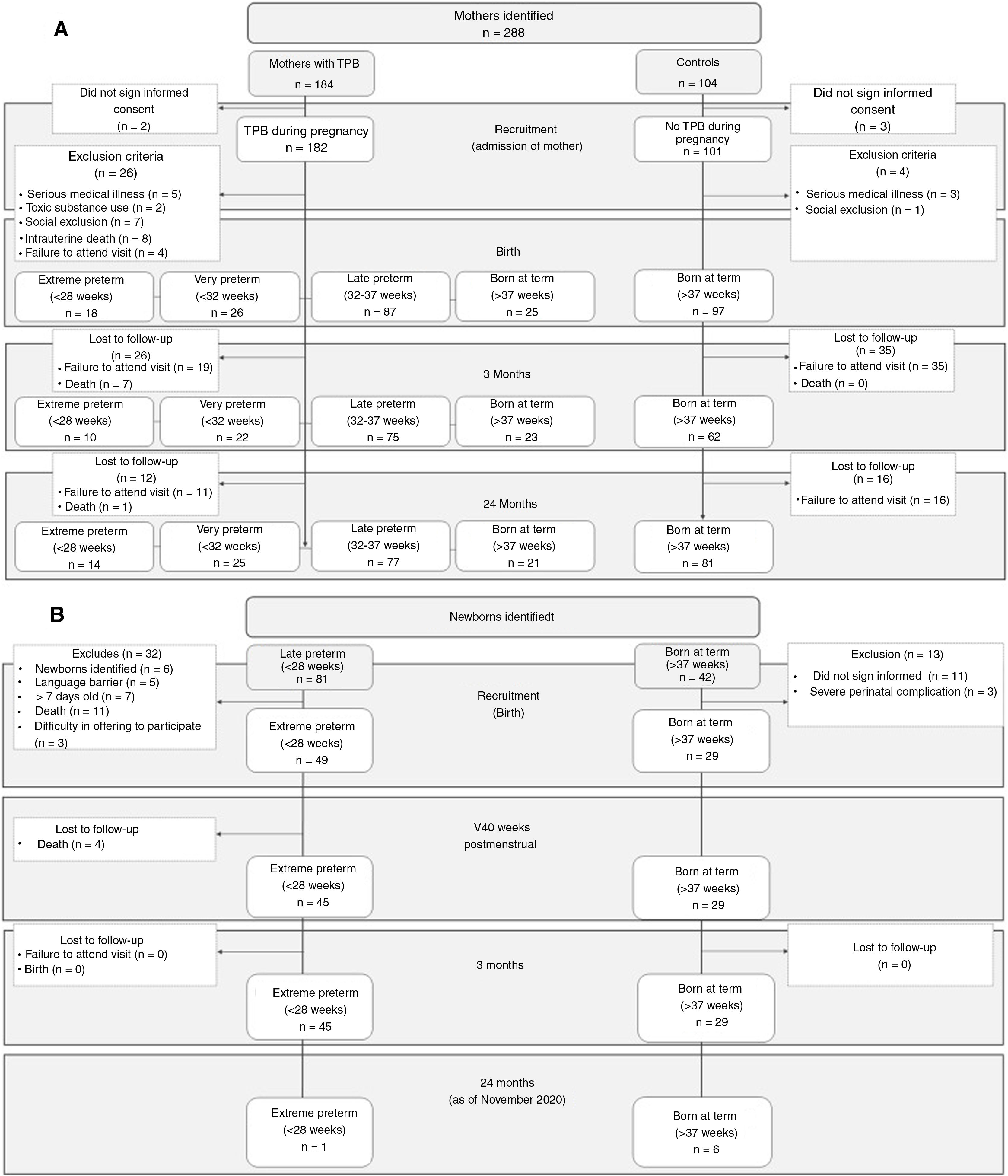
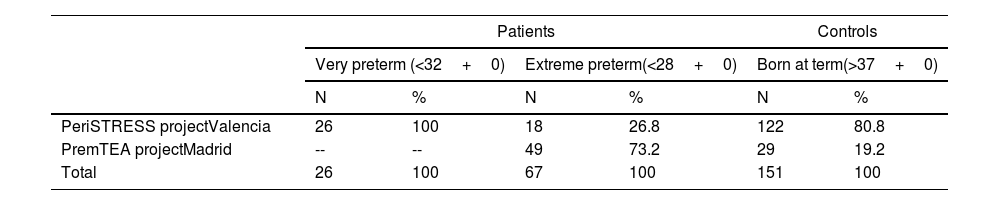
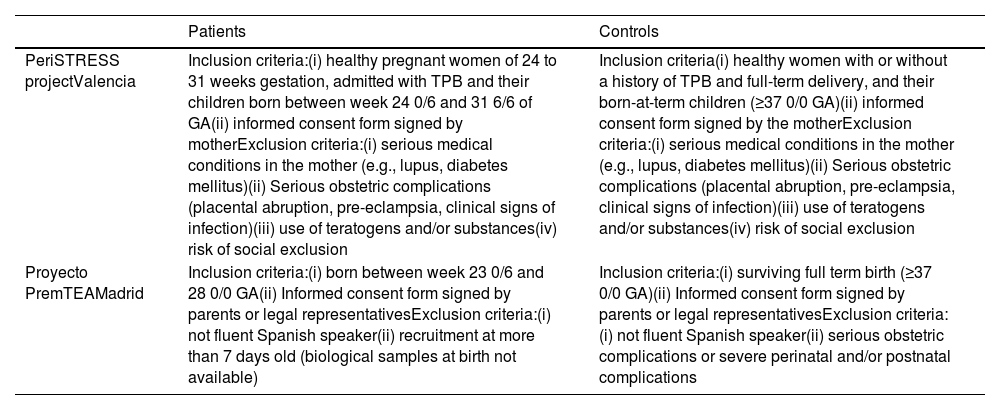
Subjects and methodsSelection procedureBetween January 2015 and November 2020, two university hospital centres in Spain, Hospital Universitario y Politécnico La Fe in Valencia and Hospital General Universitario Gregorio Marañón in Madrid, recruited a cohort of 26 VPTN and 67 EPTN, and a cohort of 151 healthy controls born at term matched by sex and date of birth to the patient group. Table 1a shows the number of patients and controls included in the study per participating centre (Table 1a), and the inclusion and exclusion criteria for patients and controls at each centre (Table 1b).
Patients and controls included by participating centre.
| Patients | Controls | |||||
|---|---|---|---|---|---|---|
| Very preterm (<32+0) | Extreme preterm(<28+0) | Born at term(>37+0) | ||||
| N | % | N | % | N | % | |
| PeriSTRESS projectValencia | 26 | 100 | 18 | 26.8 | 122 | 80.8 |
| PremTEA projectMadrid | -- | -- | 49 | 73.2 | 29 | 19.2 |
| Total | 26 | 100 | 67 | 100 | 151 | 100 |
As of 30 November 2020.
Inclusion and exclusion criteria per participating centre.
| Patients | Controls | |
|---|---|---|
| PeriSTRESS projectValencia | Inclusion criteria:(i) healthy pregnant women of 24 to 31 weeks gestation, admitted with TPB and their children born between week 24 0/6 and 31 6/6 of GA(ii) informed consent form signed by motherExclusion criteria:(i) serious medical conditions in the mother (e.g., lupus, diabetes mellitus)(ii) Serious obstetric complications (placental abruption, pre-eclampsia, clinical signs of infection)(iii) use of teratogens and/or substances(iv) risk of social exclusion | Inclusion criteria(i) healthy women with or without a history of TPB and full-term delivery, and their born-at-term children (≥37 0/0 GA)(ii) informed consent form signed by the motherExclusion criteria:(i) serious medical conditions in the mother (e.g., lupus, diabetes mellitus)(ii) Serious obstetric complications (placental abruption, pre-eclampsia, clinical signs of infection)(iii) use of teratogens and/or substances(iv) risk of social exclusion |
| Proyecto PremTEAMadrid | Inclusion criteria:(i) born between week 23 0/6 and 28 0/0 GA(ii) Informed consent form signed by parents or legal representativesExclusion criteria:(i) not fluent Spanish speaker(ii) recruitment at more than 7 days old (biological samples at birth not available) | Inclusion criteria:(i) surviving full term birth (≥37 0/0 GA)(ii) Informed consent form signed by parents or legal representativesExclusion criteria:(i) not fluent Spanish speaker(ii) serious obstetric complications or severe perinatal and/or postnatal complications |
In Figure 1, Figure 1a and b show the recruitment flowcharts of the PeriSTRESS and PremTEA projects, respectively. In both centres, patients were recruited consecutively at birth in each centre's NICU and included in the national clinical follow-up programme SEN1500.26 The PeriSTRESS project recruited healthy pregnant mothers of 24 to 31 weeks gestation, admitted to the hospital's obstetrics unit with a diagnosis of threatened preterm birth (TPB) and their infants. The PremTEA project focused on recruitment at birth of infants born less than 28 weeks 0/0 GA. In PeriSTRESS (start of recruitment 2015), a total of 26 VPTN and 18 EPTN, and a total of 122 controls (25 born to TPB+women, and 97 born to TPB- women) met the inclusion criteria and completed the initial assessment. Of these, 25 VPTN, 14 EPTN, and 102 controls completed the 2-year follow-up period (with the first follow-up visit estimated at age 6 for the first patient and the first follow-up in the first quarter of 2021). The main reasons for withdrawal from the study were death and loss to follow-up. In PremTEA (start of recruitment 2018), a total of 49 EPTN and 29 controls met the inclusion criteria and completed the initial assessment. Of these, 1 EPTN and 6 controls completed the 24-month follow-up period (as of 30 November 2020).
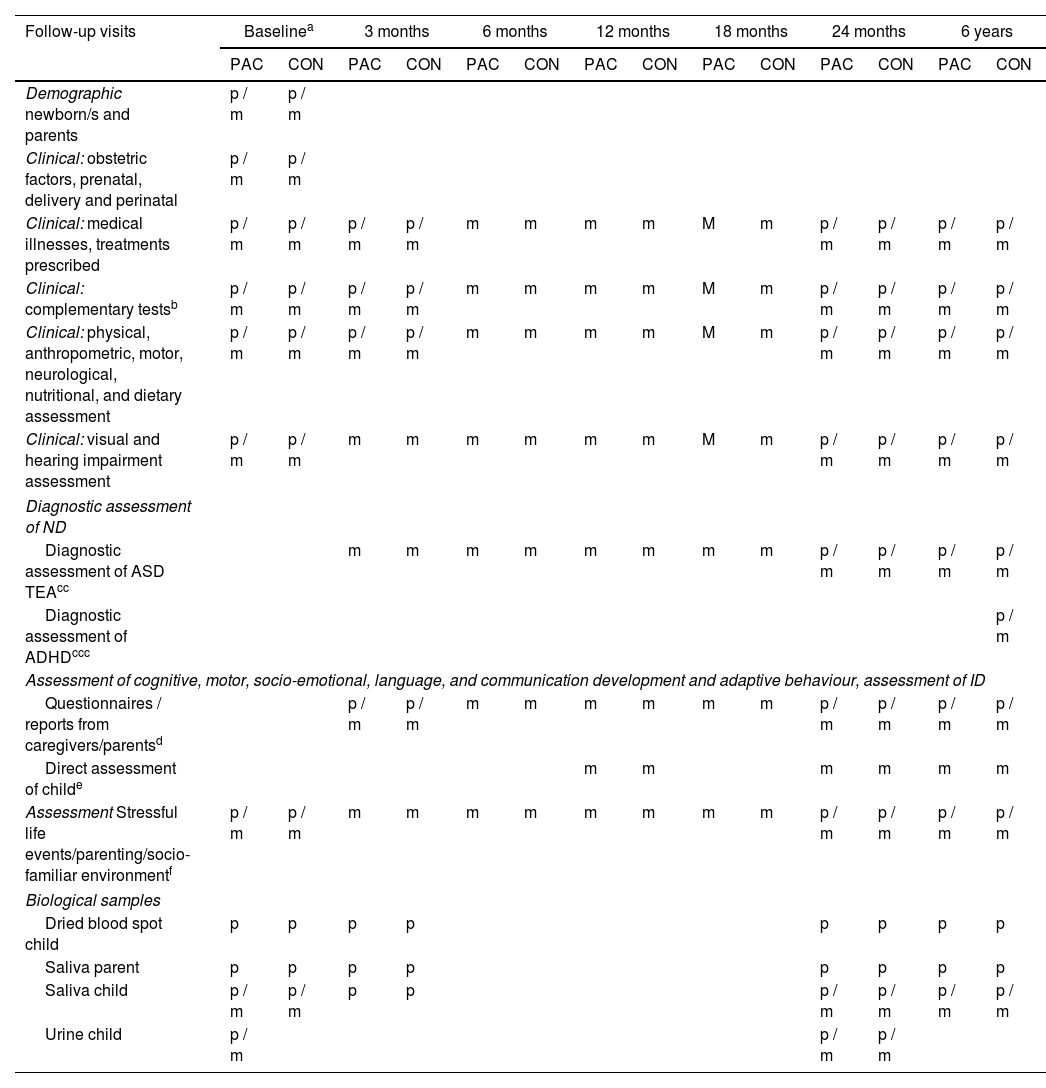
DesignThe PeriSTRESS-PremTEA study is a prospective longitudinal study in which several assessment visits are conducted, from the time of TPB (in the case of PeriSTRESS - patients) or birth (in the case of PeriSTRESS - controls, and PremTEA - patients and controls) until 6 years of age. Table 2 shows the visit schedule for each study and the assessments made at each visit.
Assessment visits for patients and controls - PeriSTRESS and PremTEA cohorts.
| Follow-up visits | Baselinea | 3 months | 6 months | 12 months | 18 months | 24 months | 6 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC | CON | PAC | CON | PAC | CON | PAC | CON | PAC | CON | PAC | CON | PAC | CON | |
| Demographic newborn/s and parents | p / m | p / m | ||||||||||||
| Clinical: obstetric factors, prenatal, delivery and perinatal | p / m | p / m | ||||||||||||
| Clinical: medical illnesses, treatments prescribed | p / m | p / m | p / m | p / m | m | m | m | m | M | m | p / m | p / m | p / m | p / m |
| Clinical: complementary testsb | p / m | p / m | p / m | p / m | m | m | m | m | M | m | p / m | p / m | p / m | p / m |
| Clinical: physical, anthropometric, motor, neurological, nutritional, and dietary assessment | p / m | p / m | p / m | p / m | m | m | m | m | M | m | p / m | p / m | p / m | p / m |
| Clinical: visual and hearing impairment assessment | p / m | p / m | m | m | m | m | m | m | M | m | p / m | p / m | p / m | p / m |
| Diagnostic assessment of ND | ||||||||||||||
| Diagnostic assessment of ASD TEAcc | m | m | m | m | m | m | m | m | p / m | p / m | p / m | p / m | ||
| Diagnostic assessment of ADHDccc | p / m | |||||||||||||
| Assessment of cognitive, motor, socio-emotional, language, and communication development and adaptive behaviour, assessment of ID | ||||||||||||||
| Questionnaires / reports from caregivers/parentsd | p / m | p / m | m | m | m | m | m | m | p / m | p / m | p / m | p / m | ||
| Direct assessment of childe | m | m | m | m | m | m | ||||||||
| Assessment Stressful life events/parenting/socio-familiar environmentf | p / m | p / m | m | m | m | m | m | m | m | m | p / m | p / m | p / m | p / m |
| Biological samples | ||||||||||||||
| Dried blood spot child | p | p | p | p | p | p | p | p | ||||||
| Saliva parent | p | p | p | p | p | p | p | p | ||||||
| Saliva child | p / m | p / m | p | p | p / m | p / m | p / m | p / m | ||||||
| Urine child | p / m | p / m | p / m | |||||||||||
m: PremTEA; p: PeriSTRESS.
PeriSTRESS project: patients (and mothers and mothers with a diagnosis of threatened preterm TPB+), collection of variables between the time of TPB+ and 48 h after delivery; controls between time of birth and 48 h after delivery. PremTEA project: patients, collection of demographic and clinical variables between birth and post-menstrual week 40; controls between birth and 7 days after delivery.
Those required by clinical indication. At baseline visit includes amplitude integrated electroencephalography (aEEG) and cerebral Doppler ultrasound, somatosensory potentials, magnetic resonance imaging (MRI) at post-menstrual week 40, collecting data on presence of periventricular haemorrhage (PVH) and periventricular leukomalacia (PVL), and presence of cerebellar lesion.
TEA PeriSTRESS diagnostic assessment: at 24-30 months, parent/caregiver questionnaire M-CHAT-R (Modified Checklist for Autism in Toddlers Revised version), followed by observation of the child and parent/caregiver interview ADI-R (Autism Diagnostic Interview Revised version) if M-CHAT-R indicates moderate/high risk and/or history suggestive of ASD. At age 6, ADI-R if history is suggestive or ASD diagnosis in previous assessments. PremTEA: at 3, 6, 12, and 18 months: assessment of ASD red flags; at 18, 24, 30 months parent/caregiver questionnaire M-CHAT-R, followed by assessment of the child Autism Diagnostic Observation Schedule – Toddler version (ADOS-Toddler) if M-CHAT-R indicates moderate/high risk and/or history suggestive of ASD; to which is added Childhood Autism Rating Scale (CARS) and ADI-R from the age of 24 months. At age 6, ADOS-2, CARS, and ADI-R if history is suggestive or a diagnosis of ASD in previous assessments. In both projects: confirmation of ASD diagnosis applying DSM-5 criteria after integrating the clinical information obtained through clinical interview and through these instruments, plus study of medical, early care, and pedagogical reports, when available.
Diagnostic assessment ADHD: in both projects, at age 6, parent/caregiver questionnaires Strengths and Difficulties Questionnaire (SDQ) and Revised Conners’ Parent Rating Scale (CPRS-R) version for children over 3 years of age. Confirmation of diagnosis of ADHD applying ICD-10 criteria after integrating the clinical information obtained through clinical interview and through these instruments, direct observation of the child, plus study of medical, early care, and pedagogical reports, when available.
Questionnaires. Common to PeriSTRESS and PremTEA: psychomotor development (Ages & Stages Questionnaires®, Third Edition (ASQ®-3), emotional dysregulation (Mary Rothbart's Temperament Questionnaires), executive function (Behaviour Rating Inventory of Executive Function – Preschool Version, BRIEF-P), behavioural problems (Child Behaviour Checklist, CBCL); global functioning/adaptive behaviour (Vineland-3 Scale). PremTEA specific: language and communication (Communication and Symbolic Behaviour Scales, CSBS; MacArthur-Bates Communicative Development Inventory); Adaptive behaviour and self-care, social-emotional and temperament questionnaires included from the Merrill-Palmer R (MP-R). In both projects: confirmation of ID diagnosis applying DSM-5 criteria after integrating the clinical information obtained through clinical interview and through these instruments, plus study of medical, early care, and pedagogical reports, when available.
At the time of recruitment, an informed consent form is signed, and minimum essential demographic data collected, leaving for later (48h after delivery in the case of PeriSTRESS, or at post-menstrual week 40 in the case of PremTEA) a full assessment that includes a more extensive record of demographic, clinical, and socio-familial data. In the PeriSTRESS project, for both patients and controls, all the assessments shown in Table 2 (baseline, 3 months, 24 months, and 6 years) are conducted face-to-face. In the PremTEA project, all patient assessments are conducted face-to-face, coinciding with the routine clinical visit of the SEN1500 follow-up programme. In the case of controls, all assessments are in person (except for the 3- and 6-month assessments, which are conducted by telephone). Exceptionally, all patient and control visits that coincided with the period of the state of alarm decreed in Spain during the COVID pandemic, from March to June 2020, were also conducted by telephone.
Analysis of the epigenome in peripheral blood and salivaIn the PeriSTRESS project, epigenome analysis is performed on both the child's peripheral venous blood cells (at baseline), extracted by a minimally invasive method called “dried blood spot”, and on saliva samples from mothers and children, obtained with sterile swabs at baseline, 3 months, 24 months, and 6 years. In the PremTEA project, epigenome analysis is performed on saliva samples from the child, obtained as close as possible to the date of birth in patients (before 7 days of life), and at post-menstrual week 40 and month 24 in patients and controls. Once the blood or saliva samples have been obtained, Qiagen's QIAamp DNA Investigator Kit (in PeriSTRESS) and PrepIT-L2P5 (in PremTEA) are used for DNA extraction, optimised for samples of this nature. The DNA samples obtained are coded and stored at -80°C according to the requirements of Law 14/2007 on Biomedical Research, and then sent to a genotyping centre for whole genome methylome analysis “WGMA” using Illumina MethylationEPIC BeadChip methylation array. To validate the methylation profile obtained, a pyrosequencing study is later performed using the Pyromark Q24 pyrosequencer from Werfen.
The statistical software R will be primarily used for processing the methylation array data, and packages designed specifically for the analysis of bioconductor methylation data such as methyAnalysis27 or others adapted for reading Illumina methylation chips such as methylumiR.28 Using these programmes, we will analyse the methylation data in the loci contained on the chip to identify regions of differential methylation, analysing significant case-control differences and making the subsequent annotation. From the results obtained, the pathways mostly involved in the onset of an ND in cases (vs. controls) will be searched for using available and appropriate software such as GREAT29 or EpiExplorer.30
Biomarkers of oxidative stress and inflammation in urineThe metabolomic study is performed only in the patient group. Urine is collected at birth at three time points: at the 1st micturition (during the first 24h of life), in the first 24-72h of life, and in the first 3-7 days of life. An additional urine sample is also collected in each case of hospital readmission. The urine sample is collected non-invasively by neonatology staff from sterile gauze in the nappy or from a urine bag in the nappy, from which 2ml of urine collected in standard Eppendorf tubes is separated. The tubes contain butylated hydroxytoluene (BHT, .25% [w/v]) to prevent further oxidation of the samples and are immediately stored at a temperature of -80°C. The samples are pseudo-anonymised (they are assigned a number dissociated from their identity and a sampling time). Once collected, all samples will be sent to the Hospital Universitario y Politécnico La Fe (frozen and on dry ice) for metabolomic processing, and the remainder will be stored following the requirements of the Law 14/2007 on Biomedical Research in the Biological Sample Collection of the Instituto de Investigación Sanitaria La Fe (IISLaFe).
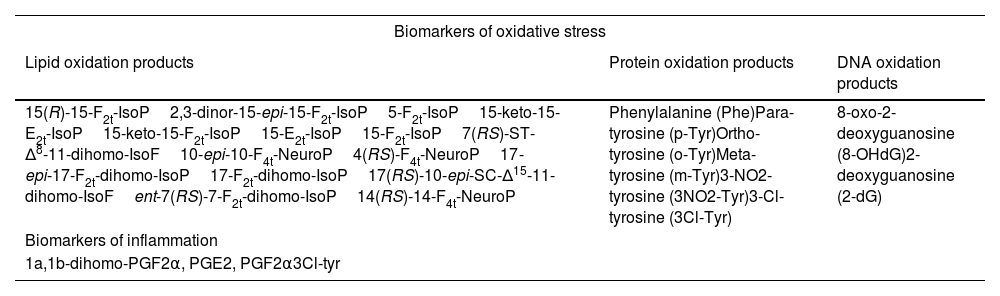
The processing will aim to determine metabolite profiles of lipid peroxidation in urine and prostaglandins, as well as oxidative damage to proteins and DNA, biomarkers that are listed in Table 3.
Urine biochemical determinations in patients: biomarker panel.
| Biomarkers of oxidative stress | ||
|---|---|---|
| Lipid oxidation products | Protein oxidation products | DNA oxidation products |
| 15(R)-15-F2t-IsoP2,3-dinor-15-epi-15-F2t-IsoP5-F2t-IsoP15-keto-15-E2t-IsoP15-keto-15-F2t-IsoP15-E2t-IsoP15-F2t-IsoP7(RS)-ST-Δ8-11-dihomo-IsoF10-epi-10-F4t-NeuroP4(RS)-F4t-NeuroP17-epi-17-F2t-dihomo-IsoP17-F2t-dihomo-IsoP17(RS)-10-epi-SC-Δ15-11-dihomo-IsoFent-7(RS)-7-F2t-dihomo-IsoP14(RS)-14-F4t-NeuroP | Phenylalanine (Phe)Para-tyrosine (p-Tyr)Ortho-tyrosine (o-Tyr)Meta-tyrosine (m-Tyr)3-NO2-tyrosine (3NO2-Tyr)3-Cl-tyrosine (3Cl-Tyr) | 8-oxo-2-deoxyguanosine (8-OHdG)2-deoxyguanosine (2-dG) |
| Biomarkers of inflammation | ||
| 1a,1b-dihomo-PGF2α, PGE2, PGF2α3Cl-tyr | ||
The determinations included in the final project may be modified depending on scientific advances in this field of research. Measurement of biomarker levels by ultra-high performance liquid chromatography coupled with mass spectrometry (UPLC-MS/MS), consisting of a Waters Acquity UPLC-Xevo TQD system. The chromatographic separation conditions using an Acquity UPLC BEH C18 column (2.1 x 50mm, 1.7μm) and a pre-column (2.1 x 5mm) are: i) binary mobile phase methanol (.01% v/v acetic acid): water (.01% v/v acetic acid, pH 3) with gradient elution; ii) flow rate .4mL/min; iii) column temperature 37°C; iv) injection volume 10 microlitres. Mass spectrometric detection is carried out by multiple reaction monitoring (MRM). To standardise results, urine creatinine levels will be determined using a colorimetric assay kit (MicroVue creatinine EIA) by spectrophotometer.
A database was created in each centre to integrate all the information available in each project and facilitate data management and exploitation. The participating groups’ clinical and demographic characteristics were compared for each of the projects. Fisher's exact test or the χ2 test was used to compare discrete categorical variables. For the comparison of quantitative variables, with parametric/non-parametric distribution, ANOVA/Kruskal-Wallis (PeriSTRESS) and Student's t test/ Mann Whitney U test (PremTEA) were used. Statistical analyses were performed with SPSS v18.31 Differences with a p-value <.05 were considered significant. All tests were two-tailed.
Ethical aspectsAll procedures used for this study comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, revised in 2008. The ethics committees of Hospital Universitario y Politécnico La Fe and Hospital General Universitario Gregorio Marañón approved the study protocols, participant information sheets, and informed consent forms. Written informed consent was obtained from all study participants or their legal representatives.
ResultsThe clinical and demographic characteristics at birth of VPTN and/or EPTN patients and controls included in the PeriSTRESS and PremTEA projects are shown in Table 4a and Table 4b, respectively.
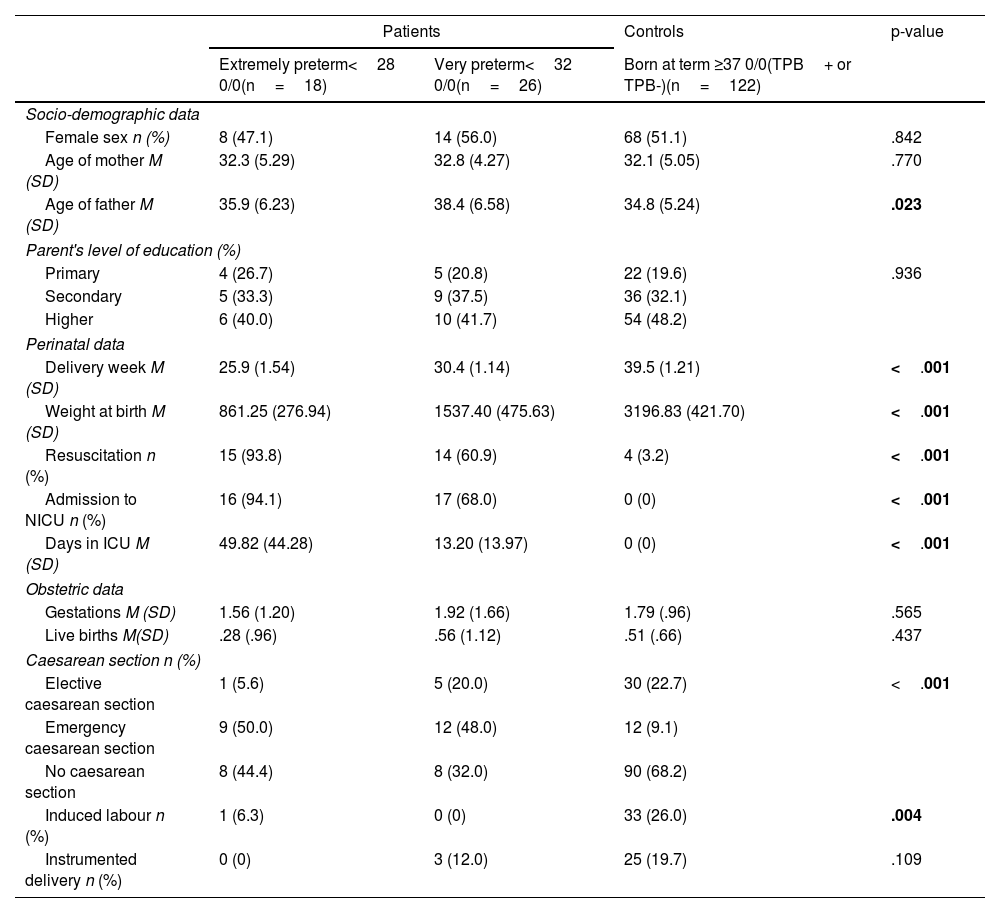
Demographic and clinical characteristics at birth of patients and controls of the PeriSTRESS – Valencia cohort.
| Patients | Controls | p-value | ||
|---|---|---|---|---|
| Extremely preterm<28 0/0(n=18) | Very preterm<32 0/0(n=26) | Born at term ≥37 0/0(TPB+ or TPB-)(n=122) | ||
| Socio-demographic data | ||||
| Female sex n (%) | 8 (47.1) | 14 (56.0) | 68 (51.1) | .842 |
| Age of mother M (SD) | 32.3 (5.29) | 32.8 (4.27) | 32.1 (5.05) | .770 |
| Age of father M (SD) | 35.9 (6.23) | 38.4 (6.58) | 34.8 (5.24) | .023 |
| Parent's level of education (%) | ||||
| Primary | 4 (26.7) | 5 (20.8) | 22 (19.6) | .936 |
| Secondary | 5 (33.3) | 9 (37.5) | 36 (32.1) | |
| Higher | 6 (40.0) | 10 (41.7) | 54 (48.2) | |
| Perinatal data | ||||
| Delivery week M (SD) | 25.9 (1.54) | 30.4 (1.14) | 39.5 (1.21) | <.001 |
| Weight at birth M (SD) | 861.25 (276.94) | 1537.40 (475.63) | 3196.83 (421.70) | <.001 |
| Resuscitation n (%) | 15 (93.8) | 14 (60.9) | 4 (3.2) | <.001 |
| Admission to NICU n (%) | 16 (94.1) | 17 (68.0) | 0 (0) | <.001 |
| Days in ICU M (SD) | 49.82 (44.28) | 13.20 (13.97) | 0 (0) | <.001 |
| Obstetric data | ||||
| Gestations M (SD) | 1.56 (1.20) | 1.92 (1.66) | 1.79 (.96) | .565 |
| Live births M(SD) | .28 (.96) | .56 (1.12) | .51 (.66) | .437 |
| Caesarean section n (%) | ||||
| Elective caesarean section | 1 (5.6) | 5 (20.0) | 30 (22.7) | <.001 |
| Emergency caesarean section | 9 (50.0) | 12 (48.0) | 12 (9.1) | |
| No caesarean section | 8 (44.4) | 8 (32.0) | 90 (68.2) | |
| Induced labour n (%) | 1 (6.3) | 0 (0) | 33 (26.0) | .004 |
| Instrumented delivery n (%) | 0 (0) | 3 (12.0) | 25 (19.7) | .109 |
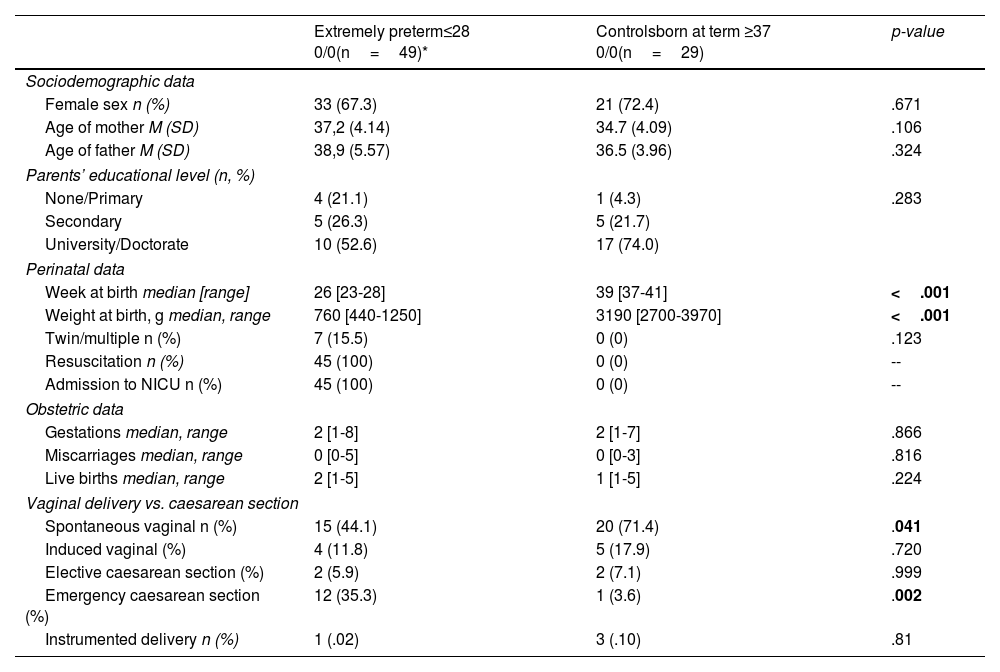
Demographic and clinical characteristics at birth of patients and controls in the PremTEA – Madrid cohort.
| Extremely preterm≤28 0/0(n=49)* | Controlsborn at term ≥37 0/0(n=29) | p-value | |
|---|---|---|---|
| Sociodemographic data | |||
| Female sex n (%) | 33 (67.3) | 21 (72.4) | .671 |
| Age of mother M (SD) | 37,2 (4.14) | 34.7 (4.09) | .106 |
| Age of father M (SD) | 38,9 (5.57) | 36.5 (3.96) | .324 |
| Parents’ educational level (n, %) | |||
| None/Primary | 4 (21.1) | 1 (4.3) | .283 |
| Secondary | 5 (26.3) | 5 (21.7) | |
| University/Doctorate | 10 (52.6) | 17 (74.0) | |
| Perinatal data | |||
| Week at birth median [range] | 26 [23-28] | 39 [37-41] | <.001 |
| Weight at birth, g median, range | 760 [440-1250] | 3190 [2700-3970] | <.001 |
| Twin/multiple n (%) | 7 (15.5) | 0 (0) | .123 |
| Resuscitation n (%) | 45 (100) | 0 (0) | -- |
| Admission to NICU n (%) | 45 (100) | 0 (0) | -- |
| Obstetric data | |||
| Gestations median, range | 2 [1-8] | 2 [1-7] | .866 |
| Miscarriages median, range | 0 [0-5] | 0 [0-3] | .816 |
| Live births median, range | 2 [1-5] | 1 [1-5] | .224 |
| Vaginal delivery vs. caesarean section | |||
| Spontaneous vaginal n (%) | 15 (44.1) | 20 (71.4) | .041 |
| Induced vaginal (%) | 4 (11.8) | 5 (17.9) | .720 |
| Elective caesarean section (%) | 2 (5.9) | 2 (7.1) | .999 |
| Emergency caesarean section (%) | 12 (35.3) | 1 (3.6) | .002 |
| Instrumented delivery n (%) | 1 (.02) | 3 (.10) | .81 |
The PeriSTRESS-PremTEA study is the first multicentre longitudinal observational longitudinal study to analyse, in VPTN, EPTN, and healthy controls born at term, the interaction between multiple environmental factors (prenatal, perinatal, and postnatal) and biological factors measured non-invasively and early (markers of oxidative stress and inflammation), as well as epigenetic pattern variations over the first six years of life, including the socio-familial environment) and biological factors measured non-invasively and early (markers of oxidative stress and inflammation), as well as variations in the epigenetic pattern throughout the first six years of the infant's life, and their association with the onset and course of NDs such as ASD, ADHD, or ID.
The present study is a unique opportunity to study highly prevalent disorders such as ASD, ADHD, or ID in their earliest stages and in a particularly vulnerable population, such as VPTN and EPTN. The ultimate aim is to describe risk factors and biomarkers that allow us to propose a theoretical model that, from a multidisciplinary perspective, brings us closer to understanding the pathogenesis and pathophysiology of these NDs or, at least, of ND subtypes with differential pathophysiological pathways. The identification of early markers that discriminate those preterm newborns who will evolve to a certain ND subtype versus those who will not, will allow us to identify the subgroup of patients in which early intervention (including, among others, early stimulation, cognitive rehabilitation, or psychoeducation) and prevention strategies can be implemented. Furthermore, if the presence of a differential pathophysiological pathway involving elements of inflammation/oxidative stress in subgroups of patients with a given ND and a history of prematurity is confirmed, this would provide the opportunity to develop new therapeutic and preventive strategies in these “at risk” groups, who, presenting similar phenotypes to other subjects with ND, would benefit from more personalised specific interventions, based on the profiles of the individual and not on the disease (e.g. use of anti-inflammatory drugs, immunomodulators, etc.). Such strategies could help to improve the prognosis and, ultimately, the functionality and quality of life of these patients, from clinical practice based on evidence, technology, and personalisation. We believe therefore that this is a scientific project of unique characteristics, which, if implemented, will bring knowledge that is currently greatly needed for these children and for society as a whole.
The results from this study may also lead to improvement of current protocols for the follow-up of preterm infants and to the possible creation of specific perinatal mental health units for the care of this at-risk population. Our results would justify the inclusion of mental health specialists in neonatal follow-up protocols who can: (i) design ND prevention programmes that consider risk factors as well as protective factors; (ii) detect ND early through comprehensive clinical and psychometric examinations, integrated within the current follow-up protocols; and (iii) design early interventions to reduce the risk of ND and based on the results of the study.
The present study has a number of limitations. Firstly, there is difficulty recruiting and following up the sample, given the high rate of deaths in the group of very preterm newborns and, more specifically, in extreme preterm newborns, with an estimated average mortality rate of 28% in the first days of life. Secondly, the large number of clinical and demographic variables collected may make statistical analyses difficult for establishing the risk and protective factors contributing to the onset and course of the different NDs. In relation to epigenetic determinations, the greatest limitation is the use of non-brain tissue in the analysis of methylation. However, as can be seen in Table 1b, the PeriSTRESS and PremTEA projects present common inclusion and exclusion criteria, as well as others specific to each project. It should be noted that there is a “potentially combinable” subsample from a methodological point of view, the subsample comprising children born below 28 0/0 weeks’ GA and healthy controls >37 0/0 weeks born to women with TPB. Inclusion/exclusion criteria would be unified in these subsamples to make them comparable. Similarly, as shown in Table 2, and despite the arduous task of homogenising the protocols of the two studies, in some cases a certain variable will not be available in both studies (e.g., Merrill-Palmer R developmental scales at 12 and 24 months only collected in PremTEA). In any case, most of the scales and times of application are common to both projects, and the fundamental variables of the study, which define the samples, prematurity, and ASD/ADHD/ID diagnosis, can be combined because they are based on a clinical diagnosis made by neonatologists and child psychiatrists/psychologists, respectively, with extensive experience in the field of the study, and using diagnostic classification nomenclatures such as DSM-5 or ICD-10. In cases where it is not possible to combine samples due to incompatibility of inclusion/exclusion criteria or incompatibility of measurement instruments, independent studies will be conducted to replicate findings, based on common study hypotheses. That said, the strengths of the PeriSTRESS-PremTEA project are its attempt to overcome the limitations of previous studies by using whole methylome analysis techniques or the study of biomarkers in urine at different times in the first hours/days of life, and its longitudinal, prospective, observational, and multicentre design.
In summary, the PeriSTRESS-PremTEA study will improve strategies for the early identification of NDs such as ASD, ADHD, or ID, as well as the study of their clinical and biological predictors, in a high-risk population such as VPTN and EPTN. This will allow the development, from a translational point of view, of early detection strategies in these particularly vulnerable population subgroups. Furthermore, if differential pathophysiological pathways for the onset of each of these NDs in preterm newborns (ASD, ADHD, ID) are confirmed, this opens the door to the design and implementation of both early intervention and prevention strategies, specific and personalised, based on the profiles of the individual and not of the disorder. Such strategies may ultimately help to improve the prognosis and functionality of these children, as well as their quality of life and that of their families. The results may also support the development and inclusion of specialists and protocols for prevention and intervention in the area of perinatal mental health in existing VPTN/EPTN monitoring and follow-up programmes such as the SEN1500 programme, through a multidisciplinary approach.
FundingWith the support of the Ministry of Science, Innovation, and Universities. Instituto de Salud Carlos III (PI17/00131, PI17/01997, PI18/01352); co-financed with European Union ERDF Funds “A way for Europe”, CIBERSAM; European Union H2020 Programme “Innovative Medicines Initiative 2 Joint Undertaking” (project code 77394; AIMS-2-TRIALS project), and ERA-NET NEURON (Network of European Funding for Neuroscience Research) Fundación Familia Alonso, Fundación Mutua Madrileña, and Fundación Alicia Koplowitz.
Authorship/collaboratorsPina-Camacho and García-Blanco contributed equally to the paper as co-senior authors.
Conflict of interestsPN, FG, DBB, MV, LPC, and AGB have received grants from the Instituto de Salud Carlos III, Ministerio de Ciencia, Innovación y Universidades. CCP, DBB, MV, LPC, and AGB have received grants from the Alicia Koplowitz Foundation. BA has received support from the Ministry of Education and Science. JMN, JGP, ERT, SZ, MA and PCC have no conflicts of interest to declare.
We would like to thank all the participants in the study and their families.