This study aims to assess the validity of the ADHD module of the Mini-International Neuropsychiatric Interview (MINI-Plus) in patients with substance use disorders (SUD), using the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID) as the external criterion.
MethodA cross sectional international multi-center study in 10 countries was conducted in treatment seeking SUD patients. A sample of 1263 patients with both MINI-Plus and CAADID was analyzed to determine the psychometric properties of the MINI-Plus.
ResultsAccording to the CAADID, 179 patients (14.2%) met criteria for adult ADHD, whereas according to the MINI-Plus 227 patients (18.0%) were identified as having adult ADHD. Sensitivity of the MINI-Plus ADHD module was 74%, specificity was 91%, positive predictive value was 60% and negative predictive value was 96%. Kappa was 0.60.
ConclusionThe MINI-Plus has acceptable criterion validity for the screening of adult ADHD in treatment seeking SUD patients.
Scientific significanceOn the basis of the results, The MINI-Plus may be used for the screening of ADHD in SUD patients.
Attention-deficit/hyperactivity disorder (ADHD) is a childhood onset disorder that frequently persists into adulthood characterized by inattention and/or hyperactivity–impulsivity.1–3 In the general population, the prevalence of ADHD in adults ranges between 2 and 5%.1,2 ADHD has been associated with many psychiatric comorbidities, including mood disorders, anxiety and substance use disorders (SUD).1–4 More than 30% of patients with ADHD also develop lifetime SUD,5 and ADHD is considered a risk factor for the development of SUD.6 On the other hand, about 20–25% of adult SUD patients fulfill criteria for ADHD.4 It should be noted, that the prevalence of ADHD in SUD patients varies due to differences between classification systems (DSM-IV vs. DSM-5), diagnostic instruments, primary substance used (alcohol vs. drugs), and treatment setting (inpatients vs. outpatients).7 In a meta-regression analysis, a substantial part of the between study heterogeneity of ADHD prevalence in SUD patients was explained by differences in the diagnostic instruments that were used.8
Diagnosing ADHD in SUD patients is a challenge given the overlap of symptoms,9 and very few diagnostic and screening instruments for ADHD have been validated in SUD patients. This poses obstacles for diagnosis and treatment of these patients.10 This is a clinically urgent issue because the correct diagnosis of ADHD in SUD patients provides a target for treatment. Currently many patients remain undiagnosed and untreated, which has serious implications on their prognosis.11 In past years, research efforts have been made to evaluate screening and diagnostic instruments for the assessment of adult ADHD in SUD patients.11–15 Despite these efforts, many questions remain and more research is needed.
The Mini-International Neuropsychiatric Interview (MINI-Plus) is a fully structured interview that provides a brief and accurate assessment of former Axis 1 and some Axis 2 psychiatric disorders in DSM-IV and ICD-10.16,17 The MINI-Plus provides an accurate diagnosis in some disorders, using a short-structured interview which is well received by patients and professionals.3,17,18 Furthermore, this interview can be performed by lay persons after a brief training.19 Comparisons between the MINI-Plus and the Structured Clinical Interview for DSM-IV-TR axis I disorders (SCID-I)20 have yielded kappa values of 0.70 or above for most diagnoses.21 In a clinical study, the MINI-Plus diagnosed comorbidities, including substance dependence, better than a clinical interview.18 The MINI-Plus has a specific DSM-IV ADHD module (for children and adults), but this interview does not distinguish between presentations of ADHD (inattentive, hyperactive/impulsive, combined). It is interesting to point out that, although the MINI-Plus items for ADHD are not identical to DSM-5 criteria, the MINI-Plus may be used for ADHD detection in adults (with 5 criteria as DSM-5 requires) as this instrument covers the current core ADHD symptoms.3 The ADHD module of the MINI-Plus has never been evaluated in patients with a comorbid SUD diagnosis and no data are available about its psychometric features.
Therefore, the present study aimed to evaluate the criterion validity of the ADHD module of the MINI-Plus among treatment-seeking SUD patients, using the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID)22 as the external criterion. The CAADID is one of the most frequently used semi-structured diagnostic interviews for the classification of the DSM-IV-TR diagnosis adult ADHD.22 It is clinically useful,23 and has shown good concurrent validity with the ADHD module of the Psychiatric Research Interview for Substance and Mental Disorders (PRISM).24
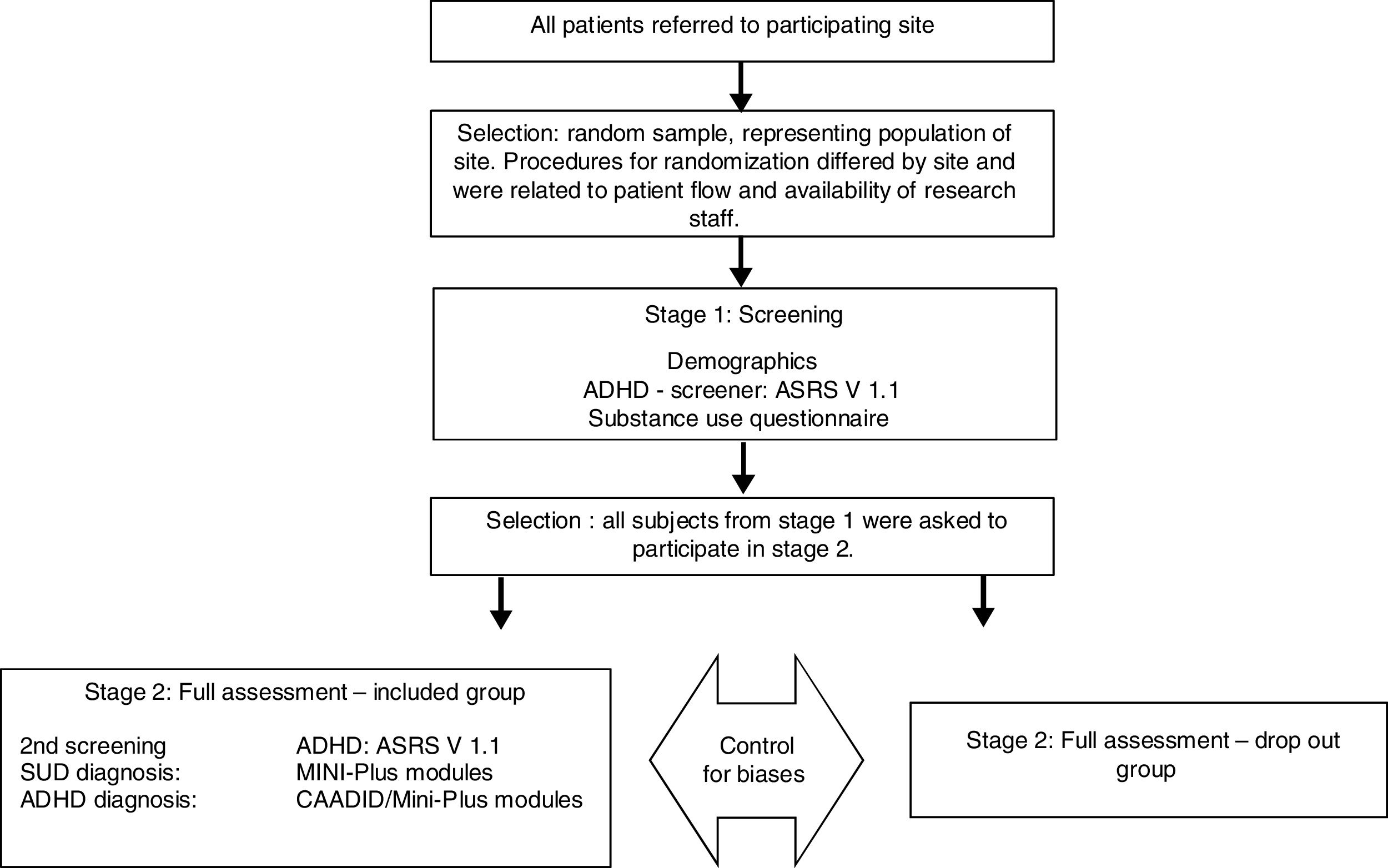
MethodsWe analyzed data from the International ADHD in Substance Use Disorders Prevalence (IASP) study;25 a cross sectional study comprising 47 addiction treatment centers from 10 countries across three continents: Australia, Belgium, France, Hungary, The Netherlands, Norway, Spain, Sweden, Switzerland and The United States.25 The IASP study used a two-staged study design, including a screening and a diagnostic stage directed at ADHD and comorbid psychiatric disorders in treatment-seeking SUD patients,7,25 see Fig. 1. The time between first and second stages was 14 days; the duration of the first stage was one hour, while the second stage required at least three consecutive visits (one-hour session), depending on the patient, in order to complete all the assessment.25 The current validation study only includes IASP participants who completed both the MINI-Plus ADHD module and the CAADID. Therefore, only participants from France, Hungary, Norway, The Netherlands, Spain, Sweden and Switzerland were included.25 Approval was granted at each center by the local medical ethical committee. All participants gave written informed consent.
ParticipantsThe IASP study included adults (age 18–65 years), who were starting a new treatment episode in an addiction treatment center between July 2008 and November 2011 (each center recruited patients for one year). The following exclusion criteria were applied: inadequate language skills, cognitive impairment, substance intoxication, acute psychiatric crisis, severe somatic problems and unwillingness to sign informed consent. Efforts were made to include subjects that were excluded initially (due to substance intoxication, acute psychiatric or medical problems) at a later date.25
ProcedureIn the two-stage design, patients that participated in stage one were invited to continue to stage two. Stage one was a screening phase that assessed sociodemographic variables, substance use and screened for ADHD using the Adult ADHD Self-Report Scale V1.1 (ASRS).26 Stage two was a diagnostic phase consisting of the CAADID and the MINI-Plus ADHD adult module (5.0 version) for the diagnosis of (adult) ADHD. The CAADID was used as the Gold Standard. Trained clinicians administered the interviews, the CAADID and MINI-Plus were administered by the same parson.
Statistical analysisCriterion validity of the MINI-Plus ADHD module was assessed by calculating the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the MINI-Plus diagnosis as a predictor of the CAADID diagnosis. We calculated a 95% confidence interval for each validity estimate. The Kappa index was calculated as a global measure of chance-corrected agreement. Statistical analyses were performed using SPSS version 20.0.
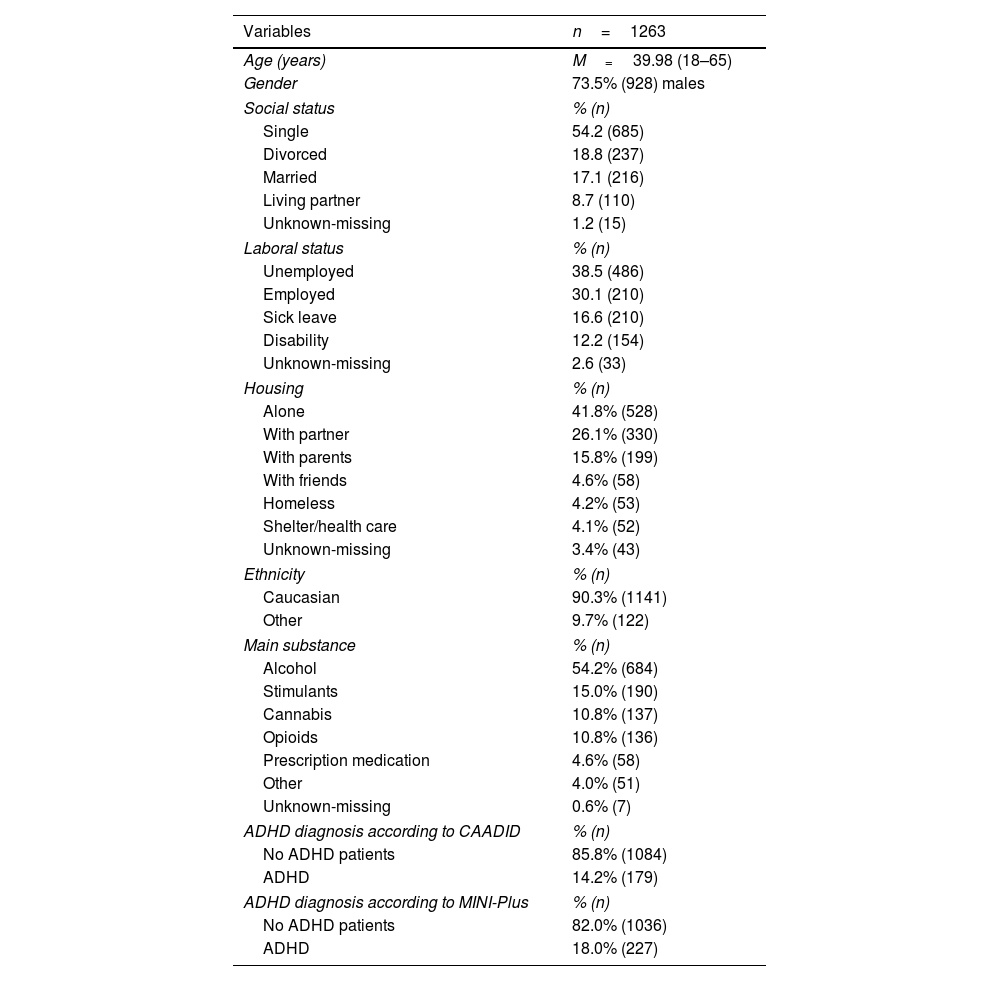
ResultsOf the original IASP sample (N=3558 subjects),27 data on both MINI-Plus ADHD module and CAADID was available for 1263 subjects (final sample for the present study). Table 1 shows the sociodemographic characteristics: mean age 40.0 years (age range=18–65), male (73.5%), Caucasian (90.3%), single (54.2%), unemployed (38.5%), living alone (41.8%). The main substances used were alcohol (54.2%), stimulants (15.0%), cannabis (10.8%), and opioids (10.8%).
Sociodemographic and clinical characteristics.
| Variables | n=1263 |
|---|---|
| Age (years) | M=39.98 (18–65) |
| Gender | 73.5% (928) males |
| Social status | % (n) |
| Single | 54.2 (685) |
| Divorced | 18.8 (237) |
| Married | 17.1 (216) |
| Living partner | 8.7 (110) |
| Unknown-missing | 1.2 (15) |
| Laboral status | % (n) |
| Unemployed | 38.5 (486) |
| Employed | 30.1 (210) |
| Sick leave | 16.6 (210) |
| Disability | 12.2 (154) |
| Unknown-missing | 2.6 (33) |
| Housing | % (n) |
| Alone | 41.8% (528) |
| With partner | 26.1% (330) |
| With parents | 15.8% (199) |
| With friends | 4.6% (58) |
| Homeless | 4.2% (53) |
| Shelter/health care | 4.1% (52) |
| Unknown-missing | 3.4% (43) |
| Ethnicity | % (n) |
| Caucasian | 90.3% (1141) |
| Other | 9.7% (122) |
| Main substance | % (n) |
| Alcohol | 54.2% (684) |
| Stimulants | 15.0% (190) |
| Cannabis | 10.8% (137) |
| Opioids | 10.8% (136) |
| Prescription medication | 4.6% (58) |
| Other | 4.0% (51) |
| Unknown-missing | 0.6% (7) |
| ADHD diagnosis according to CAADID | % (n) |
| No ADHD patients | 85.8% (1084) |
| ADHD | 14.2% (179) |
| ADHD diagnosis according to MINI-Plus | % (n) |
| No ADHD patients | 82.0% (1036) |
| ADHD | 18.0% (227) |
According to the CAADID, 179 patients (14.2%) met criteria for adult ADHD, whereas according to the MINI-Plus 227 patients (18.0%) were identified as having adult ADHD. The sensitivity of the MINI-Plus ADHD module as a predictor of the CAADID was 74.8% (95%CI=[0.68–0.80]). The specificity was 91.4% (95%CI=[0.90–0.93]). The PPV was 60.0% (95%CI=[0.52–0.65]) and the NPV was 95.6% (95%CI=[0.95–0.97]). Finally, the chance-corrected agreement between the MINI-Plus ADHD module and the CAADID showed a Kappa index of 0.60 (95%CI=[0.53–0.66]).
DiscussionIn the current study among treatment seeking SUD patients, the MINI-Plus overestimates the prevalence of adult ADHD compared to the CAADID: 18.0% vs. 14.2%. These results are similar to those reported by researches that have used MINI-Plus for studying ADHD in general population (13.8%).3 Our results could also be related to the good specificity (91%) and moderate sensitivity (74%) of the MINI-Plus. With its high NPV (96%) and moderate PPV (60%), the MINI-Plus is well-suited to detect adult ADHD patients, but patients positive for adult ADHD should be clinically assessed to prevent false positive cases of adult ADHD in treatment-seeking SUD patents. NPV and PPV values may change in samples with a different prevalence of ADHD prevalence.28 These results suggest that the MINI-Plus might be more suitable as a screener than as a diagnostic tool for ADHD in SUD patients.
Our results with the MINI-Plus are better than those with other screening instruments for ADHD in SUD patients. Van de Glind et al. (2013) studied the ASRS as a screening instrument for ADHD in a SUD population with the CAADID as the external criterion.27 The ASRS had a similar NPV (97%) but a much lower PPV (26%) in a similar population with the same prevalence.27 The ASRS had a higher sensitivity (84%) but a much lower specificity (66%) compared with the MINI-Plus. Therefore, the MINI-Plus could be cautiously considered as a better instrument than the ASRS for ADHD screening in treatment seeking SUD patients. This is because, a screening test should have a high sensitivity and good specificity, but however, NPV and PPV are key characteristics due to the fact that they describe the performance of a test in a specific population with a specific, i.e. substantial, prevalence of ADHD.29
According to the Kappa index (0,60), the agreement between the CAADID and MINI-Plus diagnoses was moderate. Previous studies on the diagnostic agreement between MINI-Plus and other diagnostic instruments (e.g., SCID-I) have shown a higher Kappa index for most diagnoses, including adult ADHD.16,21 One study found major diagnostic disagreement in 33% of cases when the MINI-Plus was used, but it was compared with an unstructured interview18 and the study did not specify whether ADHD was actively searched for.
Some studies have used the MINI-Plus to diagnose adult ADHD in other populations not selected for SUD, such as incarcerated participants,30 adult psychiatric outpatients without psychotic disorders,31 patients with mood disorders,32 psychiatric inpatients,33 and patients in an acute psychiatric ward.34 Although DSM-5 criteria for ADHD are not the same to the MINI-Plus items, the latter instrument could be used for ADHD detection in adults (with 5 criteria as DSM-5 requires) because this interview covers the current core ADHD symptoms.3 Moreover, some researches have used, analyzed and adapted the MINI-Plus to fit to DSM-5 five criteria with good results.3 To the best of our knowledge, there are no studies in SUD patients that evaluated the MINI-Plus properties for ADHD. Therefore, this is the first study of the validity of the ADHD module of MINI-Plus in SUD patients.
Adult ADHD in SUD patients has also been assessed with the PRISM,35 a diagnostic instrument that was specifically developed for this population. In a preliminary study, the ADHD section of the PRISM showed high sensitivity and specificity in SUD patients with clinical diagnosis as external criterion.24 It should be noted, however, that the PRISM requires at least two days training and that administration takes about two hours.36 In contrast, the MINI-Plus needs less training and much less time to administer.19,21,30 A specific limitation of the ADHD module of MINI-Plus is that it does not differentiate between ADHD presentations/subtypes.
The present study has both strengths and limitations. The main strengths are the large and diverse sample of treatment seeking SUD patients (that represents daily clinical practice in outpatient treatment centers) and the use of internationally available instruments within the same study protocol in different countries and treatment settings. An important limitation is the lack of clinical information to check the value of the CAADID as external criterion (“gold standard”). Besides, some authors describe that lowering the CAADID cut-off may increase ADHD diagnosis, and therefore, it modifies any comparison with other instruments37; however, in the current work, we used the validated, more conservative and recommended cut-off. Another important limitation is the lack of data on current drug use and the possible influence of ongoing drug use on the stability and validity of an ADHD diagnosis in SUD patients. However, a recent analysis of the ADHD screening data within the current study suggests that this is not a real problem.15 Finally, the administration of the CAADID and MINI-Plus by the same person could generate a confirmation bias due the evaluator could interpret or assess according to previous information.38
In conclusion, the MINI-Plus has acceptable psychometric features for the screening of adult ADHD in treatment seeking SUD patients. In order to prevent false positive diagnoses, a structured clinical assessment should be performed in all patients with adult ADHD according to the MINI-Plus.
Conflict of interestAs potential conflict of interests, the author(s) disclosed the following data: Raul F. Palma-Alvarez has received fees to give talks for Exeltis, Lundbeck, and Takeda. Lara Grau-López has received fees to give talks for Janssen-Cilag, Lundbeck, Servier, Otsuka, and Pfizer. Miquel Casas has received fees to give talks for Janssen-Cilag, Bristol-Mayers Squibb, Ferrer-Brainfarma, Pfizer, Reckitt-Benckiser, Lundbeck, Otsuka, Servier, Lilly, Shire, GSK, Rovi and Adamed. He has received financial compensation for his participation as a member of the Janssen-Cilag, Lilly, Shire, Lundbeck, Otsuka, Ferrer and Rovi board. Josep A. Ramos-Quiroga was on the speakers’ bureau and/or acted as consultant for Eli-Lilly, Novartis, Shire, Lundbeck, Ferrer and Rubió in the last 3 years. He also received travel awards (hotel and air tickets) for taking part in psychiatric meetings from Rubió, Ferrer, Shire and Eli-Lilly. The ADHD Program chaired by him received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Eli-Lilly, Shire, Janssen, Rovi, Lundbeck, and Rubió.
The other authors do not have any conflict of interest to declare. The authors alone are responsible for the content and writing of this paper.
The IASP study was funded by several institutions in each country. The ICASA Foundation developed the study and coordinated with each institution the regional funding process. The funding sources did not have any influence on the study (research protocol, sampling, issues, analyses, publication). Australia: a strategic grant from Curtin University of Technology (Perth, Western Australia) funded the IAS screening phase. Belgium: the IASP project in this country received private funding. Hungary: No direct funding was received, it was supported by a grant from The European Union and European Social Fund (under agreement to finance the project) no. TÁMOP 4.2.1./B-09/1/KMR-2010-0003. The Netherlands, Amsterdam: no external funding was obtained. The participating institute, Arkin, paid for the costs involved. Norway, Bergen Clinics Foundation: 50% of funding was provided by the Regional Research Council For Addiction in West Norway (Regionalt kompetansesenter for rusmiddelforskning I Helse Vest (KORFOR); the remaining 50% was supported by Bergen Clinics Foundation (Staff and infrastructure). Norway, Fredrikstad: The IASP was funded by the hospital (Sykehuset Østfold HF) not with money, but with 50% of the salary of the participants, then by two sources outside the hospital: The Regional center of Dual Diagnosis and the social – and Health directory. Spain, Barcelona: Financial support was received from Plan Nacional sobre Drogas, Ministerio de Sanidad y Política Social (PND 0080/2011), the Agència de Salut Pública de Barcelona and the Departament de Salut, Government of Catalonia, Spain. Sweden, Stockholm: The study was funded by the Stockholm Center for Dependency Disorders. Switzerland, Berne/Zurich: The IASP in Switzerland was funded by the Swiss Foundation of Alcohol Research (Grant # 209). USA, Syracuse: no funding was obtained. The funding sources did not have influence on: who participated as an author in this study; the research protocol; the sampling of data; the topics chosen for publications; the analyses of the data; the content of the publication.