The gold-standard for fibrosis diagnosis in non-alcoholic fatty liver disease (NAFLD) is liver biopsy, despite its invasive approach, sampling limitations and variability among observers. The objective was to validate the performance of non-invasive methods (Fibroscan™; APRI, FIB4 and NAFLD score) comparing with liver biopsy in the evaluation of liver fibrosis in patients with NAFLD.
Material and methodsNAFLD patients ≥18 years of age who were submitted to liver biopsy were included and evaluated at two reference tertiary hospitals in Brazil with transient hepatic elastography (THE) assessment through Fibroscan™, APRI, FIB4 and NAFLD scores were determined. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive values for the diagnosis of advanced fibrosis were calculated to evaluate the performance of these non-invasive methods in NAFLD patients, adopting liver biopsy as the gold standard.
ResultsA total of 104 patients were studied. At three different cutoff values (7.9, 8.7 and 9.6kPa) THE presented the highest sensitivity values (95%, 90% and 85% respectively), and the highest NPV (98%, 96.4% and 95.1% respectively) for the diagnosis of advanced fibrosis. It also presented the highest AUROC (0.87; CI 95% 0.78–0.97).
ConclusionWhen compared to the gold standard, transient hepatic elastography presented the best performance for the diagnosis and exclusion of advanced fibrosis in patients with NAFLD, overcoming APRI, FIB4 and NAFLD score.
Non-alcoholic fatty liver disease (NAFLD) is a clinically relevant condition. It presents an evolutionary potential, including the development of nonalcoholic steatohepatitis (NASH), cirrhosis and inherent complications such as hepatocellular carcinoma [1–4].
NAFLD prevalence in the general population is unknown, mainly due to the high number of asymptomatic patients. It has been reported that it occurs in 20% of the population and it is already considered the most frequent liver disease in the United States of America [5–7]. In high-risk groups, its prevalence is higher, and may exceed 90% in morbid obese candidates for bariatric surgery, 69% in type-2 diabetic patients and 50% in dyslipidemic patients [8,9]. A NAFLD prevalence of 90.4% was previously reported in obese individuals prospectively evaluated for bariatric surgery in our center [10].
The diagnosis of NAFLD is based on the presence of steatosis on imaging or liver biopsy, as long as there is no alcohol consumption (less than 30g/day for men and 20g/day for women) [11], secondary causes for steatosis or coexisting liver disease [8,9]. The current gold standard method for NAFLD diagnosis is liver biopsy. Nevertheless, it is an invasive method, has sampling limitations and presents variability among observers [12].
The use of noninvasive models to predict liver fibrosis may be an option for the staging of the disease, although not totally satisfactory. Serum markers have been shown to present a good accuracy in the determination of advanced fibrosis in viral hepatitis [13–15]. However, the lack of precision in the determination of more incipient fibrosis and the paucity of studies in NAFLD are limitations of these tools [16].
The most promising noninvasive method for assessing liver fibrosis currently appears to be transient hepatic elastography (THE) (Fibroscan™). The technique is fast, reproducible and does not cause discomfort or risks to the patient. The cutoff points vary according to the etiology of liver disease and most studies have been performed on patients with chronic hepatitis C [14,17,18], and have not yet been fully validated for NAFLD [19–29]. Indeed, this is the first study that evaluates the accuracy of elastography in Brazilian NAFLD population.
Due to its practicality and low cost, scores derived from serological tests such as the APRI score (aspartate aminotransferase – AST to Platelet Ratio Index), the FIB4 score (consider age, AST, alanine aminotransferase – ALT and platelets) and NAFLD score (consider age, body mass index, AST, ALT, albumin, platelets and the presence of diabetes/insulin resistance) – also deserve to be highlighted [8,9].
The aim of the present study was to validate the performance of non-invasive methods (Fibroscan™, APRI, FIB4 and NAFLD scores) in assessing liver fibrosis in patients with NAFLD comparing to liver biopsy as a gold standard.
2Material and methodsPatients with NAFLD from the Gastroenterology/Hepatology outpatient clinic of Santa Casa de Misericórdia, Porto Alegre, and from the Clementino Fraga Filho University Hospital at the Federal University of Rio de Janeiro, two reference tertiary hospitals in Brazil, were prospectively evaluated from January 2016 to January 2017 and a convenience sample was obtained for the study.
Inclusion criteria were age greater than or equal to 18 years; diagnosis of NAFLD by liver biopsy [30,31] and THE (Fibroscan™, Echosens, France) performed with no more than 3 months apart from liver biopsy. Exclusion criteria were diagnosis of hepatitis B or C; presence of significant alcohol consumption (more than 30g/day for men and 20g/day for women) [11]; other causes of chronic liver disease, secondary NAFLD and hepatocellular carcinoma.
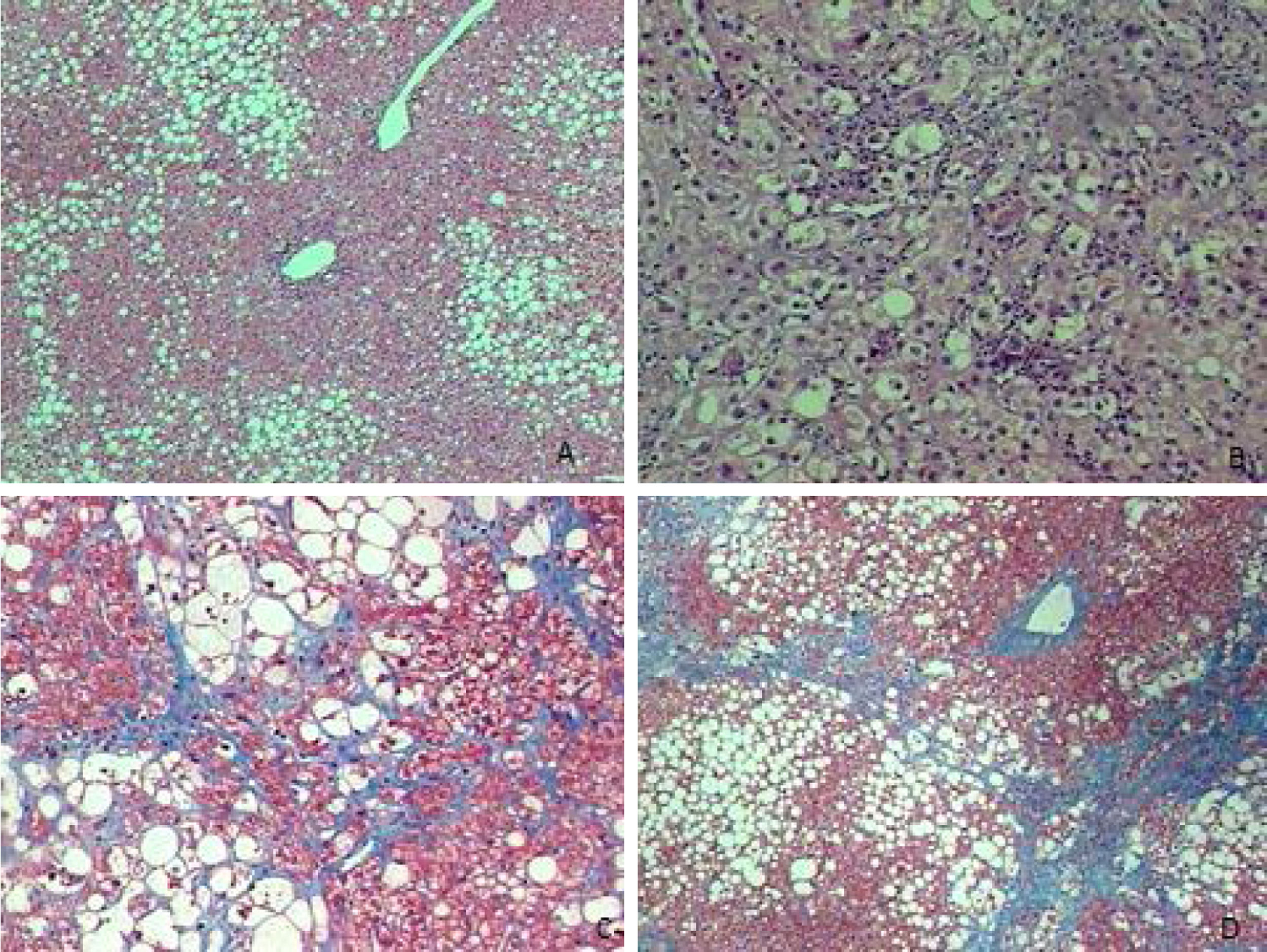
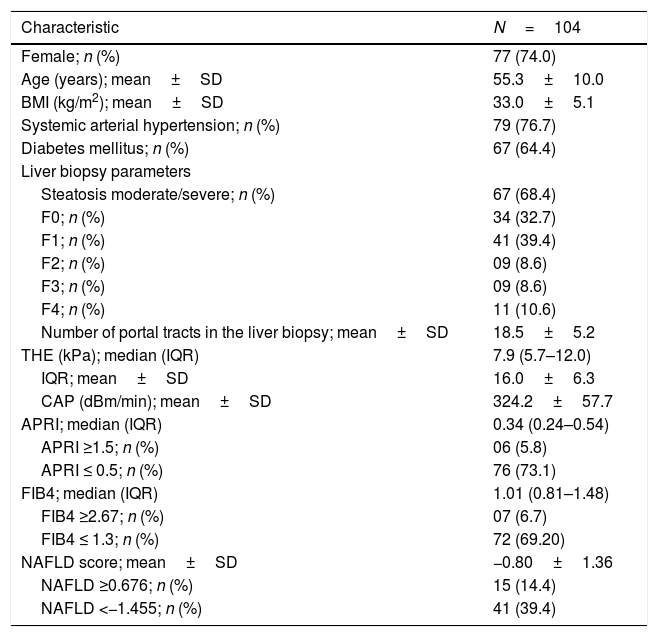
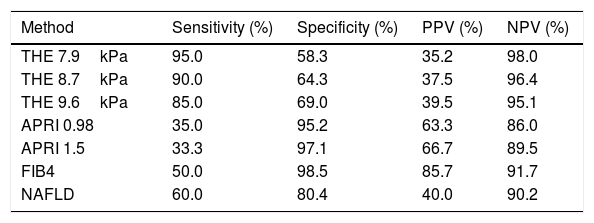
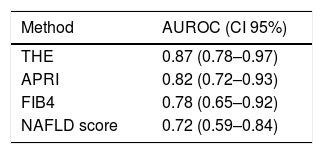
Liver biopsies were obtained through an ultrasound-guided technique using a Tru-cut needle. Histological slides were coded and read by two expert pathologists, who were unaware of the patient's identity and history. A minimum length of 15mm of the biopsy specimen or the presence of at least 10 complete portal tracts was required. Steatosis was assessed as the percentage of hepatocytes containing fat droplets (minimum 5%) and evaluated as a categorical variable. The Kleiner classification [30] was used to stage fibrosis from 0 to 4. Advanced fibrosis was considered for fibrosis stage ≥F3.
The studied population was stratified into two groups considering the staging of fibrosis at liver biopsy: with (≥F3) and without advanced fibrosis (
Weight, height and body mass index (BMI) were measured in all patients and laboratory tests (ALT, AST, total and direct bilirubin, platelets, albumin, glucose and insulin) were also performed to calculate noninvasive scores (APRI, FIB4 and NAFLD).
APRI score was calculated using the AST and platelet variables [31]. For the evaluation of significant fibrosis, APRI score was categorized into absence (value ≤0.5) or presence (value ≥1.5) of significant fibrosis [32]. A cutoff value of 0.98 was also evaluated as suggested by Kruger et al. [33] specifically for NAFLD.
FIB4 score was calculated considering age, AST, ALT and platelet values. For the evaluation of advanced fibrosis, FIB4 score was categorized into absence (value ≤1.3) or presence (value ≥2.67) of advanced fibrosis [34].
NAFLD score included age, BMI, insulin resistance or diabetes mellitus type 2, aminotransferases, platelets and albumin values [16]. For the exclusion of advanced fibrosis, a NAFLD score cutoff value under −1.455 was adopted. A NAFLD score cutoff value higher than 0.675 was considered for the diagnosis of advanced fibrosis [8,9].
Transient hepatic elastography was performed by a specialized physician experienced with the procedure (at least 500 examinations performed). The physician was blinded for patient data. A Fibroscan™ device – Echosens (Paris) was used and the results were expressed in kilopascals (kPa). The exam was performed after a 2-h fast. Procedures were considered reliable and included for analysis only when they presented at least 10 valid shots, a success rate of at least 60% and an interquartile range (IQR) of liver stiffness (LS) value under or equal to 30%. The XL probe was used when it was not possible to estimate LS with the M probe. To evaluate THE performance, three different cutoff values were used as proposed by Wong et al. [19,20] with the M probe (cutoffs of 7.9, 8.7 and 9.6kPa). When the XL probe was used the 7.9 probe M cutoff was substituted by 7.2 as suggested by Wong et al. [21].
The study was submitted and approved by the local Ethics Committee, and all patients signed the Informed Consent.
2.1Statistical analysisSensitivity, specificity, positive predictive (PPV) and negative predictive values (NPV) were calculated for each test in comparison to liver biopsy.
The symmetry of the variables was assessed using the Kolmogorov–Smirnov test. Variables with symmetrical distribution were described by mean and standard deviation. Variables with asymmetric distribution were described by the median and the interquartile range (25th and 75th percentiles).
The area under the curve (AUROC) with the respective confidence interval 95% (CI 95%) was calculated for all tests.
The categorical variables were described by frequencies and percentages.
The software used in the analysis was SPSS v.23.0 (IBM, United States).
3ResultsOne hundred and four patients were included. Mean age was 55.3±10.0 years and mean BMI 33.0±5.1kg/m2. The majority (74.0%) of patients were female (Table 1).
Clinical characteristics of 104 patients submitted to liver biopsy and non-invasive diagnostic tests.
| Characteristic | N=104 |
|---|---|
| Female; n (%) | 77 (74.0) |
| Age (years); mean±SD | 55.3±10.0 |
| BMI (kg/m2); mean±SD | 33.0±5.1 |
| Systemic arterial hypertension; n (%) | 79 (76.7) |
| Diabetes mellitus; n (%) | 67 (64.4) |
| Liver biopsy parameters | |
| Steatosis moderate/severe; n (%) | 67 (68.4) |
| F0; n (%) | 34 (32.7) |
| F1; n (%) | 41 (39.4) |
| F2; n (%) | 09 (8.6) |
| F3; n (%) | 09 (8.6) |
| F4; n (%) | 11 (10.6) |
| Number of portal tracts in the liver biopsy; mean±SD | 18.5±5.2 |
| THE (kPa); median (IQR) | 7.9 (5.7–12.0) |
| IQR; mean±SD | 16.0±6.3 |
| CAP (dBm/min); mean±SD | 324.2±57.7 |
| APRI; median (IQR) | 0.34 (0.24–0.54) |
| APRI ≥1.5; n (%) | 06 (5.8) |
| APRI ≤ 0.5; n (%) | 76 (73.1) |
| FIB4; median (IQR) | 1.01 (0.81–1.48) |
| FIB4 ≥2.67; n (%) | 07 (6.7) |
| FIB4 ≤ 1.3; n (%) | 72 (69.20) |
| NAFLD score; mean±SD | −0.80±1.36 |
| NAFLD ≥0.676; n (%) | 15 (14.4) |
| NAFLD <−1.455; n (%) | 41 (39.4) |
SD=standard deviation; BMI=body mass index; THE=transient hepatic elastography; IQR=interquartile range; kPa=kilopascals; CAP=controlled attenuation parameter; NAFLD=non-alcoholic fatty liver disease.
Liver biopsies were representative, with 18.5±5.2 portal tracts. All patients presented steatosis in liver biopsy, but moderate or severe steatosis was present in 68.4%, and steatohepatitis in 76.0%. In addition, fibrosis was diagnosed at stages F0 to F2 in 80.8% of patients and at advanced fibrosis or cirrhosis in 19.2% of patients (Table 1 and Fig. 1).
Besides that, THE results presented a median value of 7.9kPa (5.7–12.0kPa) with an IQR of 16.0±6.3 and a mean controlled attenuation parameter result (CAP) of 324.2±57.7dBm/min. The results of APRI, FIB4 and NAFLD scores are also presented in Table 1.
When APRI, FIB4 and NAFLD scores were compared to the 3 different cutoff values analyzed for THE using the liver biopsy as the gold standard (Table 2), it is observed that THE presented the highest sensitivity and NPV for every different cutoff points evaluated. Similarly, the best AUROC was obtained with the THE (Table 3).
Diagnostic performance of THE, APRI and FIB4 to identify advanced fibrosis in relation to liver biopsy in NAFLD patients (n=104).
| Method | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| THE 7.9kPa | 95.0 | 58.3 | 35.2 | 98.0 |
| THE 8.7kPa | 90.0 | 64.3 | 37.5 | 96.4 |
| THE 9.6kPa | 85.0 | 69.0 | 39.5 | 95.1 |
| APRI 0.98 | 35.0 | 95.2 | 63.3 | 86.0 |
| APRI 1.5 | 33.3 | 97.1 | 66.7 | 89.5 |
| FIB4 | 50.0 | 98.5 | 85.7 | 91.7 |
| NAFLD | 60.0 | 80.4 | 40.0 | 90.2 |
THE=transient hepatic elastography; kPa=kilopascals; PPV=positive predictive value; NPV=negative predictive value; NAFLD=non-alcoholic fatty liver disease.
AUROC for the identification of advanced fibrosis according to THE, APRI, FIB4 and NAFLD score in relation to liver biopsy (n=104).
| Method | AUROC (CI 95%) |
|---|---|
| THE | 0.87 (0.78–0.97) |
| APRI | 0.82 (0.72–0.93) |
| FIB4 | 0.78 (0.65–0.92) |
| NAFLD score | 0.72 (0.59–0.84) |
THE=transient hepatic elastography; AUROC=area under the curve; CI=confidence interval; NAFLD=non-alcoholic fatty liver disease.
It has been widely reported that blood fibrosis tests and transient hepatic elastography (THE) require further validation in NAFLD [28,29,35–37]. The present study shows that THE should be considered a good non-invasive method for exclusion of advanced liver fibrosis, overcoming other established tests as APRI and FIB4 and even NAFLD score and might be used as the preferred non-invasive tool to assess NAFLD-related fibrosis.
However, other non-invasive tests that have been assessed in the present study may also be considered as useful tools, especially in low income countries with difficult assess to elastography. APRI, FIB4 and NAFLD scores do not require any additional special examination for their calculation, which facilitates their application with a low cost in daily NAFLD clinic. They might be used even before elastography in the first clinic evaluation to estimate the fibrosis stage of NAFLD patients. Both NAFLD score, FIB4 score and THE had also been considered suitable for clinical use by the recent guidelines published by the American Association for the Study of Liver Diseases (AASLD) [9] or the European Association for the Study of the Liver (EASL) [11]. Hence, in the setting where both methods are available, THE may be more useful since it has the highest sensitivity and negative predictive value for the evaluation of fibrosis as shown in the present study.
NAFLD score has been widely used score in clinical practice [8,9] despite its limitation in the detection of low-stage fibrosis. Using values below the lowest (−1.455) or above the highest (0.675) cutoff point, it is able to predict absence or presence of advanced fibrosis in 75% of the patients evaluated, and it is possible to avoid liver biopsy in most cases [16]. However, in this study, NAFLD score presented a low sensibility and positive predictive value. Although the mean NAFLD score in the present study was −0.80, higher than the cut-off value that excludes the presence of advanced fibrosis, 46.2% of the patients were in the gray zone.
In the study of Kruger et al. [33] it was observed that the performance of the APRI score presented a sensitivity of 75% and specificity of 86% when using a cutoff point of 0.98 for detection of advanced fibrosis in patients with NAFLD. However, in the present study, the APRI score showed a very low sensitivity when both cutoff points of 1.5 or 0.98 were evaluated.
Several non-invasive algorithms based on clinical and biochemical variables have been developed to detect individuals with NAFLD and advanced fibrosis. However, major drawbacks include reduced accuracy for detection of earlier fibrosis stages and the high proportion of patients with undetermined results. These non-invasive scoring systems includes NAFLD score, FIB-4, APRI and BARD score. Despite these biochemical tests presented good diagnostic efficacy, many patients fall in-between the lower and upper threshold values (indeterminate results), and many factors such as age, diabetes, and prevalence of fibrosis may influence their diagnostic performance [38].
Transient hepatic elastography has been successful in identifying advanced fibrosis in patients with hepatitis B and C viruses, and has shown high sensitivity and specificity also in patients with NAFLD [20,21,26,27]. The present study indicates that THE may be a good method to exclude advanced fibrosis because of the high NPV, similar to what was previously described [11]. However, the 95%CI of the AUROC were wide, and the overlapping should limit the interpretation of significance of the data. This method has also the advantage of being able to categorize the different stages of fibrosis, increasing the performance [9].
Three different cutoffs were analyzed in the present study, and all of them presented high sensitivity and NPV. The best performance was observed with the cutoff of 7.9kPa. However, the best specificity was obtained with the 9.6kPa cutoff, although very similar to the other cutoffs. Many different cutoffs have been evaluated, and it should be noted there is a great controversy about the best cutoff to predict F3 or F4 in NAFLD patients [22–24,27]. Corroborating these findings, a combination of two or more noninvasive methods has been recommended to provide additional diagnostic accuracy and to avoid diagnosis by hepatic biopsy [11].
The validity of THE in the diagnosis of fibrosis in patients with NAFLD has been evaluated in a recent meta-analysis by Hashemi et al. [39]. Seven articles were included, consisting of 698 patients. The authors showed that as fibrosis increases, the sensitivity (87.5%, 93.7% and 96.2% for F2, F3 and F4 respectively) and specificity (78.4%, 91.1% and 92.2% for F2, F3 and F4 respectively) also improve, concluding that THE can be considered to exclude liver cirrhosis, but more studies are required to confirm the results [39].
A cross-sectional study evaluated eight non-invasive serum tests (BARD, NAFLD fibrosis score, FibroMeterNAFLD, APRI, FIB4, FibroTest, Hepascore, FibroMeterV2G) and THE in 452 NAFLD patients with liver biopsy. The authors identified THE and FibroMeterV2G as the most accurate methods for the noninvasive diagnosis of fibrosis in patients with NAFLD [27].
As possible limitations of this study, we can highlight that it was developed in tertiary hospitals, and included predominantly female patients. However women are usually the predominant population in NAFLD studies [40]. In this study there were also a predominance of low levels of fibrosis, and this may have influenced the poor performance of the tests. Besides that, the high BMI can negatively affect the results, as suggested by Fujimori et al. [41].
In conclusion, the present study suggests transient hepatic elastography may be a good tool for the diagnosis and exclusion of advanced fibrosis in patients with NAFLD. Transient hepatic elastography presented the highest sensitivity and highest NPV for advanced fibrosis, overcoming APRI, FIB4 and NAFLD scores. These results should be useful in clinical practice, in order to avoid liver biopsy. However, more studies are needed with a greater number of patients to confirm these results.AbbreviationsNAFLD non-alcoholic fatty liver disease non-alcoholic steatohepatitis transient hepatic elastography aspartate aminotransferase alanine aminotransferase body mass index kilopascals interquartile range liver stiffness positive predictive value negative predictive value area under the curve American Association for the Study of Liver Diseases European Association for the Study of the Liver standard deviation controlled attenuation parameter confidence interval
None.
Author contributionsCristiane V. Tovo, Cristiane A. Villela-Nogueira and Nathalie C. Leite conceptualized the study. Carine L. Panke, Gabriela Z. Port, Sabrina Fernandes, Caroline Buss, Gabriela P. Coral, Ana C. Cardoso, Claudia M. Cravo, Fernanda L. Calçado, Guilherme F.M. Rezende, Frederico C. Ferreira, João M.A. Neto and Renata de M. Perez collected the data. Cristiane V. Tovo, Carine L. Panke, Cristiane A. Villela-Nogueira and Nathalie C. Leite analyzed the data. Cristiane V. Tovo, Carine L. Panke, Cristiane A. Villela-Nogueira, Nathalie C. Leite, Henrique S. Moraes-Coelho and Angelo A. de Mattos wrote the manuscript. All the authors revised and approved the final version.
Conflict of interestNone.