Editado por: Sonia Roman
Más datosHepatitis B virus (HBV) is endemic in many parts of the world and is a significant cause of chronic liver damage and hepatocellular carcinoma. HBV therapeutics vary according to the disease stage. The best therapeutic option for patients with end-stage liver disease is liver transplantation, while for chronic patients, HBV infection is commonly managed using antivirals (nucleos(t)ides analogs or interferons). However, due to the accessibility issues and the high cost of antivirals, most HBV patients do not have access to treatment. These complications have led researchers to reconsider treatment approaches, such as nutritional therapy. This review summarizes the nutrients reported to have antiviral activity against HBV and their possible mechanism of action. Recent studies suggest resveratrol, vitamin E, lactoferrin, selenium, curcumin, luteolin-7-O-glucoside, moringa extracts, chlorogenic acid, and epigallocatechin-3-gallate may be beneficial for patients with hepatitis B. The anti-HBV effect of most of these nutrients has been analyzed in vitro and in animal models. Different antiviral and hepatoprotective mechanisms have been proposed for these nutrients, such as the activation of antioxidant and anti-inflammatory pathways, regulation of metabolic homeostasis, epigenetic control, activation of the p53 gene, inhibition of oncogenes, inhibition of virus entry, and induction of autophagosomes. In conclusion, scientific evidence indicates that HBV replication, transcription, and expression of viral antigens can be affected directly by nutrients. In the future, these nutrients may be considered to develop appropriate nutritional management for patients with hepatitis B.
Hepatitis B infection affects the liver and is caused by the hepatitis B virus (HBV) [1]. Currently, HBV infection is considered one of the most important health problems in the world. In 2019, there were 296 million people with chronic HBV infection and 820,000 deaths [2]. In most cases, HBV infection is asymptomatic and self-limiting. However, about 90% of infants and 2–6% of adults with hepatitis B can develop chronic hepatitis B [3]. In the long term, these patients can develop liver cirrhosis and hepatocellular carcinoma [4]. The therapeutic methods for patients with HBV vary according to disease stage. For chronic patients, the infection can be managed using antivirals (nucleos(t)ides analogs and interferons), while for patients with end-stage liver disease, the best therapeutic option is liver transplantation [4].
The most common antivirals to treat HBV infection are lamivudine, adefovir, telbivudine, entecavir, tenofovir, interferon alfa-2b and peginterferon alfa-2a [5]. In 2021, the cost of one year of treatment with lamivudine was US$1296 (150 mg tablet), while one year with entecavir cost US$2866 (500 mg tablet), and tenofovir cost US$4130 (25 mg tablet). Also, an injection of interferon alfa-2b (5 million IU/mL) and peginterferon alfa-2a (180 µg/0.5 mL) can cost around US$74 and US$250, respectively [6]. Notably, the cost of these treatments can increase since some antiviral drugs must be taken in combination to reduce the risk of resistance mutations. In the United States, the average price of a liver transplant is US$163,438 (US$145,277–181,598) [7], and the ablation of one or more liver tumors by radiofrequency costs at least US$50,000 [8]. These procedures are expensive, and their success depends on the patient's clinical and nutritional status. A study in HBV patients with liver transplants due to hepatocellular carcinoma found that life expectancy is 76% in the first year, and 49% of people lived at least five years after transplantation [9].
Globally, it is estimated that 22% of patients with hepatitis B are under treatment [2]. Although, another study estimates that only 4.5% of those who are eligible for HBV treatment have access to it [5]. These findings suggest that most people infected with HBV do not have access to treatment. This problem has led researchers to reconsider treatment approaches, such as medical nutrition therapy that uses diet to help manage chronic conditions[10]. There is no specific nutritional therapy guideline for managing hepatitis B infection to date. However, several studies have analyzed the relationship between nutrients and the clinical outcome of viral hepatitis. In patients with hepatitis C, a consumption of ≥ 4.9% of polyunsaturated fatty acids has been associated with low viral load levels, whereas consumption ≥ 21.5 g/day of fiber was related to high viral load[11]. Moreover, vitamin D deficiency is common in HBV patients with liver cirrhosis and high viral load [12]. Another important association was found in children with HBV treated with interferon alfa-2b, whose zinc levels were higher in responders than in non-responders [13]. Also, in vitro and in vivo studies have proposed that the resveratrol, vitamin E, lactoferrin, selenium, curcumin, luteolin-7-O-glucoside, moringa extracts, chlorogenic acid, and epigallocatechin-3-gallate may be beneficial for patients with hepatitis B (Table 1). Thus, this study aimed to describe these nutrients’ antiviral properties and possible mechanisms of action.
Nutrients and foods with potential antiviral effect against hepatitis B virus.
| Nutrient | Food | Effect | Study design | Reference |
|---|---|---|---|---|
| Resveratrol | Grapes, plums, peanuts, apples, blueberries | Antitumor activity | In vitro, In vivo | [16] |
| Vitamin E | Sunflower seeds, almonds, peanuts, spinach, broccoli, and mango | ↓ALT levels and ↓ DNA viral | humans | [19, 20] |
| Lactoferrin | Milk | ↓HBsAg y ↓ADN viral | In vitro | [28, 29] |
| Selenium | Tuna, sardine, shrimp, beef steak, turkey, beef liver, chicken, and rice | ↓ Incidence of primary liver cancer | In Humans | [35] |
| Curcumin | Plant (Curcuma longa) | ↓ ADN y ↓ARN viral, antitumor | In vitro, In vivo | [41, 44] |
| Luteolin-7-O-glucoside | Lettuce (Lactua sativa) | ↓HBsAg, ↓ADN, ↓ARN viral | In vitro | [51] |
| Moringa extracts | Plant (Moringa oleífera) | ↓ADN y ↓ARN viral, antifibrotic | In vitro | [58, 60] |
| Chlorogenic acid | Coffee (Coffea sp.) | ↓ HBsAg, ↓HBeAg, antifibrotic and antitumor | In vitro | [65, 67, 68] |
| Epigalocatequin-3-gallate | Tea (Camellia sinensis) | ↓ HBsAg, ↓HBeAg, ↓ADN, and ↓ARN viral | In vitro | [73, 74, 76] |
ALT: Alanine Aminotransferase; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; sp.: specie
Resveratrol is a polyphenol found naturally in grapes, plums, peanuts, apples, blueberries, and their deviates, such as wine or butter [14]. Resveratrol is well known for its many health benefits (anti-inflammatory and antioxidant), but it also has antiviral activity against many viruses, including HBV [15,16]. Resveratrol at a concentration of 100 µM decreased hepatocarcinoma cell proliferation by 67% without cytotoxicity in HBV-induced hepatocellular carcinoma cells [16]. Similar results were found in vivo, where HBV-hepatocarcinoma xenograft mice treated with resveratrol injections showed a significant decrease in tumor volume compared to those treated with vehicle. It has been proposed that the anticancer molecular mechanism of resveratrol may be through the arrest of tumor cells in the G1 phase since this nutrient can decrease the levels of cyclin D1 mRNA (Fig. 1) [16]. Currently, the curative effect of resveratrol (1000 mg/day) combined with interferon or entecavir is being investigated in patients with chronic HBV patients [17]. The most important expected effects of these therapies are the loss, decrease, or seroconversion of HBV antigens, accompanied by decreased levels of HBV viral load [17]. These studies are registered at https://clinicaltrials.gov with the identification numbers NCT03546530 and NCT03509688.
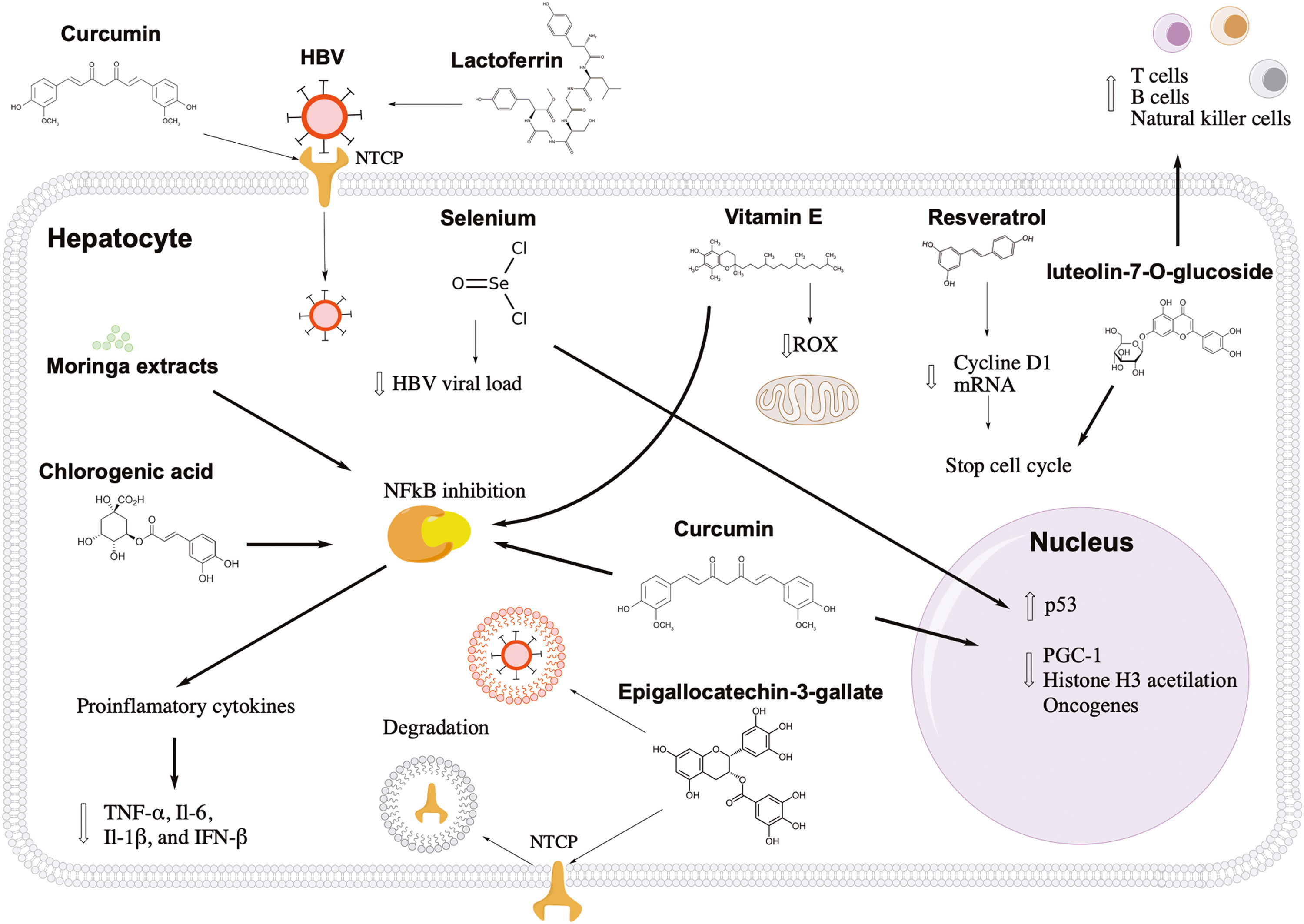
Antiviral and hepatoprotective mechanisms of nutrients against hepatitis B. Lactoferrin and curcumin inhibit the first stage of HBV infection, blocking the viral and hepatocyte receptor, respectively. Epigallocatechin-3-gallate induces the degradation of the entry receptor and viral particles through the induction of autophagosomes. Resveratrol and luteolin-7-o-glucoside show an antitumor effect, arresting the cell cycle of infected cells. Curcumin can activate tumor suppressor genes, such as p53, and decrease factors that favor the transcription of viral genes. In addition, luteolin-7-o-glucoside can induce the proliferation of immune response cells. Liver inflammation can also be controlled with vitamin E, moringa extracts, chlorogenic acid, and curcumin through NFkB pathway inhibition. Also, vitamin E can reduce reactive oxygen species in the mitochondria.
Vitamin E is another antioxidant that may be beneficial for patients with hepatitis B. Vitamin E is a fat-soluble vitamin found in sunflower seeds (7.4 mg/serving), almonds (6.8 mg/serving), peanuts (2.2 mg/serving), spinach (1.9 mg/serving), broccoli (1.2 mg/serving), and mango (0.7 mg/serving) [18] . The recommended daily vitamin E intake for people over 14 is 15 mg, while lactating women need more than 19 mg [18]. There is no evidence of toxic effects of vitamin E from natural dietary sources [18]. A pilot study in patients showed that vitamin E supplementation (600 mg/day) for 15 months helped normalize alanine aminotransferase levels and negativize HBV viral load in 47% and 53% of cases, respectively [19]. Also, a meta-analysis suggests that vitamin E increases the probability of hepatitis B e antigen (HBeAg) seroconversion in children with chronic HBV hepatitis [20]. The hepatoprotective mechanism of vitamin E in patients with hepatitis B may be due to its antioxidant properties preventing cell damage caused by reactive oxygen species during the immune response and metabolism (Fig. 1) [21]. Also, vitamin E reduces the production of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor-alpha, and C-reactive protein [22], which have been linked to the progression of liver disease [23].
4LactoferrinLactoferrin is a protein found in milk, and it has been related to a wide range of biological effects, including antiviral, antibacterial, and anti-inflammatory [24]. It seems that lactoferrin can inhibit infections caused by herpes simplex virus type 1, human immunodeficiency virus (HIV), respiratory syncytial virus, rotavirus, poliovirus, echovirus, influenza A, parainfluenza, hepatitis C virus (HCV), and HBV [24,25]. Lactoferrin can also help reduce the cytokine storm in patients with the severe acute respiratory syndrome of coronavirus 2 (SARS-CoV-2) [26]. Camel lactoferrin (Camelus dromedarius) at a concentration of 0.25 mg/mL can block HCV infection in cell culture [27]. Similar antiviral effects were found in HBV-infected cell culture pretreated with 1 mg/mL of lactoferrin [28]. In 2021, it was confirmed that breast milk extracts could inhibit HBV infection in cell culture; these extracts (10 mg/mL) had an inhibition efficacy between 80% and 90%, while the extracts of bovine milk (10 mg/mL) showed limited inhibitory activity [29]. Lactoferrin can prevent HBV infection in the first phase of the viral cycle, avoiding the entry of HBV into the hepatocyte (Fig. 1). One study in vitro demonstrated that lactoferrin could directly bind to the hepatitis B surface antigen (HBsAg), inhibiting its activity [29].
5SeleniumSelenium is an important element for antioxidant enzymes in the body [30]. This trace element can be obtained through natural sources, such as tuna (92 mg/portion), sardine (45 mg/portion), shrimp (40 mg/portion), beefsteak (33 mg/portion), turkey (31 mg/portion), beef liver (28 mg/portion), chicken (22 mg/portion), and rice (19 mg/portion) [31]. The recommended intake of selenium for people over 19 is 55 mg/day, while the maximum tolerable intake is 400 mg/day [31] . Some studies have shown that selenium may have antiviral activity against porcine circovirus type 2 and influenza [32,33]. In patients with HIV, selenium supplementation could suppress HIV viral load and improve CD4 count [34]. In a study in two Chinese populations with a high prevalence of HBV infection, the effect of selenium supplementation was evaluated for eight years. During follow-up, there was a 35.1% reduction in the incidence of primary liver cancer in the treated population compared with those without selenium supplementation [35]. The antiviral effect of selenium is probably due to the activation of the tumor protein 53 (p53) and p73 genes, which suppresses the activity of HBV promoters and enhancers, inhibiting the HBV transcription and replication, as well as suppression of HBsAg and HBeAg antigens (Fig. 1) [36]. Another study investigated the effect of 200 µg selenium supplementation on the response rate to the HBV vaccine in diabetic patients. The researchers found a 74.2% protection rate in the selenium-treated group compared to 48.4% in the control group [37]. These data suggest that adequate amounts of selenium in the diet could benefit patients with hepatitis B, reducing the risk of primary liver cancer and improving the immune response to the HBV vaccine.
6CurcuminCurcumin is a natural compound found in the root of Curcuma longa[38]. One kilogram of curcumin costs US$4.8 and can be consumed by infusions or as a condiment. Oral consumption between 8 and 12 gr/day of curcumin is well tolerated in healthy people [39]. Several studies suggest that curcumin can be beneficial for patients with hepatitis B. In HBV-infected cells, curcumin at a concentration of 150 µM can reduce the expression of HBsAg and hepatitis B core antigen (HBcAg) by 65% and 45%, respectively [40]. In addition, the combination of curcumin (150 µM) and lamivudine (1mM) increased the inhibition level up to 75% [40]. Another study, in cells treated with 200 mg/L and 500 mL/L of curcumin, found an 80% inhibition of HBsAg secretion and suppression of HBV replication and transcription [41]. Also, it has been demonstrated that Curcuma longa extracts (30 µg/mL) can decrease the level of HBV covalently closed circular ADN (cccDNA) in cell culture [42]. The cccDNA is a nuclear form of the HBV genome and is the main obstacle to curing hepatitis B infection [43]. One study placed curcumin in lipid vesicles (phytosomes) and administered them to transgenic mice expressing HBV antigens [44]. This new dietary treatment reduced the total volume of tumors without drastically altering body weight and ALT levels in transgenic mice [44].
Currently, there are six HBV-antiviral mechanisms associated with curcumin: control of metabolic factors, epigenetic control, activation of p53, inhibition of oncogenes, anti-inflammatory effect, and inhibition of entry (Fig. 1). HBV can use the PPARG coactivator 1 (PGC-1) protein, which regulates metabolic homeostasis in the liver [45]. HBV uses this protein to enhance the transcription of its own genes [40]. In cell culture, it has been shown that curcumin promotes the degradation of PGC-1 resulting in the inhibition of HBV expression [40]. Regarding epigenetic control, a study in cell culture showed that curcumin treatment (20 µmol/L) could reduce up to 57.7% and 75.5% the levels of HBsAg and cccDNA, respectively [46]. These reductions depended on histone H3 acetylation level and curcumin treatment, suggesting that curcumin can inhibit HBV transcription through deacetylation of cccDNA-bound histones [46]. Curcumin can also increase the expression of p53, which directly suppresses the HBV promoters and enhancers [41]. Also, p53 can arrest the cell cycle and induce apoptosis of infected cells [47].
On the other hand, the mammalian target of rapamycin (mTOR) is a gene associated with the development of hepatocellular carcinoma [48]. An HBV-transgenic mouse model demonstrated that phytosomal curcumin suppressed mTOR gene expression and has been proposed as chemopreventive in patients with chronic HBV [44]. Curcumin is well known for its anti-inflammatory properties due to its inhibition of the activation of the nuclear factor kappa B (NFkB) signaling cascade [44]. Recently, it has been published that curcumin can bind directly to the sodium-taurocholate co-transporting polypeptide (NTCP), which is the host receptor for HBV entry [49]. These results indicate that curcumin can compete with HBsAg preventing viral internalization in the first step of HBV infection [49].
7Luteolin-7-O-glucosideLuteolin-7-O-glucoside is a nutrient in lettuce leaves (Lactua sativa) [50]. Lettuce is one of the most consumed foods globally. 100 g of lettuce is made up of water (94.6 g), carbohydrates (3.29 g), proteins (1.23 g), and fiber (1.19 g). Its main ions are potassium (247 mg), calcium (33 mg), phosphorus (30 mg) and magnesium (14 mg) [50]. A study in China showed that Lactua sativa variety Lollo Rossa (red leaf lettuce) extracts inhibited by 82.5% of the HBsAg expression in cell culture [51]. This study also showed that Lactua sativa extracts combined with lamivudine or interferon significantly reduced the HBV replication and transcription compared with those cell cultures treated with only lamivudine or interferon [51]. The direct use of luteolin-7-O-glucoside in cell culture reduced 82.5%, 51%, and 44.7% the expression of HBsAg, HBV DNA, and HBV RNA, respectively [51]. The authors proposed that the antiviral effect of luteolin-7-O-glucoside may be due to its antioxidant properties since it reduced reactive oxygen species in the mitochondria [51]. In addition, luteolin-7-O-glucoside has antitumor and immunomodulatory properties. Luteolin-7-O-glucoside at a concentration of 200 µM arrested 34% of hepatocarcinoma cells in the G2/M phase [52]. Another study in cell culture found that heat-treated luteolin-7-O-glucoside can increase the proliferation of T, B, and natural killer (NK) cells and decrease macrophage-mediated toxicity (Fig. 1) [53].
8Moringa extractsMoringa oleífera is a plant native to India, but it is currently cultivated in many tropical and subtropical regions [54]. Powdered moringa leaves can be added directly to food or diluted in water. Moringa oleífera extracts have beneficial effects against various viral infections, such as SARS-CoV-2, HIV, influenza, and HBV [55–59]. In a study with rats intoxicated with carbon tetrachloride (CCL4), Moringa oleífera extracts reduced liver enzymes, albumin, and the degree of inflammation compared to the control group [60]. In HBV-infected cell models, Moringa oleífera leaf extracts decreased 80% the expression of cccDNA, while the extracts of its seeds only reduced 50% the expression of cccDNA [61]. The beneficial effects of moringa extracts may vary depending on the HBV genotype. One study showed that HBV genotype C had better inhibition at RNA pre-genomic levels than HBV genotype H in cell cultures [58] . The hepatoprotective properties of Moringa oleífera may be due to the cocktail of bioactive molecules, including vitamins, polyphenols, alkaloids, tannins, and saponins [58]. It is thought that moringa extracts may reduce the risk of progression to liver fibrosis since they can inhibit the NFkB cascade pathway and decrease the levels of inflammatory cytokines (TNF-α, IL-6, IL-1β, and IFN-β) (Fig. 1) [62,63].
9Chlorogenic acidChlorogenic acid is found in coffee, which, when it breaks down, forms quinic and caffeic acids [64,65]. The hepatoprotective effect of coffee is associated with the concentrations of chlorogenic acid and its derivatives. Considering an 8-ounce cup, moderate consumption of coffee can be between 3 and 5 cups/day [66]. A study in 328 individuals with chronic hepatitis B observed that patients with high coffee consumption had low levels of aspartate aminotransferase (ALT) and decreased levels of liver fibrosis [67]. In an endemic area for hepatitis B, the lifetime consumption of more than 20,000 cups reduces the risk of developing hepatocellular carcinoma [68]. In addition, a significant reduction in HBV DNA levels was observed in those chronic hepatitis B patients who consumed three or more cups of coffee per day [69]. The effect of coffee extracts on inhibiting HBV infection has been studied in cell cultures and animal models. With crude extracts of coffee, it was possible to inhibit HBV replication and secretion of hepatitis B antigens in cell culture [65]. In a duckling model, oral administration of chlorogenic acid, caffeic acid, and quinic acid reduced the Duck Hepatitis B virus DNA levels in serum by 31.8%, 12.6%, and 5.11% ten days after treatment [65]. The anti-HBV effect of coffee is associated with anti-inflammatory properties.
The antiviral-HBV mechanism of coffee extracts remains unknown. Although it has been proposed that its anti-inflammatory properties would be one of the most important [70]. A study in vitro showed that chlorogenic acid reduces the mRNA expression of pro-inflammatory cytokines (TNF-α, IL-6, and COX2), probably by suppressing the NFkB signaling pathway (Fig. 1) [70]. Besides, in a cirrhotic model, coffee extracts could attenuate the progression of liver fibrosis through inhibition of hepatic stellate cell adhesion and activation [71].
10Epigallocatechin-3-gallateEpigallocatechin-3-gallate is one of the most promising compounds for the nutritional management of patients with hepatitis B infection [72]. Epigallocatechin-3-gallate is found in extracts from the leaves and buds of the tea plant Camellia sinensis[72]. There are four types of tea: black, green, oolong and pu-erh. It is recommended to drink six cups/day weighing 0.6 g per 100 mL of water (80 °C) or consume in the morning a concentrated cup weighing 2 g in 100 mL of water [72]. In humans, it was demonstrated that consuming 1-6 cups/day of green or black tea significantly increases plasma antioxidant levels after 1 h of consumption [72].
A cell culture study reported that epigallocatechin- 3- gallate is the most important compound against HBV in green tea [73]. Epigallocatechin-3-gallate at a concentration of 50 µM inhibited HBV entry by more than 80% without cytotoxicity in the cell culture [74]. Similar results were found in cultures transfected with HBV genotypes A, B, C, and D [74]. A treatment with 100 µM epigallocatechin-3-gallate decreased the expression of HBsAg and HBeAg by 58.6% and 48.5%, respectively, maintaining cell culture viability [73]. In addition, HBV viral load levels were dependent on epigallocatechin-3-gallate concentration [73]. In vitro, epigallocatechin-3-gallate was better at inhibiting HBsAg and HBeAg expression than lamivudine [75]. Also, two daily injections of epigallocatechin-3-gallate (50 mg/kg) could reduce HBV DNA and HBsAg levels in serum and liver tissues of the HBV-infected human liver chimeric mice [76]. In addition to the antioxidant effect, two anti-hepatitis B molecular mechanisms of epigallocatechin-3-gallate have been studied in vitro. First, epigallocatechin-3-gallate inhibits the first step of the HBV life cycle, stimulating the degradation or endocytosis of NTCP, which is a viral receptor [73]. The second antiviral mechanism of epigallocatechin-3-gallate is the induction of autophagosomes [77,78]. Autophagosomes are double-membrane structures that store intracellular components for degradation through fusion with lysosomes [77]. HBV can naturally induce phagosomes and then escape from them. In vitro, a study showed that epigallocatechin-3-gallate could induce special autophagosomes unfavorable for HBV replication (Fig. 1) [78].
11Potential anti-HBV nutrients in endemic foods of AmericaAlthough the nutrients mentioned above have been studied in other parts of the world, records in the database Phenol-explorer and US Food data central indicate that resveratrol, vitamin E, selenium, luteolin-7-O-glucoside, chlorogenic acid, and epigallocatechin-3-gallate can be found naturally in some staple Amerindian foods [79,80]. A summary of the concentrations of each compound are shown in Table 2. Resveratrol is found naturally in cocoa (Theobroma cacao) and peanuts (Arachis hypogaea). The highest vitamin E concentrations are found in sunflower seeds (Helianthus annuus) and raw green chili (Capsicum annuum). Sunflowers seeds (Helianthus annuus) are also an important source of selenium, followed by chia seeds (Salvia hispanica), raw green chili (Capsicum annuum), amaranth (Amaranthus), and maize (Zea mays) [79,80]. An animal source of selenium is turkey (Meleagris gallopavo), first domesticated by the Mesoamerican cultures [81]. The highest concentrations of luteolin-7-O-glucoside were found in the plant known as Mexican oregano (Lippia graveolens Kunth) [79]. Both chlorogenic acid and its derivative, caffeic acid, are mainly found in sunflower seeds (Helianthus annuus), potatoes (Solanum tuberosum), and red tomatoes (Lycopersicon esculentum Mill.) [79,80]. According to our review, the Amerindian beverage with the highest concentrations of epigallocatechin-3-gallate is cocoa (Theobroma cacao), followed by smaller amounts in avocado (Persea americana) and custard apple or “chirimoya” (Annona cherimola) [79,80]. Interestingly, the Mexican oregano plant (Lippia graveolens Kunth), beans (Phaseolus vulgaris), chili (Capsicum annuum), cocoa (Theobroma cacao), nopal (Opuntia ficus-indica), pumpkin (Cucurbita maxima), and maize (Zea mays) are recorded in the Cruz-Badianus codex [82,83], indicating that these plants have been used for their medicinal properties during hundreds or thousands of years. For example, archaeological and genetic evidence indicates that maize has been consumed by Mesoamerican populations from approximately 5400 to 9000 years ago [84,85]. On the other hand, HBV shows several degrees of adaptation to human populations [86]. Native Mexican patients infected with HBV genotype H tend to have lower viral load levels compared to other circulating genotypes, subtle liver enzyme abnormalities, absence of HBsAg, and rarely liver cancer [87]. Similar results have been observed in the Amazon Basin, where the HBV genotype F is the most prevalent, and its infection is less aggressive in Amerindian communities [88,89]. These characteristics could be the result of a balance between the immune response and viral replication that favors the survival of both the host and HBV. In this sense, an extended period of time is needed to develop this high degree of adaptation [86], considering that HBV jumped to humans around 33,600 years ago [90]. These findings are significant since the efficacy of nutritional therapy may depend on food availability, capacity to absorb anti-HBV nutrients, HBV genotype, and population ancestry.
Concentration of potential anti-HBV nutrients in Amerindian foods.
With the current antiviral drug therapy, hepatitis B infection is still almost impossible to cure due to the persistence of the cccDNA and the ability of the viral DNA to integrate into the host´s genome. In addition, no study has shown that patients with hepatitis B can be cured with nutritional therapy alone. However, developing a nutritional therapy combined with pharmacological treatment could increase the probability of a cure if HBV infection is diagnosed early. Nutritional therapy could be an alternative for naïve patients while they gain access to antivirals. Also, anti-HBV nutrients could be used as adjuvant therapy in those who achieved a functional cure, decreasing the risk of hepatitis B reactivation.
In this work, we summarized the nutrients with potential antiviral action against HBV that affect different steps of the HBV life cycle (Fig. 1). Most of these nutrients are found in accessible foods such as grapes, plums, peanuts, apples, blueberries, sunflower seeds, almonds, peanuts, spinach, broccoli, mango, tuna, sardines, shrimp, milk, coffee, and tea (Table 1). These nutrients and foods could be considered to develop a nutritional guide for managing patients with hepatitis B, which can be personalized to the foods available in each geographical region. The goals of this guideline wound be to improve the patient's nutritional status, reduce HBV viral load, improve response to antiviral therapy, and prevent the progression of liver fibrosis [10]. These goals are ambitious; however, with the development of DNA sequencing techniques, chromatography, Big Data analysis, and the application of artificial intelligence, it will be possible to accelerate the discovery of anti-HBV nutrients and propose new treatment strategies.
13ConclusionsScientific evidence indicates that the HBV replication, transcription, and expression of viral antigens can be affected directly by nutrients. In the future, these nutrients may be the basis for developing a nutritional guide for managing patients with hepatitis B infection.
Author contributionsA. Jose-Abrego conceived, investigated, and drafted the original manuscript. I. Rivera-Iñiguez wrote, investigated and reviewed the manuscript. L.A. Torres-Reyes wrote and reviewed the manuscript. S. Roman conceived, wrote, supervised, and visualized the manuscript. All authors contributed with intellectual content, critically revised, and approved the final version of the manuscript.
S. Roman is responsible for the integrity of the work as a whole.
FundingThis research did not receive any specific grant from public, commercial, or not-for-profit funding agencies. Fundacion Clínica Medica Sur funded the publication of this article.