Mycoplasma pneumoniae is a frequent cause of respiratory infections in school children and adolescents. Epidemiological suspicion is important, since there are no specific symptoms or signs to help in diagnosing infection caused by this agent.
ObjectiveTo determine the variation in prevalence over the last 10 years of M. pneumoniae IgM seropositivity according to age, particularly in pre-schoolers.
MethodThe results of M. pneumoniae IgM serological testing between January 2004 and December 2013 were analysed. Variables such as gender and month and year of sample processing were studied according to age groups (<5, 5–18, 19–50, 51–70 and >70 years of age).
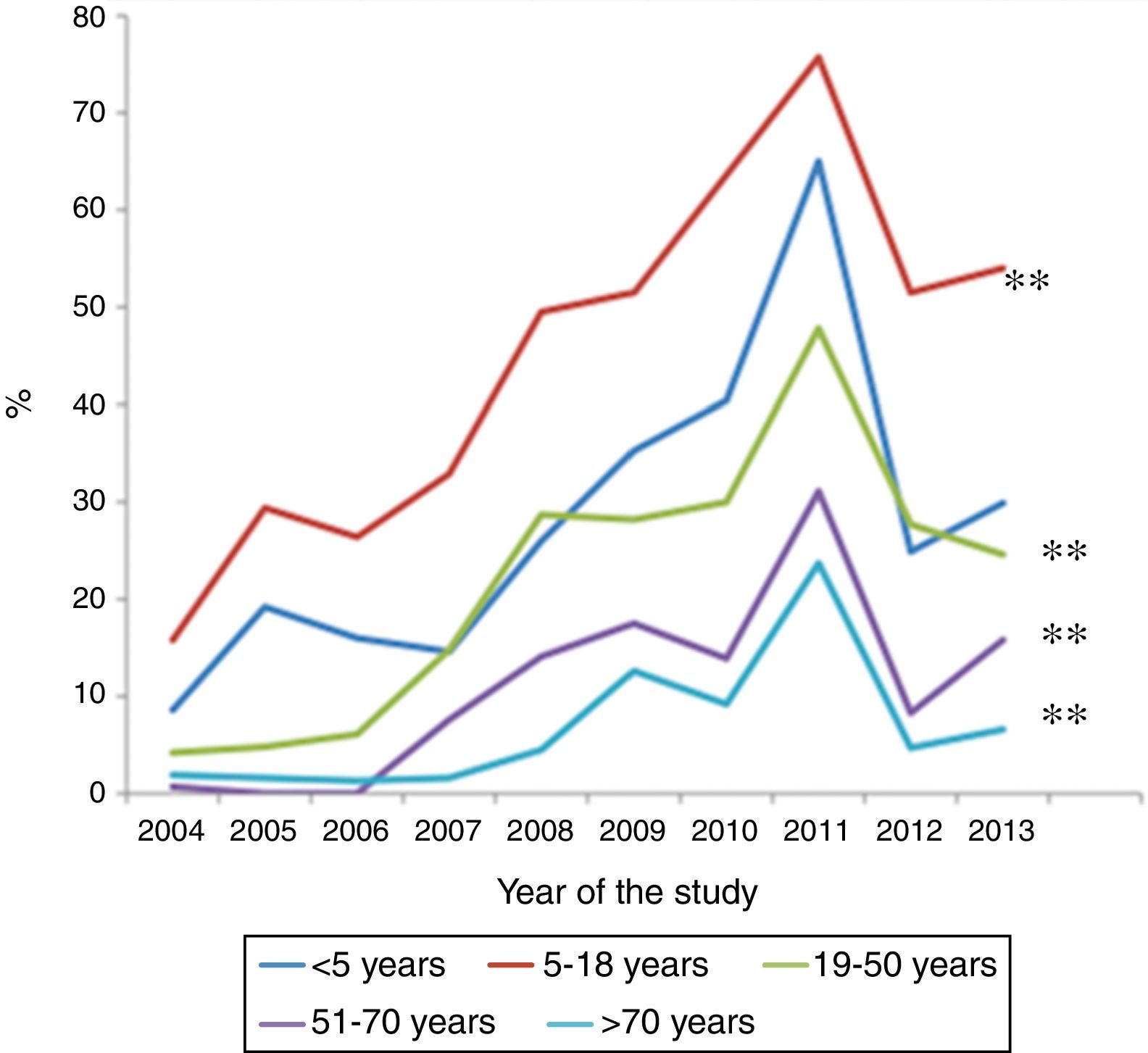
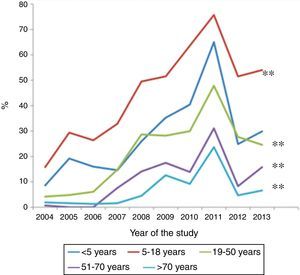
ResultsOf a total of 20,020 serological samples, 31.9% proved positive for M. pneumoniae. All age groups showed increases in percentage seropositivity over the last 10 years, although the most significant increase corresponded to the 5–18 years group (from 15.8% to 54%), followed by children <5 years of age (from 8.6% to 30%). Seropositivity was significantly higher in women in all age groups, except in those over 50 years of age.
ConclusionChildren under five years of age were the group with the second highest increase in seropositivity.
Mycoplasma pneumoniae is a common cause of respiratory infections in children, and is recognised as having been one of the aetiologies of atypical pneumonia over the last 50 years.1,2 In children, M. pneumoniae is responsible for 10%–40% of all cases of community-acquired pneumonia.2,3 Although the infection is mild and self-limiting, some patients of all ages can develop severe and fulminating disease. M. pneumoniae can also manifest with extra-pulmonary symptoms.4 Since there are no specific clinical symptoms or signs of help in diagnosing community-acquired pneumonia caused by this agent, epidemiological suspicion proves very important.5 The early diagnosis of this microorganism allows specific antimicrobial therapy and limits its spread within the community.6
Up until the early 1990s it was thought that M. pneumoniae was a pathogen that causes pneumonia in patients over five years of age. Since then, however, specialised diagnostic techniques have shown that M. pneumoniae may play an important role as a cause of upper and lower respiratory tract infections also in children under five years of age.2,7 A study has shown that in the last 16 years, the average age of M. pneumoniae seropositivity in patients with prolonged respiratory infections has decreased from 7 years to 2.9 years.8 The more recent outbreaks in Korea have shown a peak in the early years of life in comparison to the situation found in the past.9 Another study has reported the peak incidence of M. pneumoniae pneumonia in children to be in the 4–6 years age range.2 Furthermore, the prevalence of M. pneumoniae pneumonia among hospitalised children under five years of age has been found to be 17%.10
All these changes in the prevalence of M. pneumoniae should have inferences in the current treatment guidelines. However, there is little information on the relationship between this infection and age distribution in Latin America,8,11 and no previous epidemiological studies have been made in the concrete case of Chile. A single study conducted in ambulatory clinics in Santiago (Chile) reported a 2% prevalence of M. pneumoniae pharyngeal carrier status in 185 asymptomatic children.6
The aim of this study is to determine the variation in prevalence over the last 10 years of M. pneumoniae IgM seropositivity according to age, particularly in pre-schoolers. Our working hypothesis is that seropositivity in children under five years of age has increased over the last 10 years in Santiago (Chile).
MethodsA cross-sectional study was made between 2004 and 2013 in our clinical university laboratory (UC-Christus C.M. San Joaquin, Santiago, Chile). Inclusion criteria: all samples collected for M. pneumoniae IgM serological testing requested by the attending physician were analysed. A browser was added with the program Query Builder (v. 6.0.7.0.0) to highlight all the results of M. pneumoniae IgM serological testing performed during this period. The result of such serological testing was considered to be positive based on the cut-off value used in our laboratory employing enzyme immunoassay (ELISA IgM M. pneumoniae Test System®, Zeus Scientific, Raritan, NJ, USA) testing during 2004–2008, or indirect immunofluorescence (IFI M. pneumoniae IgM IFI Antibody Test System®, Zeus Scientific, Raritan, NJ, USA) during 2009–2013. The sensitivity and specificity of these techniques were 89.1% and 92.8% for the ELISA, and 100% and 97.5%, respectively, for the IFI. The IFI technique was reported positivity with a cut-off point >1/16 dilutions, and indeterminate when the solution could not be clearly defined as either positive or negative.
The following patient parameters were analysed: age, gender, year and month of processing of the test, and the origin of the test sample (ambulatory or in-hospital). For the purposes of this study, the results were stratified according to five age groups: patients under 5 years of age, 5–18 years of age, 19–50 years of age, 51–70 years of age, and over 70 years of age.
The Ethics Committee of the Pontificia Universidad Catolica de Chile approved the study protocol (# 13-052).
Statistical analysisIn assessing the differences between positive versus negative M. pneumoniae serology, the chi-squared test was used for categorical variables and the Student t-test for continuous variables. A p-value of <0.05 using a two-tailed test was taken to indicate significance. Logistic regression analysis was performed to discard confounding variables in the analysis of IgM seropositivity over time. The SPSS® version 17.0 statistical package (IBM, Armonk, NY, USA) was used throughout.
ResultsDuring the 10-year study period, a total of 20,020M. pneumoniae serological tests were retrieved from the database (54.3% corresponding to tests in females). Ambulatory patients were more frequently involved (83.2%) than hospitalised patients. A total of 29.4% (n=5886) of the study sample corresponded to children under the age of five years; while 35.6% (n=3824) corresponded to children between 5 and 18 years of age; 19.1% (n=7127) to patients between 19 and 50 years of age; 10.4% (n=2082) to patients between 51 and 70 years of age; and 5.4% (n=1081) to patients >70 years of age. The largest annual number of requested tests corresponded to the year 2011 with 2866 tests (13.3%) and the lowest to the year 2004 with 1481 tests (7.4%).
Of the total serological tests, 31.9% yielded a positive result (n=6385), while 64.5% proved negative (n=12,915) and 3.6% were indeterminate (n=720). The age group with the highest percentage M. pneumoniae seropositivity was the 5–18 years age group (45.6%), followed by the patients under five years of age (31.4%). The lowest percentage seropositivity corresponded to patients over 70 years of age (7.4%). From the age of 18 years to the oldest patients, percentage seropositivity was found to decrease with increasing age (from 24.3% to 7.4%).
Over the 10-year study period, all age groups showed an increase in percentage seropositivity, although the two groups with the highest increments were the 5–18 years age group (from 15.8% to 54%), followed by the patients under five years of age (from 8.6% to 30%) (p<0.001) (Fig. 1). Among the children under five years of age, those over two years of age showed a greater increase than those under two years of age (33% versus 17%, respectively; p<0.001). The group with the lowest increment corresponded to the patients over 70 years of age.
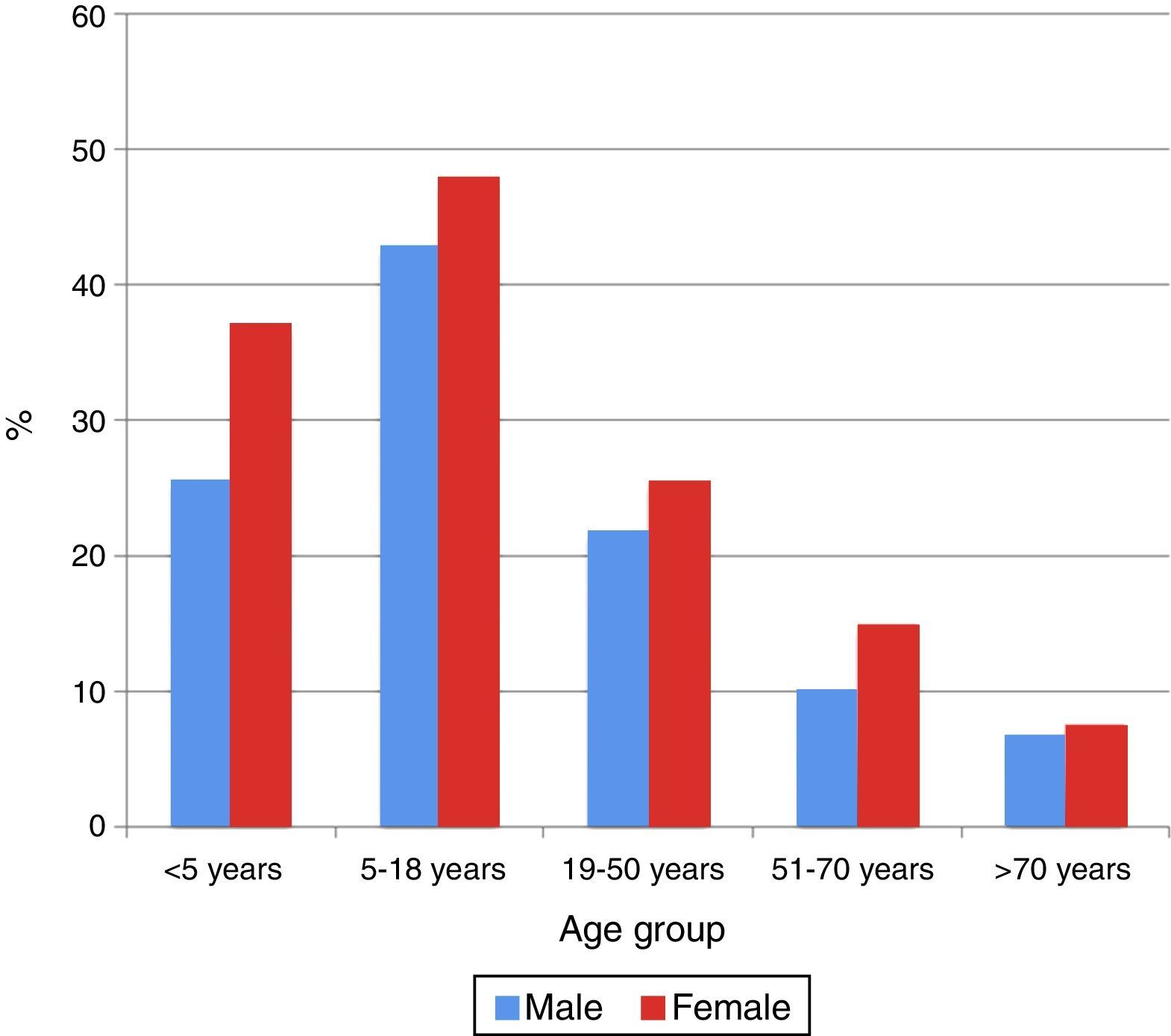
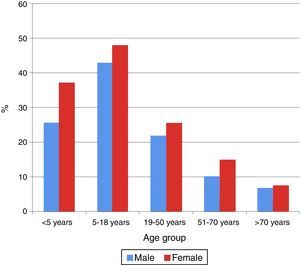
In all the age groups women showed the highest percentage seropositivity (p<0.05), except in the 51–70 years age group, where no significant gender differences were observed (p=0.47) (Fig. 2).
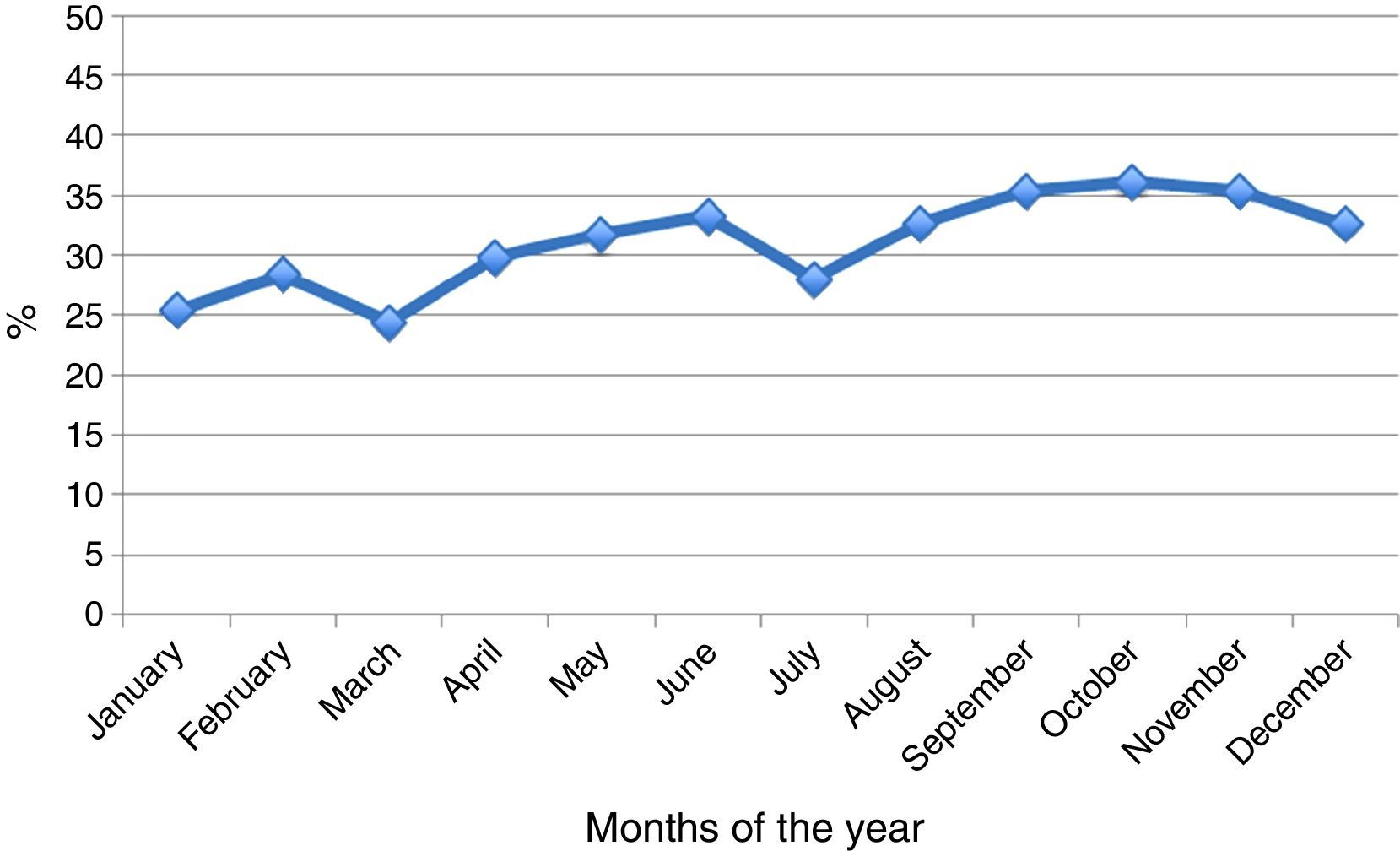
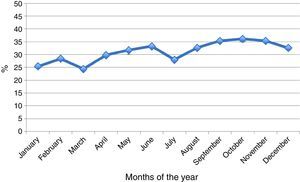
As regards seasonal distribution in the course of the year, increased seropositivity was recorded in May, June, August, September and October. In general terms, a seropositivity increment of 0.5% monthly was observed over the years (Fig. 3).
DiscussionThe present study recorded a constant increase in the prevalence of positive M. pneumoniae serological tests in all age groups over the course of the 10-year study period. Following the patients between 5 and 18 years of age, the second highest increment corresponded to those younger than five years of age (particularly infants between 2 and 4 years of age).
A number of factors may explain these findings, such as a progressive increase in the incidence of infections caused by this microorganism or an increase in clinical suspicion on the part of physicians over time. An important observation supporting this latter explanation is the fact that the requests for serological testing increased on a constant basis year-to-year from 2004 to 2013. Another possible explanation is that the new virological diagnostic techniques (e.g., molecular PCR) would cause the request for M. pneumoniae serological testing to be made in more selected patients than in the past.
On the other hand, our results support the change in the classical conception that M. pneumoniae is infrequent in the first years of life.7 The cause of the observed increase among children under the age of five years may be the more frequent existence of pre-school nurseries.8 Accordingly, changes in therapy for respiratory infections in patients under the age of five years should be taken into account.3,8,10 A recent nationwide surveillance in Taiwan reports that children young than five years of age with community-acquired mycoplasma pneumonia have a significantly longer hospitalisation, higher intensive care unit admission rates and more complications than older children.11
In our study the greatest frequency of infection by M. pneumoniae corresponded to the female gender in patients less than 50 years of age. This is consistent with the literature, which describes an increase in frequency in this gender between 25 and 40 years of age.3 The larger percentage of ambulatory origin can be interpreted as resulting from the fact that handling and suspicion of this infection occur mainly in the ambulatory care setting. However, due to the characteristics of the study, it is not known whether these patients needed further hospitalisation.
M. pneumoniae is endemic all over the world, at any time and in any season, although it is a little more frequent in the Northern hemisphere towards the end of summer and the beginning of autumn. Furthermore, outbreaks are common and occur in cycles of 4–7 years.3,4,12,13 In Chile (located in the Southern hemisphere) there was an increase in positive test results in May and June (autumn), follow by August, September and October (end of winter and early spring). These latter months have not been frequently cited.3,13 The prevalence of all clinical syndromes caused by M. pneumoniae varies from 2% during an endemic year to 35%–10% in epidemic periods.3 In our study we observed an increase in percentage seropositivity in the year 2011 (from 20.5% to 60% in the period 2005–2011) that could be interpreted as an outbreak – although this can only be corroborated by follow-up data. Interestingly, in 2011 there was an increase also in England, Wales and Finland (e.g., Finland registered a 4-fold increment from 2005 to 2011).14,15
A strong point of our study is the great number of tests performed in the same laboratory during these 10 years. However, the study also has some weaknesses. Firstly, obtaining the results referred only to the test caused a loss of information as to whether the seropositive results had any clinical repercussion (e.g., severity or need for treatment). Secondly, this is a single-centre study, and the results therefore cannot be generalised. On the other hand, two different laboratory techniques were used for M. pneumoniae testing (ELISA and IFI), although their sensitivity and specificity were similar. In turn, it is not known whether one same patient had two M. pneumoniae IgM samples during the acute infection episode (since negative conversion may take some time). Lastly, it is known that serological tests are difficult of interpret in the case of acute infection due to M. pneumoniae, especially when based on a single sample. However, in children the sole presence of IgM antibodies is a good indicator of acute infection.16 Currently a very useful but expensive and not very widespread technique for the diagnosis of M. pneumoniae infection is real time-PCR (RT-PCR) testing in respiratory fluid samples. A recent study found that during the acute phase of the disease, the detection of IgM antibodies in combination with RT-PCR allows for a precise and reliable diagnosis of M. pneumoniae infection in children.17 Another new available technique is the Ribotest® (a rapid antigen kit for the detection of M. pneumoniae ribosomal protein L7/L12 using immunochromatographic assay), however their sensitivity is only 60% compared with RT-PCR.18 In contrast, the study of the protein MPN474 (which plays an important role in cytoadherence of the cells during M. pneumoniae infection) has a high sensitivity (around 90.7%).19
In conclusion, following the 5–18 years age group, children under five years of age have shown the greatest increase in M. pneumoniae seropositivity over the last 10 years. It is therefore important for clinicians to suspect infection due to M. pneumoniae in this age group in order to ensure adequate diagnosis and treatment.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Source of fundingNone.
Financial disclosureThe authors have no financial relationships relevant to this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors thank Dr. Cecilia Perret for her scientific review of the manuscript, and Mr. Joe Perkins for his editorial assistance.