Seasonal allergic rhinitis (SAR) is characterised by an inflammation consequent to allergen exposure. Nitric oxide may be involved in allergic inflammation.
ObjectiveThis study evaluated the serum nitrite concentrations in SAR patients during and outside pollen exposure in order to estimate activity of nitric oxide synthases.
MethodsOne hundred and two (56 females, 46 males, median age: 28.7 years) were included in this study: 56 with SAR evaluated outside the pollen season and so without allergic inflammation and symptoms, and 46 with SAR evaluated during the pollen season with symptoms. Serum concentrations of nitrite were measured and in those patients exposed to pollens, results were compared to scores of the Visual Analogue Scale for nasal obstruction perception.
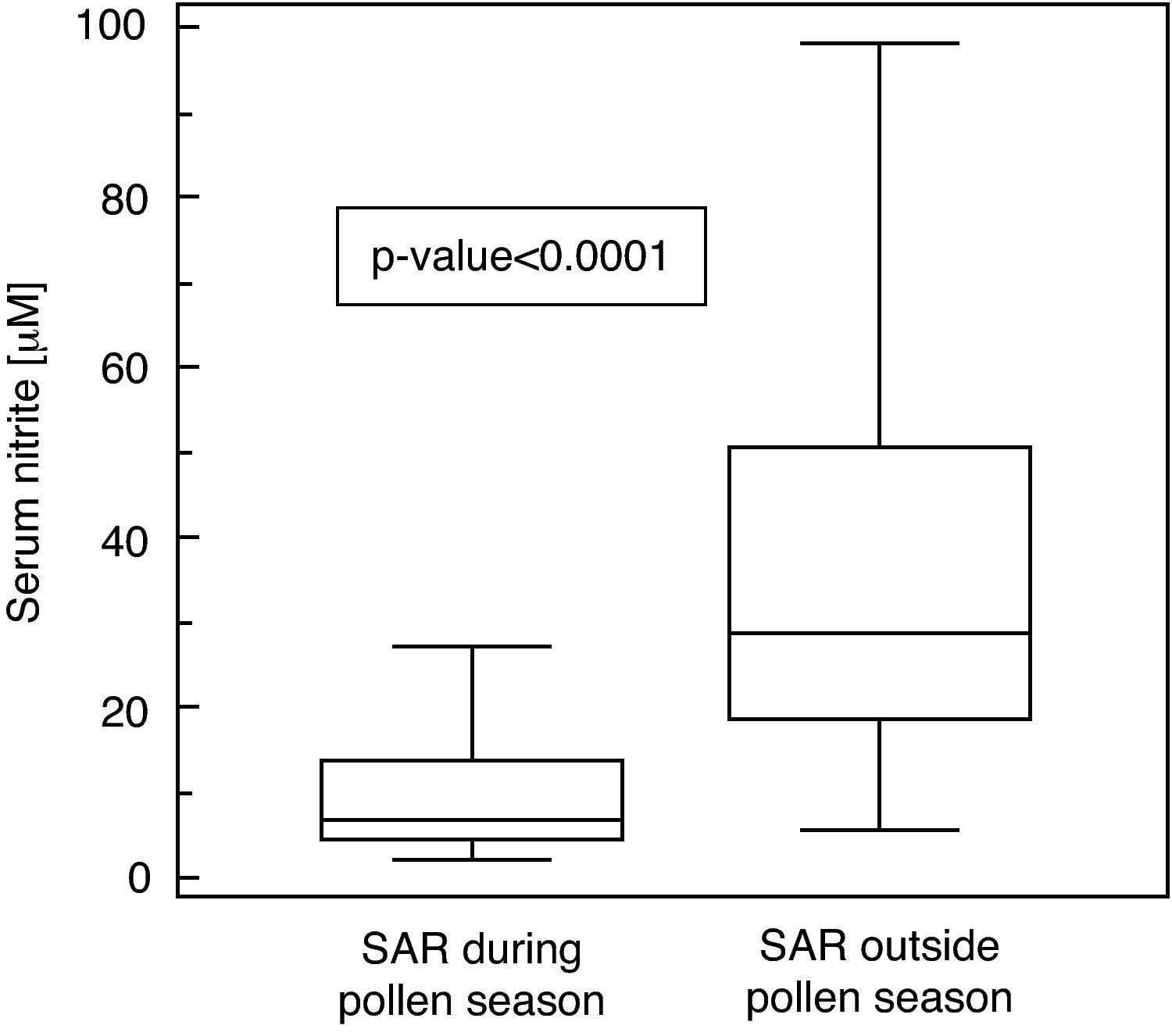
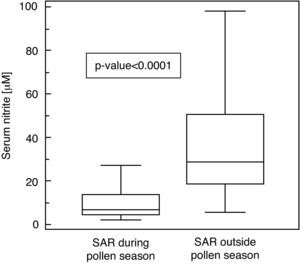
ResultsSerum nitrite concentrations were higher in SAR patients evaluated outside the pollen season (U=−6.78; p<0.0001), moreover, there was a significant relationship between nasal obstruction perception and nitrite in patients evaluated during the pollen season.
ConclusionThis preliminary study demonstrates that serum nitric oxide metabolism depends on allergen exposure.
Allergic rhinitis (AR) is typically an immune-mediated disease, characterised by a dysregulation of T-cell responses. An inflammatory cascade characterises AR: the allergen exposure induces mast cell activation by allergen-specific IgE. Activated mast cells release pro-inflammatory cytokines and chemotactic factors which induce an accumulation of inflammatory cells, including eosinophils, neutrophils, basophils, and lymphocytes in nasal mucosa.1 These inflammatory cells start a vicious cycle by producing more cytokines, chemotactic factors, and free radicals, including superoxide anion (O2−).2–4 These events cause an enhanced vascular permeability and persistent inflammation of the nasal mucosa, finally resulting in obstruction. Nasal obstruction may be considered the main relevant symptom in AR.5 Moreover, with the progression of mucosal oedema, a compression of blood vessels occurs, thus the blood flow declines and renders in turn an ischemic insult to the nasal mucosa.6 Both mucosal oedema and ischaemia determine the objective finding at rhinoscopy characterised by a pale and swollen mucosa. Nasal obstruction is generated by vasodilation, the secretion of nasal glands and by exudation from the mucosal vasculature. A dense network of nerves surrounding these glands and blood vessels is believed to regulate nasal function. Recently, nitric oxide (NO) has been shown to be a co-transmitter of acetylcholine in parasympathetic nerve fibres of the central peripheral nervous system and can also modulate cholinergic effects in the vascular system and glands: the so-called nitrergic innervation.7,8 When considering the causes for the reduced nasal blood flow, it may include both the decrease in NO production and the mechanical compression, since NO is a potent vasodilating factor, generated by constitutive NO synthase in the endothelial cells.9,10 It has been reported that NO is reduced when the endothelium is impaired.11 However, few studies have addressed the role of NO in AR. A first study reported an increased concentration of NO, in the exhaled air in untreated AR patients compared with normal subjects.12 Increased NO production in nasal lavage fluid in the nose of patients with perennial AR was documented.13 A recent study investigated the serum concentrations of nitrite in patients with AR,14 nitrite concentrations presenting an oxidation product of NO and other nitrogen-containing compounds.
This preliminary study was performed in order to compare serum nitrite concentrations in seasonal AR (SAR) patients during and outside the pollen exposure season.
Materials and methodsOne hundred and two patients (56 females, 46 males, median age: 28.7 years) were included in this study: 56 with SAR evaluated outside the pollen season and so without allergic inflammation and symptoms, and 46 with SAR evaluated during the pollen season with symptoms. AR diagnosis was made according to the Allergic Rhinitis and its Impact on Asthma (ARIA) document.15 All allergic patients were sensitised to pollens alone and suffered from moderate to severe persistent Allergic Rhinitis since at least four years ago.
The Ethical Committee approved the study and informed consent was obtained by each of the subjects.
Visual Analogue Scale (VAS) was used to quantify the subjective feeling of nasal obstruction, as previously reported,16 in patients exposed to pollen allergens. It ranges from 0 (no obstruction) to 10 (complete obstruction). Patients were asked to position a cross on a line corresponding to their own perception of nasal obstruction.
Concentrations of the stable NO metabolite nitrite were determined in serum applying the Griess reaction assay.17,18 Using a Griess reaction system (Promega), sulphanilamide was quantitatively converted to a diazonium salt by reaction with NO2− in acid (phosphoric acid) conditions. The diazonium salt was then coupled to N(1-naphthyl) ethylenediamine dihydrochloride (NED), forming an azo dye that was read at 540nm in a spectrophotometer.
Statistical analysis was performed using the statistical software package Medcalc 9 (Frank Schoonjans, BE). Descriptive statistics were first performed, and quantitative parameters were reported as the medians (MD) with interquartile ranges (IQR) due to the skewed distribution. Comparison of the nitrite concentrations in the two groups of patients was performed by the non-parametric Wilcoxon test. Correlation between quantitative variables was evaluated by means of the Spearman's correlation coefficient (rs). All tests were two sided and a p-value less than 0.05 was considered statistically significant.
ResultsSerum nitrite concentrations significantly differed between SAR patients evaluated outside the pollen season (MD: 28.9μM, IQR: 18.9-50.9μM) compared to those obtained during the pollen season (MD: 7.25μM, IQR: 4.4-13.9μM; U=−6.78, p<0.0001; Fig. 1).
Serum concentration of nitrite (μM) in patients with seasonal allergic rhinitis evaluated during and outside the pollen season. Data are represented as medians (horizontal lines), interquartile ranges (boxes), ranges (vertical lines, excluding outliers) and p-value between the groups (U=−6.78; p<0.0001).
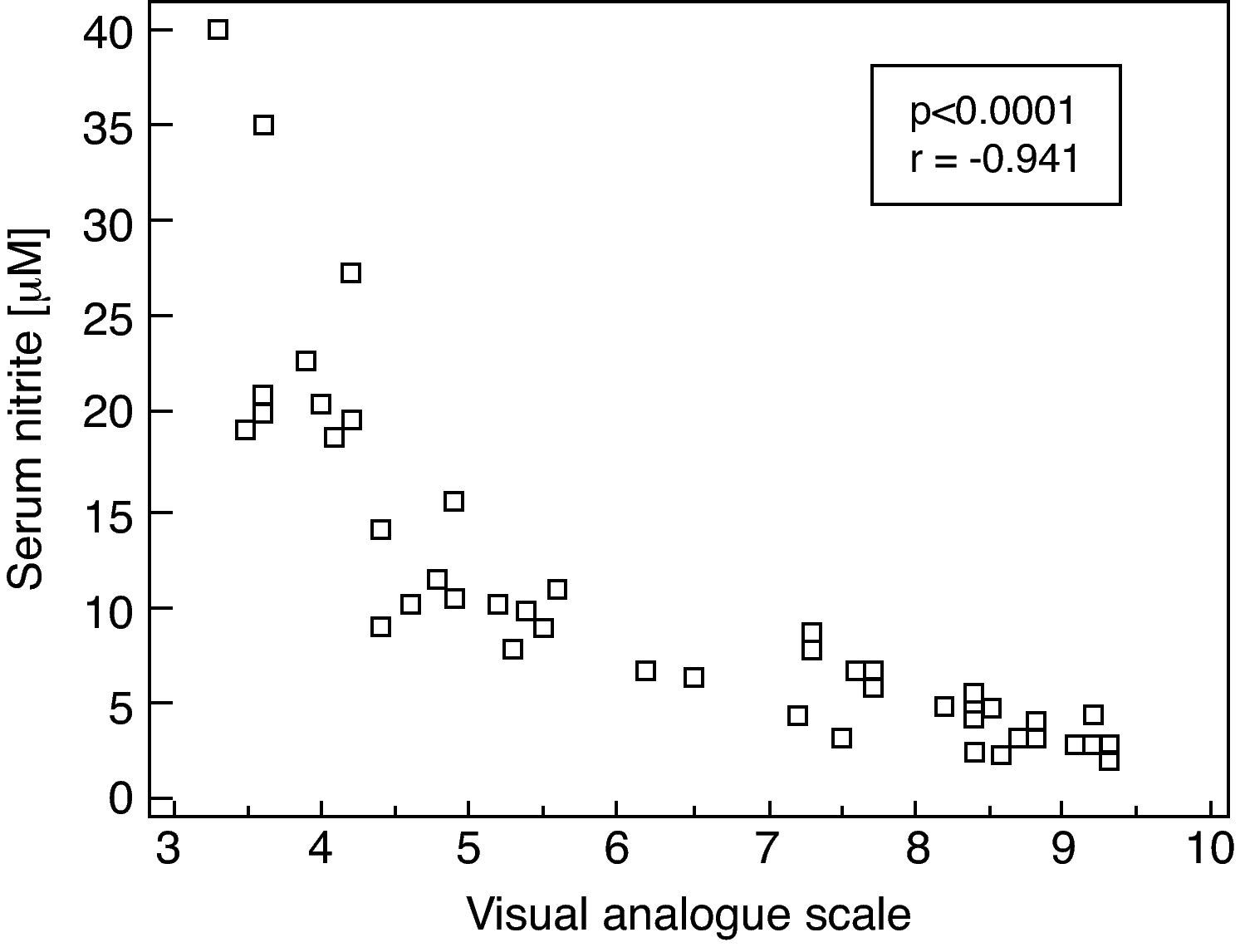
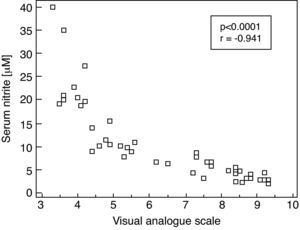
Moreover, there was a significant correlation between serum nitrite concentrations and VAS in SAR patients evaluated during the pollen season (rs=−0.941, p<0.0001. Fig. 2)
Correlation between serum concentration of nitrite (μM) and intensity of symptoms as judged by the Visual Analogue Scale in patients with seasonal allergic rhinitis evaluated during the pollen season; Spearman's correlation coefficient (rs) and p-value are given (rs=−0.941; p<0.0001).
Allergic Rhinitis is sustained by an inflammatory reaction. Various populations of effector and regulatory T-cells have been shown to play a crucial role in allergic inflammation, mainly concerning Th2-type cells.19 Th2-derived cytokines, such as IL-4 and IL-13, are the primary pathogenic factors in inducing, maintaining, and amplifying inflammatory allergic inflammation. IL-4 and IL-13 orchestrate allergic inflammation promoting IgE synthesis, up-regulating adhesion molecules selective for eosinophil recruitment, and causing increased mucus production and airway hyperreactivity. Therefore, a cascade of inflammatory phenomena sustains the symptom occurrence, mainly concerning nasal obstruction. In fact, nasal obstruction severity is directly related to the intensity of allergic inflammation. Pollen allergy is characterised by more intense severity of both symptoms and nasal inflammation than AR due to perennial allergens.20 In this regard, the inflammatory oedema easily induces a compression of nasal blood vessels. This pathophysiological mechanism could partially explain the findings of this study.
Our study confirms and extends the earlier data reported by Unal et al. on a small group of SAR patients presenting with higher nitrite concentrations than healthy controls.14 Moreover, we demonstrated a significant correlation between nitrite concentrations and symptom severity as assessed by the VAS. Increased nitrite concentrations could indicate higher activity of NOS in SAR patients outside the pollen season, which is suppressed during the pollen exposure season. According to the hypothesis of a shifted balance of Th1/Th2-type immunity during acute allergic responses, one might expect diminished production of Th1-type cytokine interferon–γ (IFN-γ) in the patients during seasonal exposure. So the decline of nitrite concentrations during the pollen season could be well explained by an increased Th2-type cytokine profile with lower IFN-γ production. Such speculation would also fit to the earlier described elevation of serum tryptophan concentrations in SAR patients outside the pollen season.21,22 It is well documented that NO production strongly interferes with expression of tryptophan-degrading enzyme indoleamine(2,3)-dioxygenase (IDO).23 Thus, when nitrite concentrations are higher in SAR patients outside the pollen season and due to higher NO production, an inhibition of IDO is likely and as a consequence tryptophan concentrations would increase. But still one has to keep in mind that increased nitrite concentrations can develop not only when NO production is manipulated, they represent a summary readout for the turn-over of nitrogen-containing amino acids and are thus of low specificity for NOS, and further studies are clearly needed to clarify the issues discussed.
Moreover, patients evaluated during the pollen season have an intense nasal inflammation that could reduce the production of NO. To support this hypothesis there is a relationship between NO metabolites and perception of obstruction. The more intense the obstruction is, the more NO metabolism is impaired.
However, this preliminary study has two main limitations: (i) serum NO metabolites were assessed in two different groups of patients and not in the same patients during and out of the pollen season, (ii) serum NO metabolites concentrations were not compared with nasal NO. On the other hand, the aim of the current preliminary study was to consider a more easy and available procedure (such as serum assessment) than nasal NO measurement, which requires specific instruments.
In conclusion, this preliminary study demonstrates that serum NO metabolism depends on allergen exposure. Therefore, further studies should be performed to confirm these preliminary findings.
Conflict of interestThere is no conflict of interest for any author.
The authors wish to thank Vania Giunta and Mara DeAmici (Clinica Pediatrica, Fondazione IRCCS Policlinico S. Matteo, Pavia) and Maria Gleinser (Innsbruck Medical University) for outstanding statistical and technical support.
The authors confirm that the manuscript has not been published elsewhere and is not under consideration for publication elsewhere, as well as all authors approved the paper.