Chronic liver inflammation may lead to hepatic cirrhosis, limiting its regenerative capacity. The clinical standard of care is transplantation, although stem cell therapy may be an alternative option. The study aim was to induce endogenous hematopoietic stem cells (HSCs) with granulocyte colony stimulating factor (G-CSF) and/or intravenous administration of adipose tissue-derived mesenchymal stem cells (MSCs) to decrease hepatic fibrosis in an experimental model.
Material and methodsA liver fibrosis model was developed with female Wistar rats via multiple intraperitoneal doses of carbon tetrachloride. Three rats were selected to confirm cirrhosis, and the rest were set into experimental groups to evaluate single and combined therapies of G-CSF-stimulated HSC mobilization and intravenous MSC administration.
ResultsTreatment with MSCs and G-CSF significantly improved alanine amino transferase levels, while treatment with G-CSF, MSCs, and G-CSF+MSCs decreased aspartate amino transferase levels. Hepatocyte growth factor (HGF) and interleukin 10 levels increased with MSC treatment. Transforming growth factor β levels were lower with MSC treatment. Interleukin 1β and tumor necrosis factor alpha levels decreased in all treated groups. Histopathology showed that MSCs and G-CSF reduced liver fibrosis from F4 to F2.
ConclusionsMSC treatment improves liver function, decreases hepatic fibrosis, and plays an anti-inflammatory role; it promotes HGF levels and increased proliferating cell nuclear antigen when followed by MSC treatment mobilization using G-CSF. When these therapies were combined, however, fibrosis improvement was less evident.
Chronic liver inflammation may be caused by viruses, alcohol, autoimmunity, and fatty liver disease, leading to cirrhosis [1–3]. Hepatic failure occurs when hepatic progenitor cell niches burn out, limiting the organ's regenerative capacity [4]. The clinical standard of care is liver transplantation, though the low donor availability and high costs make this option less practical. Another treatment option is stem cell therapy [5,6] based on two strategies: (1) mobilization of endogenous hematopoietic stem cells (HSCs) induced by administration of granulocyte colony stimulating factor (G-CSF) or (2) inoculation of exogenous HSCs or mesenchymal stem cells (MSCs) [7].
MSCs are multipotent, non-hematopoietic stromal cells. They probably reside in a perivascular niche in vivo and can be isolated from various organs and tissues, including adipose tissue (AT) [8]. MSCs can contribute to the direct production of new hepatocytes, can promote tissue repair by secreting trophic molecules, are immunomodulatory, have antifibrotic properties, and inhibit activation of hepatic stellate cells [9]. In bone marrow (BM), MSCs comprise around 0.001–0.08% of cells and mobilize to the peripheral circulation following experimental injury [8,10]. AT is also a good source of MSCs, with a high proliferative potential [11,12]; one gram of AT has 500 times more MSCs than BM and a better proliferative capacity than BM-derived MSCs [13,14]. Clinical studies using AT-derived MSCs showed improved histology and liver function in human and animal subjects [15–19]. Furthermore, the easy access to subcutaneous AT, the ability to repeatedly sample this tissue, and the uncomplicated enzyme-based isolation procedures make AT the most attractive source of MSCs [20].
Another strategy for stem cell therapy is to stimulate the mobilization of HSCs from BM into the blood stream through administration of G-CSF [21]. Mobilization of endogenous HSCs using G-CSF is also a promising treatment for acute and chronic liver damage in animal models [16,22–24]. BM-resident HSCs can be mobilized into the peripheral blood at a low rate under specific stimuli, such as tissue injury [16,25,26], or at high rates with pharmacologic priming with cytostatic drugs, chemokines, or hematopoietic cytokines [27,28]. G-CSF is a hematopoietic growth factor that mediates HSC mobilization to peripheral blood and represents the most widely used mobilizing agent [29]. Several reports have suggested that G-CSF mobilized HSCs contribute to liver repair in acute and chronic liver injury models [30,31]. The synergistic effect of HSC and MSC therapies have been observed in heart failure patients and in neovascularization processes in bioengineered bone [32,33].
Thus, the study aim was to use endogenous HSCs via G-CSF and/or intravenous administration of AT-derived MSCs to improve liver function and decrease hepatic fibrosis in an experimental model.
2Material and methods2.1Isolation and characterization of MSCs derived from adipose tissueWe obtained MSCs from inguinal fat AT of male rats (2 months of age, 225±25g body weight). The fat was collected in phosphate buffered saline (BioWest, France) containing 2% antibiotic/antimycotic (BioWest) and incubated for 1h at 4°C. AT was homogenized and subsequently digested with collagenase type I (0.1%) (Gibco, USA) for 30min at 37°C under constant stirring. After two phases were visualized, the lower phase was collected and centrifuged at 1500rpm for 3min. The supernatant was removed, and the pellet was washed three times with sterile phosphate buffered saline at 1500rpm for 3min. The cell pellet was suspended in Dulbecco's Modified Eagle's Medium (BioWest), 10% fetal bovine serum (BioWest) and 1% antibiotic/antimycotic (BioWest). The medium was changed every 3 d until the monolayer of adherent cells reached 80% confluence. Cell passaging was performed using 0.25% trypsin-ethylenediaminetetraacetic acid solution (BioWest) until the third passage, after which the cells were used for transplantation according to procedures described by Tobergte et al. [34].
Expression of the markers CD105, CD90, CD45, CD34, and β-actin as an internal control was determined by quantitative real-time polymerase chain reaction using the primers: CD105 (5′-TGG TCT TTT CGA ACG AGA ATG-3′/5′-AGC CGG AGG ACA ATG CTT TTG G-3′); CD90 (5′-TTT ATC AAG GTC CTT ACT CTA GCC-3′/5′-CAG TCA CAG AGA AAT GAA GTC C-3′); CD45 (5′TCC ACG GGT ATT CAG CAA GTT TC-3′/5′-CCA GAT CAT CTT CCA GAA GTC ATC-3′); CD34 (5′-AAG ATC TTG GGA GCC ACC AGA G-3′/5′-TAG CCC TGG CCT CCA CCA TTC-3′); and β-actin (5′-GTC ACC TGG GAC GAT ATG G-3′/5′-AAG TCT AGG GCA ACA TAG CAC AG-3′). Ribonucleic acid extraction of MSCs from AT during the third passage was with Trizol® (Invitrogen, USA), performed according to the manufacturer's instructions, and complementary deoxyribonucleic acid was synthesized using SuperScript™ III First-Strand Synthesis SuperMix (Invitrogen, USA), SYBR® GreenER™ quantitative real-time polymerase chain reaction SuperMix (Invitrogen, USA) and analyzed with the method.
2.2Animal model of liver fibrosis and stem cell therapy administrationWe used female Wistar rats with a body weight of 225±25g raised with free access to water and food and on 12-h light/dark cycles. All studies were performed according to the Mexican Official Regulation (NOM-062-ZOO-1999) regarding the technical specifications for the production and care of laboratory animals. The research protocol was approved by the Institutional Animal Ethics Committee at the School of Medicine and the “Dr. José Eleuterio González” University Hospital, Universidad Autónoma de Nuevo Leon, (reference number: HI14-001).
To induce the liver fibrosis model, 15 female Wistar rats were injected intraperitoneally with carbon tetrachloride (CCl4) (Sigma–Aldrich, USA) at doses that increased weekly at 500, 570, 650, 850, and 1000mg 3× weekly over 8 weeks (wks), according to the modified protocol described by Muriel et al. [35]. At the end of the 8 wks, CCl4 was stopped, and we randomly selected three rats to confirm histopathologically that they had developed cirrhosis (see below). Each group included 3 Wistar rats. The negative control group received mineral oil intraperitoneally, and the CCl4 group was the positive control for the experimental cirrhosis. The next three groups received the experimental treatment at week (wk) 9. In all groups, CCl4 was stopped at wk 8. Group CCl4+G-CSF received 3 doses of G-CSF 300μg/kg/3d at wk 9 (days 1, 2, and 3). Group CCl4+MSC received 1 dose of 3×106 MSC at wk 9 (day 1), and group CCl4+G-CSF+MSC received 3 doses of G-CSF 300μg/kg/3d at wk 9 (days 1, 2, and 3) and 1 dose of 3×106 MSC at wk 10 (day 1).
2.3Determination of CD34+ surface markerWe evaluated circulating CD34+ before and after G-CSF treatment in all groups. Erythrocytes were lysed using BD FACS™ lysing solution (BD Biosciences, San José, CA) according to the manufacturer's instructions. The anti-CD34 antibody (Abcam, USA) was added in 1:50 proportion and incubated for 30min at room temperature. The stained cells were analyzed with Accuri C6 flow cytometer and CFlow plus software (BD Biosciences).
2.4AST, ALT, and growth factor and cytokine analysisBlood samples were drawn from all groups at 8 and 16 wks to perform aspartate aminotransferase (AST) and alanine aminotransferase (ALT) using standard commercial biochemical assay kits (Ilab Plus 300, Instrumentation Laboratory, Italy). The hepatocyte growth factor (HGF) (R&D Systems, USA) and transforming growth factor beta (TGF-β) (Abcam PLC, Cambridge, UK) were determined with enzyme linked immunosorbent assay using the multi-detection microplate reader (Biotek Instruments, USA). Interleukin 1beta (IL-1β), interleukin 6 interleukin 10 (IL-10), and tumor necrosis factor alpha (TNF-α) (Merck, Darmstadt, Germany) were measured by a Luminex analyzer (Merck). All analyses were run according to the manufacturer's instructions.
2.5Histopathological analysisAt 16 wks, the rats were sacrificed by cervical dislocation and the livers removed by dissection, fixed in 10% formalin, and embedded in paraffin wax. Tissues were stained with hematoxylin and eosin Masson's trichrome, and sirius red. Histopathological analysis was performed using the Olympus BH-2 optical microscope (Olympus Optical, USA). The degree of hepatic fibrosis was based on a histological grading scale using the METAVIR score [36]. The fibrosis was graded on a 5-point scale from F0 to F4.
Image analysis was performed to determine fibrosis score on micrographs of sirius red stained sections. Thirty fields per slide area were analyzed using ImageJ program version 2.0.0-rc-43/1.50E.
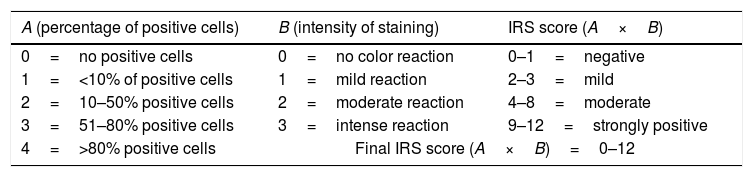
2.6Immunohistochemical study of proliferating cell nuclear antigen expressionParaffin-embedded samples at 8 and 16 wks were stained for immunohistochemistry with primary antibody proliferating cell nuclear antigen expression (PCNA) (1:4000) (Abcam, Cambridge, UK) with the mouse- and rabbit-specific HRP/DAB (ABC) detection immunohistochemistry kit (Abcam, Cambridge, UK) according to the manufacturer's instructions. The immunoreactive score was calculated as the product of the positive cell proportion score (0–4) multiplied by the staining intensity score (0–3) (Table 1) [37].
Immunoreactivity scores (IRS).
| A (percentage of positive cells) | B (intensity of staining) | IRS score (A×B) |
|---|---|---|
| 0=no positive cells | 0=no color reaction | 0–1=negative |
| 1=<10% of positive cells | 1=mild reaction | 2–3=mild |
| 2=10–50% positive cells | 2=moderate reaction | 4–8=moderate |
| 3=51–80% positive cells | 3=intense reaction | 9–12=strongly positive |
| 4=>80% positive cells | Final IRS score (A×B)=0–12 | |
The results are presented as mean±standard deviation (SD). Between-group differences were assessed for statistical significance with Student's t-test and p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS 19.0 computer software package (SPSS Inc, Chicago, IL).
3Results3.1Specific markers of MSCs derived from ATFrom the third passage cell culture, MSCs were characterized from a culture of adherent cells with fusiform appearance. Relative gene expression of specific markers for MSCs and HSCs were measured by quantitative real-time polymerase chain reaction in complementary deoxyribonucleic acid. Relative to expression of β-actin messenger ribonucleic acid, specific markers of MSC CD90+ and CD105+ were 60.5±0.1% and 24.8±0.1%, respectively. Low messenger ribonucleic acid expression of hematopoietic markers CD34 4±1.5% and CD45 10.4±0.4% was detected.
3.2Expression CD34+ in peripheral bloodThe population of CD34+ was analyzed before and 24h after treatment with G-CSF. Treatment induced a significant mobilization of hematopoietic CD34+ cells in: CCl4+G-CSF (0.20±0.17% before vs. 1.50±0.50% after treatment, p<0.01) and CCl4+G-CSF+MSC (0.57±0.25%, before vs. 1.70±0.36% after treatment, p<0.05). We did not find differences in either the control (0.20±0.10% before vs. 0.21±0.05% after saline, p=NS) or CCl4 (0.13±0.06%, before vs. 0.016±0.05%, after saline, p=NS).
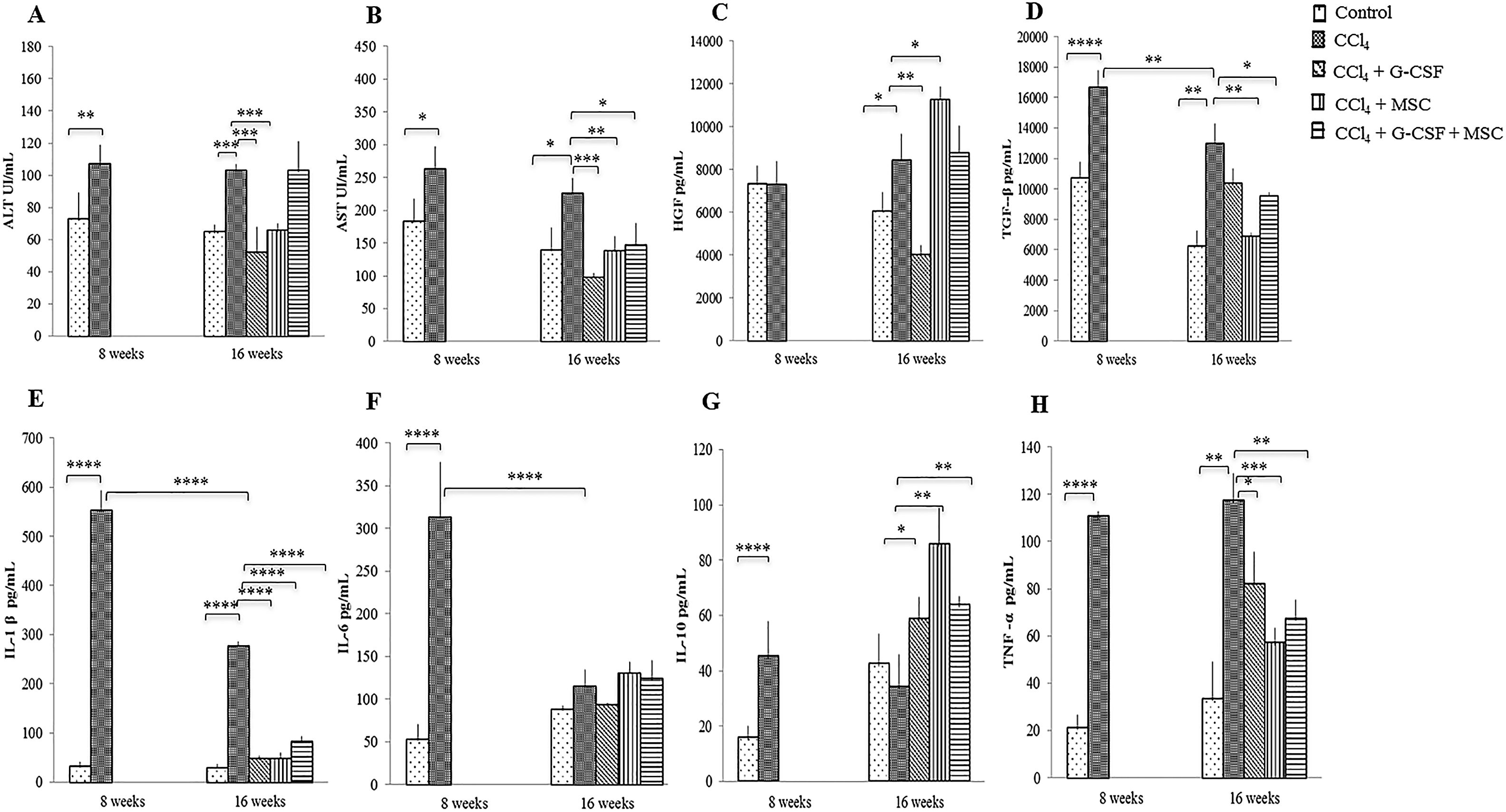
3.3AST and ALTAST and ALT were significantly decreased in the groups treated with CCl4+G-CSF and CCl4+MSC compared with the CCl4 group at 16 wks (p<0.001). In addition, AST decreased in CCl4+G-CSF+MSC (p<0.05) compared with the CCl4 group (Fig. 1A, B).
Changes in serum levels of liver enzymes, growth factors and inflammatory cytokines of the groups evaluated before treatment and after 8 and 16 weeks. Liver enzymes: (A) Alanino aminotransferase (ALT); (B) Aspartato aminotransferase (AST). Growth factors: (C) Hepatocyte growth factor (HGF); (D) Transforming growth factor β (TGF-β). Inflammatory cytokines: (E) Interleukin 1β (IL-1β); (F) Interleukin 6 (IL-6); (G) Interleukin 10 (IL-10); (H) Tumor necrosis factor α (TNF-α). Mean±SD (n=3). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
HGF levels of the positive control (CCl4) remained similar at 8 and 16 wks (p=NS) (Fig. 1C), whereas they decreased significantly at 16 wks in CCl4+G-CSF (p<0.01). Levels of HGF significantly increased in CCl4+MSC at 16 wks vs. CCl4 group (p<0.05) (Fig. 1C). There was no significant difference between TGF-β at 8 vs. 16 wks in the control group. However, a significant decrease in TGF-β levels was observed between 8 and 16 wks in the CCl4 group (p<0.01). At 16 wks a significant decrease in TGF-β levels was seen in CCl4+MSC (p<0.01) and CCl4+G-CSF+MSC (p<0.05) (Fig. 1D).
3.5Production of serum inflammatory cytokinesA natural recovery in IL-1β and interleukin 6 seemed to occur at 16 wks (p<0.001) in the CCl4 group (Figs. 1 E,F). However, the three treatment groups had significant decreases in IL-1β (p<0.001) (Fig. 1E), in contrast to interleukin 6 levels that were not altered by treatment groups (Fig. 1F). IL-10 was increased at 16 wks in all treatment groups: CCl4+G-CSF (p<0.05), CCl4+MSC, and CCl4+G-CSF+MSC vs. CCl4 (both p<0.001) (Fig. 1G). TNF-α levels were decreased in all treatment groups: CCl4+G-CSF (p<0.05), CCl4+MSC, and CCl4+G-CSF+MSC (both p<0.001) (Fig. 1H).
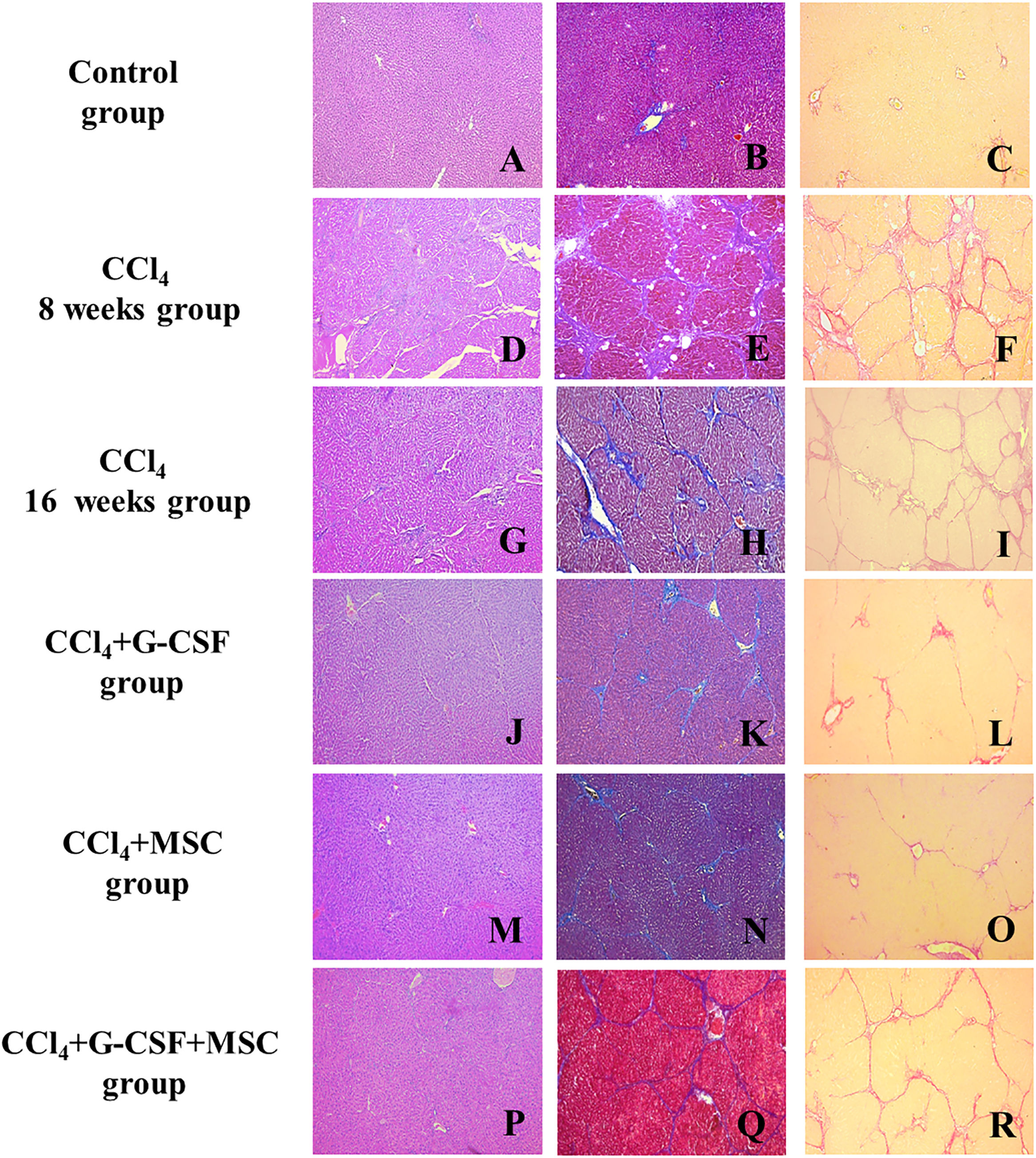
3.6Histopathological examinationThe CCl4 group showed hepatocellular degeneration, with presence of medium and small nodules with well-formed fibrous bands at 8 wks (METAVIR F4) (Fig. 2D–F). However, at 16 wks, bridging fibrosis with the presence of medium nodules with thin fibrous bands was observed (METAVIR F3) (Fig. 2G–I). At 16 wks, the CCl4+G-CSF and CCl4+MSC groups showed presence of septa and thin bridging (METAVIR F2) vs. CCl4 at 8 wks (Fig. 2J, K, L, M, N, O). At 16 wks, the CCl4+G-CSF+MSC group showed presence of septa, thin bridging, small and medium nodules, and decreased fibrosis to F3 vs. CCl4 at 8 wks, but no difference was found compared with the CCl4 group at 16 wks (METAVIR F3) (Fig. 2P, Q, R).
Histopathological analysis of liver tissue of the groups evaluated before treatment and after 8 and 16 weeks. Left side, hematoxylin & eosin (H&E); center, Masson's trichrome (MT); right, sirius red (SR); A (H&E), B (MT), C (SR): Control; D (H&E), E (MT), F (SR): CCl4 8 weeks; G (H&E), H (MT), I (SR): CCl4 16 weeks; J (H&E), K (MT), L (SR): CCl4+G-CSF 16 weeks; M (H&E), N (MT), O (SR): CCl4+MSC 16 weeks; P (H&E), Q (MT), R (SR): CCl4+G-CSF+MSC 16 weeks. Amplification (100×).
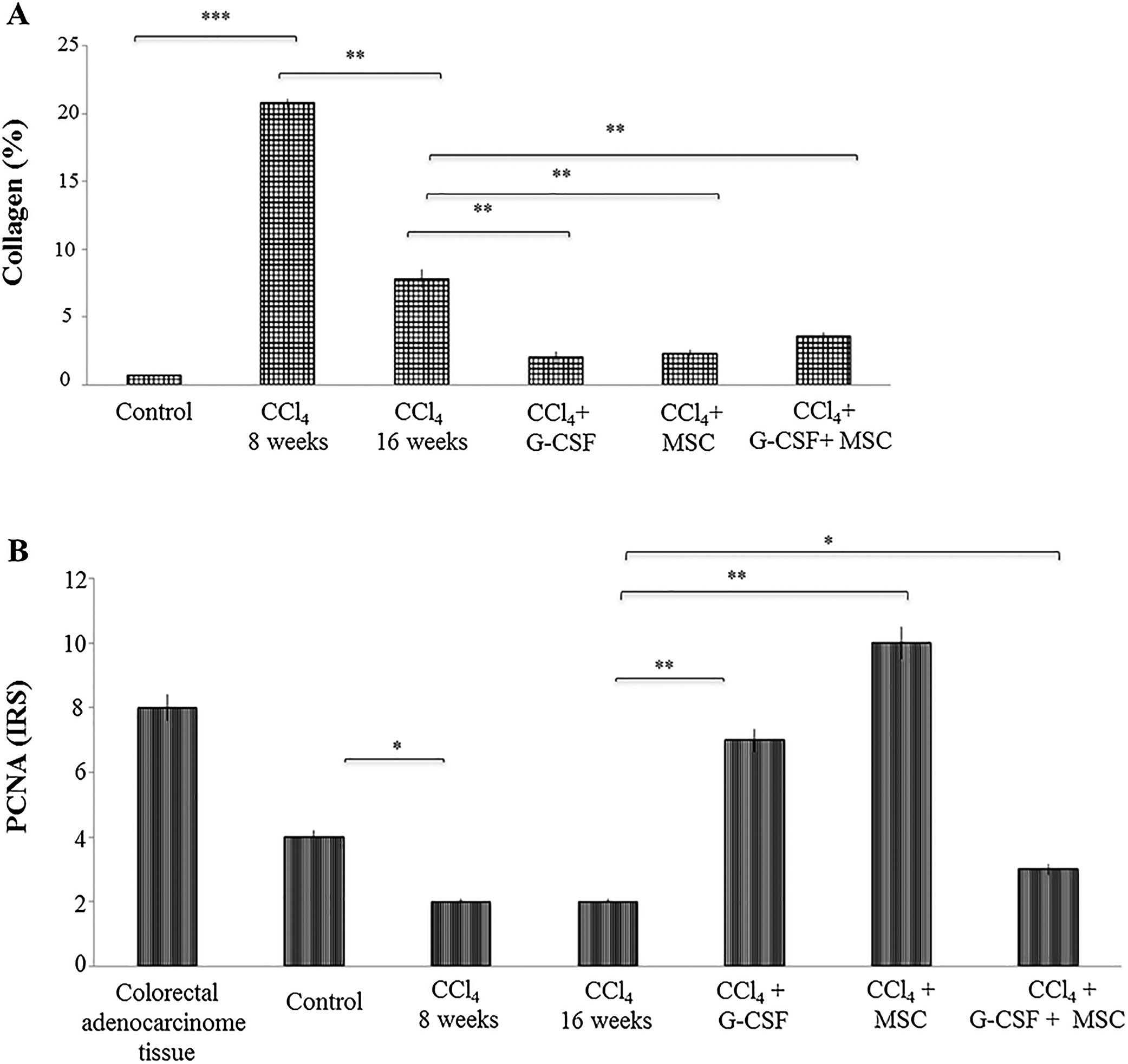
In the CCl4 group, the percentage of collagen was significantly increased by 20.81±0.31% at 8 wks compared with the control group, 0.67±0.01% (p<0.0001). In the former, there was a spontaneous reduction of collagen quantification to 7.81±0.68% at 16 wks (p<0.001). However, in the treated groups there was further collagen reduction at 16 wks: CCl4+G-CSF, 2.02±0.45%; CCl4+MSC, 2.32±0.22%; CCl4+G-CSF+MSC, 3.57±0.29% (p<0.001) (Fig. 3A).
Quantification of collagen in liver tissue and immunohistochemical analysis of the groups evaluated after treatment at 16 weeks. (A) Quantification of collagen percentage stained with sirius red. (B) Immunohistochemistry proliferating cell nuclear antigen (PCNA) in liver tissue: evaluation of staining intensities. Mean±SD (n=3). Significant differences in both analysis. *p<0.05, **p<0.001.
In the CCl4 group, PCNA expression decreased (p<0.05) at 8 and 16 wks compared with the control group. In the treated groups, PCNA expression was significantly increased in CCl4+G-CSF (p<0.001), CCl4+MSC (p<0.001), and CCl4+G-CSF+MSC (p<0.05) compared with CCl4 group at 16 wks (Fig. 3B).
4DiscussionIsolated treatment with AT-derived MSCs showed better improvement in liver function compared with G-CSF-mobilized HSC. MSC had the best results when comparing the three therapies against the positive control, on HGF, TGF-β, and IL-10 (p<0.05, p<0.01 and p<0.01, respectively) levels. However, differences were not observed in either biopsied tissue or quantification of collagen. HGF and TGF-β levels reflect better conditions for the regenerative process; this may be seen before the histological changes. Less improvement was observed when these therapies were combined. In all treatment groups, IL-1β and TNF-α decreased, likely reflecting an anti-inflammatory effect. Likewise, an elevation in IL-10 was observed, which may reflect downregulation of inflammatory cytokines. In addition, treatment of experimental cirrhosis with MSCs stimulated the highest levels of HGF and inhibited TGF-β, which may reflect a better homing capacity of MSC than of HSC.
MSCs derived from AT were characterized by adherence to plastic and expression profiles of specific markers according to Lofty et al. [14], who showed high expression of CD90 and CD105 and low expression of CD34 and CD45. We found that G-CSF induces mobilization of HSC expressing CD34+ surface markers. Accordingly, Mark et al. [23] observed an increase in CD34+ population in an experimental, acute model of CCl4 using G-CSF.
Increased hepatic enzymes (ALT and AST), TGF-β [38,39], IL-1β, and TNF-α pro-inflammatory cytokines are parameters associated with liver damage, fibrosis, and active inflammation [40,41]. Similarly, in a chronic injury model of CCl4 treated with MSCs [42], there was an improvement in liver function and diminished ALT and AST. In addition, treatment with G-CSF [16] ameliorated liver damage and decreased ALT [16,39,42]. ALT is a more specific enzyme of liver damage than AST. However, in our model, improvement was reflected by a decrease in AST in all treatment groups, whereas ALT improvement was seen in CCl4+G-CSF and CCl4+MSC but not in CCl4+G-CSF+MSC. With these experiments, it is impossible to determine why there is a different response between ALT and AST.
A decrease in TGF-β may be associated with diminished fibrosis [43]. In our model, both CCl4+MSC (p<0.01) and CCl4+G-CSF+MSC (p<0.05) groups had a significant impact on decreasing TGF-β. Changes in HGF and TGF-β serum levels may not be reflected in liver histology in our model, possibly because a short period of observation was used to define whether these growth factors predict the evolution of fibrosis. In our model, the increase in HGF and decrease in TGF-β in the three groups suggests an adequate response to therapy, with elevated HGF indicating repair and decreased TGF-β indicating decreased fibrosis.
In vitro studies have shown that MSCs in culture can produce HGF and inhibit hepatic stellate cells [22] and in vivo studies of experimental fibrosis have shown that MSCs increase levels of circulating HGF [22,42], stimulating regeneration and accelerating hepatocyte proliferation [31,39]. In the current study, MSCs stimulated the highest increase of HGF, possibly reflecting damage repair. Further, PCNA increased in MSCs and G-CSF (p<0.001) and G-CSF+MSC (p<0.05) groups. Li et al. [9] addressed the therapeutic effects of MSC and HSC in an experimental cirrhosis model, and placed emphasis on MSC having a greater homing capacity for the injured liver and greater capacity to promote hepatocyte proliferation.
In another liver fibrosis model treated with MSCs [43], IL-1β and TNF-α decreased, consistent with our findings. It has also been shown previously that TNF-α decreases with G-CSF treatment [16]. In addition, in this study, combined therapy showed a significant decrease in TNF-α (p<0.0001), possibly indicating a strong anti-inflammatory effect.
MSCs and HSCs have immunomodulatory properties and play important roles in liver injury. Studies of kidney and lung injury and fulminant hepatic failure models have demonstrated that IL-10 plays an anti-inflammatory role [44–46]. A possible anti-inflammatory role of IL-10 was observed in all treatment groups. Previous studies with a similar model with MSC treatment reported an increase in IL-10 [42,43]. Discrepancies across studies relate to the mechanisms that mediate immunosuppression, which may be due to different experimental conditions or the origin of the MSCs [47]. Although these mechanisms are not wholly understood, MSCs are able to inhibit the proliferation of T cells, to inhibit the production of interferon gamma and TNF-α, and to induce an increase in interleukin 4 and IL-10 levels. Cumulatively, these findings indicate a pro-inflammatory immune response in an anti-inflammatory state, which is favored by stimulation of the Treg lymphocytes and alterations in dendritic cell activities [47].
The MSCs are also able to interfere in the differentiation, maturation, and function of dendritic cells, causing a blockade in the immature state and altering secretion of cytokines. For example, MSCs can reduce production of proinflammatory cytokines interleukin 12, interferon gamma, and TNF-α and increase production of anti-inflammatory cytokines such as IL-10 [48].
Treatment with MSCs and G-CSF improves liver fibrosis in CCl4 chronic liver injury models [24,50,51]. Similarly, our results showed a decrease from F4 to F2 in the METAVIR score and diminished collagen fibers, especially when treatments were used separately. Several mechanisms by which these cells might contribute to decreased fibrosis have been proposed, including their differentiation into hepatocytes, their fusion with endogenous hepatocytes, and a proliferative paracrine effect on hepatocytes [49].
HSCs participate in hepatic proliferation and repair after injury [52–56] although the true contributions of BM stem cells to liver regeneration has been questioned [31,57,58]. Evidence suggests that rather than transdifferentiation of HSCs to tissue-specific stem cells or fusion of donor with host cells, replenishment of damaged tissues occurs through activation of endogenous progenitors and repair mechanisms mediated by paracrine secretion of soluble factors by BM cells [59–61]. G-CSF, by means of forced circulation of large numbers of HSCs, has been extensively investigated for its hepatic regenerative effect, both in animal models of liver injury [30,31,62] and clinical trials [63–65].
In our model, the CCl4 group at 8 wks, compared to the CCl4 group at 16 wks, decreased from F4 to F3. Muriel et al. [35] reported that the degree of fibrosis increased linearly with duration of CCl4 treatment, but spontaneous regression of fibrosis was similar after 2 or 3 months of chronic intoxication, and discontinuation of the toxin for two months produced a significant but relatively small reduction in fibrosis.
In this study, the G-CSF+MSC combination showed less improvement compared to separate use of MSCs and G-CSF treatments. A synergistic or additive effect was not observed. In previous studies, a synergistic effect was demonstrated when MSCs in combination with G-CSF were successfully used as a treatment for ulcerative colitis in rats, suggesting that G-CSF increased recruitment of MSCs [49]. However, in a study by Li et al. [9], the synergistic effect of MSCs and HSCs (derived from non-autologous BM) treatment was not observed in liver injury; this group obtained better results with MSCs than with HSC in mice. We used G-CSF to promote HSC CD34+ autologous mobilization as a possible increase in recruitment of AT MSCs. We did not observe the additive or synergistic effect between MSCs and G-CSF therapies in this chronic CCl4 model. However, these experiments were not designed to explain some mechanisms.
In conclusion, our results showed that MSC treatment improved liver function, diminished inflammatory activity, decreased hepatic fibrosis, played an anti-inflammatory role, promoted HGF production, and increased PCNA following treatment with HSC mobilization using G-CSF. However, combined therapies showed less improvement in liver fibrosis. It will be necessary to carry out experiments using higher doses and administration frequencies. Larger experimental groups will also allow assessment of the capacity of these therapies to reduce liver fibrosis.AbbreviationsALT alanine aminotransferase aspartate aminotransferase adipose tissue bone marrow carbon tetrachloride granulocyte colony stimulating factor hepatocyte growth factor hematopoietic stem cells interleukin 1beta interleukin 10 mesenchymal stem cells proliferating cell nuclear antigen transforming growth factor beta tumor necrosis factor alpha weeks week
This work was supported by the Department of Immunology and Virology and the Liver Unit, Department of Internal Medicine, “Dr. José E. Gonzalez” University Hospital of Universidad Autónoma de Nuevo Leon and PAICYT reference number: SA167-15.
Conflict of interestThe authors declare that they have no conflicts of interest.