Hepatitis B virus infection and chronic kidney disease are prevalent and remain a major public health problem worldwide. It remains unclear how infection with hepatitis B virus impacts on the development and progression of chronic kidney disease.
AimTo evaluate the effect of infection with HBV on the risk of chronic kidney disease in the general population.
Material and MethodsWe conducted a systematic review of the published medical literature to determine if hepatitis B infection is associated with increased likelihood of chronic kidney disease. We used the random effects model of DerSimonian and Laird to generate a summary estimate of the relative risk for chronic kidney disease (defined by reduced glomerular filtration rate and/or detectable proteinu-ria) with hepatitis B virus across the published studies. Meta-regression and stratified analysis were also conducted.
ResultsWe identified 16 studies (n = 394,664 patients) and separate meta-analyses were performed according to the outcome. The subset of longitudinal studies addressing ESRD (n = 2; n = 91,656) gave a pooled aHR 3.87 (95% CI, 1.48; 6.25, P 0 0.0001) among HBV-in-fected patients and no heterogeneity was recorded. In meta-regression, we noted the impact of male (P = 0.006) and duration of follow-up (P = 0.007) upon the adjusted hazard ratio of incidence of chronic kidney disease (including end-stage renal disease). No relationship occurred between HBV positive status and prevalent chronic disease (n = 7, n = 109,889 unique patients); adjusted odds ratio, were 1.07 (95% CI, 0.89; 1.25) and 0.93 (95% CI, 0.76; 1.10), respectively.
ConclusionsHBV infection is possibly associated with a risk of developing reduced glomerular filtration rate in the general population; no link between HBV sero-positive status and frequency of chronic kidney disease or proteinuria was noted in cross-sectional surveys.
Chronic kidney disease is a growing public health issue worldwide. The prevalence of chronic kidney disease, defined by a reduction in glomerular filtration rate and/or increased urinary albumin excretion, exceeds 10% of the adult general population, according to some population-based studies.1 Conventional risk factors for chronic kidney disease include demographics (aging, gender), lifestyles (smoking, alcohol intake, physical exercise), and co-morbidities (diabetes mellitus, arterial hypertension, anaemia, overweight);2 also, chronic hepatitis C virus infection has been recently associated to the risk of chronic kidney disease in the general population3 and among HIV-infected individuals.4 However, the mechanisms underlying the current frequency of chronic kidney disease in the general population of developed world remain unclear.
Hepatitis B virus (HBV) infection is an important cause of liver disease and cancer and infects about 400 million individuals worldwide. In addition to its effects in the liver, extra-hepatic manifestations may be observed in up to 20% of patients infected with HBV, in both acute and chronic infections. Manifestations related to HBV include mixed cryoglobulinemia vasculitis, polyarteritis nodosa, and renal disease.5 The association between HBV infection and glomerular disease has been already explored by various authors; the most common type of HBV-related glomerulonephritis is membranous nephropathy, particularly in the Asian continent.6 Also, chronic hepatitis B serum promotes apoptotic damage in human renal tubular cells.7 Either insulin resistance8 and oxidative stress9 have been related to HBV infection; both conditions may contribute to renal injury.
Whether HBV-infected individuals have increased risk for development and progression of chronic kidney disease has not been appropriately investigated. The aim of this study was to review the available evidence on the link between HBV infection and frequency of chronic kidney disease (low estimated glomerular filtration rate and/or detectable proteinuria) at population-based level by performing a systematic review of the literature with a meta-analysis of clinical observational studies.
Material and MethodsThis work is in agreement with the Preferred Reporting Items for Systematic reviews and Meta-Analyses state-ment10 (Supplementary file 1).
Search strategy and data extractionTwo authors (F.F., and F.M. D.) independently reviewed English-language citations from the national Library of Medicine’s Medline database from 1970 through July 1, 2015. Data on HBsAg status were not available before 1970, when the first assay for HBsAg was manufactured. We conducted our search by four Medline databases engines (Embase, Grateful Med, Ovid, and PubMed). Our Medline search was limited to human studies. We applied the following algorithm in medical subject heading and in free text words: (“HEPATITIS B” or “HEPATITIS B VIRUS INFECTION” or “HBsAg POSITIVE STATUS” or “HBc ANTIBODY POSITIVE STATUS”) and (“CHRONIC KIDNEY DISEASE” or “CKD” or “END-STAGE RENAL DISEASE” or “ESRD” or “LOW GLOMERULAR FILTRATION RATE” or “RENAL IMPAIRMENT” or “RENAL INSUFFICIENCY” or “RENAL FAILURE” or “PROTEINURIA” or ‘‘GLOMERULONEPHRITIS’’) and (“RELATIVE RISK” or “RISK RATIO” or “RR” or “ODDS RATIO” or “OR” or “HAZARD RATIO” or “HR” or “INCIDENCE”). An additional search was performed with electronic searches of the Cochrane Library; manual searches of selected specialty journals were done to identify all pertinent literature. Reference lists from qualitative topic reviews and published clinical studies were also searched. It was previously demonstrated that a Medline search alone might not be sensitive enough.11 Data on study design, study period, patient characteristics, HBV prevalence, and kidney disease outcomes were abstracted. Authors of selected papers were contacted to obtain missing data and only data from individuals with known HBV status were included in the meta-analysis. Consensus was achieved for all data. Studies were compared to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. Eligibility and exclusion criteria were pre-specified. Our search was limited to human studies that were published in the English literature.
Inclusion criteriaStudies were included if they met the following inclusion criteria:
- •
They presented original data from cohort and cross-sectional studies.
- •
The outcome of interest was clearly defined as incidence or prevalence of chronic kidney disease, i.e., reduced glomerular filtration rate and/or detectable proteinuria in the adult general population according to HBV serological status; and
- •
They provided adjusted risk estimates and their confidence intervals.
We considered both case-control studies and cohort studies as eligible for inclusion in the analysis. We included studies where the diagnosis of HBV infection was done by testing for HBsAg in serum and/or HBc antibody. Information on HBV serological status was collected at the time of enrolment. If data on the same population were duplicated in more than one study, the most recent study was included in the analysis.
Ineligible studiesStudies were excluded if they reported inadequate data on the association between chronic kidney disease and HBV sero-positive status (e.g., incomplete information on HBV status or renal outcomes). Unpublished studies, studies that were only published in abstract form or as interim reports were excluded; letters and review articles were not considered for this systematic review.
Quality assessmentThe quality of the 13 studies was appraised using a scale adapted from the ‘Newcastle/Ottawa Scale (NOS)’.12 The Newcastle-Ottawa scale is a scoring system that assesses every aspect of an observational epidemiologic study from a methodological point of view. When a study included relevant information that could be associated with the NOS, one point was added. Seven items in cross-sectional studies and eight items in cohort and case-control studies that could be related to the NOS were identified. Therefore, cross-sectional studies assigned 8-10, 6-7, 4-5, or 0-3 points (stars) were evaluated as very good, good, satisfactory or unsatisfactory studies, respectively. Similarly, cohort/case-control studies with 7-9, 5-6, 4 and 0-3 points (stars) were identified as very good, good, satisfactory or unsatisfactory, respectively. We carried out subgroup analyses based on those studies provided with very good quality. Data extraction and quality scoring were performed independently by two reviewers (F.F. and F.M. D.) and the results were merged by consensus. The complete protocol for quality scoring is available on-line (Supplementary file 2).
Outcomes measuresWe performed separate meta-analyses according to the outcome. One meta-analysis included longitudinal studies addressing the incidence of chronic kidney disease or end-stage renal disease; and another regarded cohort studies assessing the prevalence of CKD. An additional meta-analysis was performed for prevalent proteinuria. Staging of chronic kidney disease was categorized according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) definition, and estimated glomerular filtration rate was calculated using the 4-variable MDRD equation.13 The primary end point was to provide adjusted estimates of the risk (and 95% CIs) of incidence (or prevalence) of chronic kidney disease in the general population according to HBV serological status. Multivariate analysis was made to estimate the independent effect of HBV positive status on the frequency of chronic kidney disease after adjustment for potential confounders (cov-ariates) (e.g., age, gender, race/ethnicity, diabetes melli-tus, and others). Longitudinal studies adopted Cox regression analysis to assess the independent predictors of the incidence of chronic kidney disease; multiple logistic regression analyses were done in cross-sectional surveys. An additional end point was the adjusted estimate of the risk (and 95% CIs) of prevalence of pro-teinuria in the adult general population according to HBV serological status. Cox proportional hazard regression analysis was carried out to assess the effect of HBV sero-positivity per se on the incidence of chronic kidney disease after adjustment for differential follow-up time and distribution of potential confounders.
Data synthesis and analysisWe weighted the study-specific log odds ratios for case control and cross-sectional studies, and log hazard ratios for longitudinal studies by the inverse of their variance to obtain a pooled effect estimate and its 95% confidence intervals. For each study, we used the estimate of the effect measure that was adjusted for the largest number of con-founders. We present both fixed-effects and random-effects pooled estimates but use and report the latter when heterogeneity was present. We used the random-effects approach, as described by DerSimonian and Laird.14 Co-chrane Q-test was used for quantifying the heterogeneity.15 The I2 statistic, which is the percentage of total variation across studies due to heterogeneity rather than chance, was also calculated.16 The null hypothesis of this test is the absence of heterogeneity. We explored the origin of heterogeneity by restricting the analysis to subgroups of studies defined by study characteristics such as country of origin, CKD stage, and others. Heterogeneity was also evaluated by meta-regression in order to look at the effect of potential and continuous covariates on the outcome of interest. Subgroup or stratified analyses and meta-regression were pre-specified. We performed random-effects meta-regres-sion using the method of moments or maximum likelihood approaches where appropriate, a single predictor is allowed in each model (simple meta-regression). Publication bias was assessed by the Egger test for funnel-plot asymmetry. All analyses were done with the statistical package Comprehensive Meta-Analysis (CMA), version 2.0 (Biostat Inc., USA, 2005). The 5% significance level was adopted for á risk. Every estimate was given with its 95% CIs.
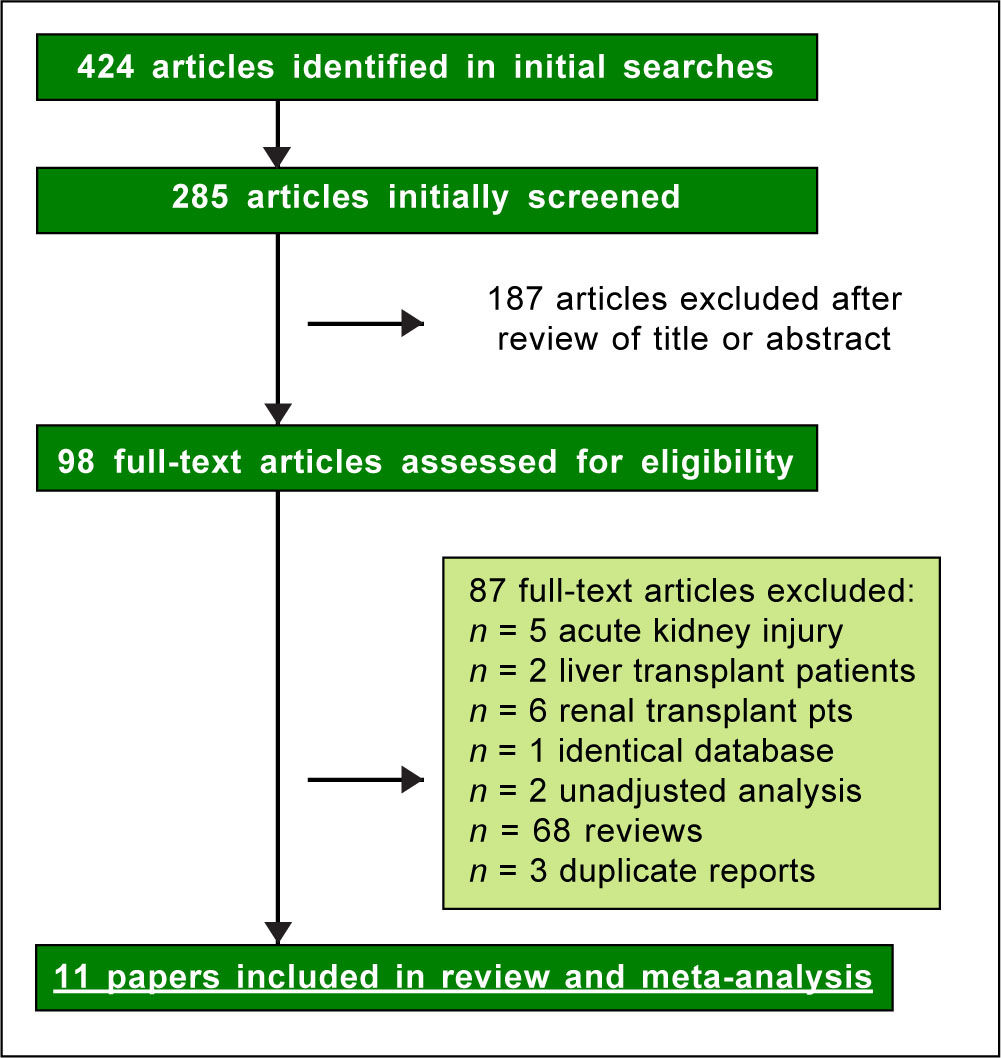
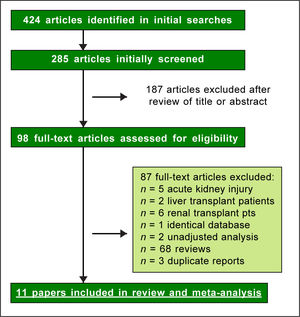
ResultsLiterature reviewAs shown in Figure 1, we retrieved 424 articles and 98 full-text papers were assessed for eligibility. The list of the papers is reported in the supplementary file 3. Sixteen studies met our inclusion criteria, they were published in 11 papers19-30 (Figure 1) and carried out in 3 countries. Two longitudinal papers addressed various outcomes (the risk of chronic kidney disease and end-stage renal disease) in the same population.20-21 Four papers23-25,28 gave information on the prevalence of chronic kidney disease and proteinuria in the same population. Thus, some studies contributed data on more than one kidney disease outcome, but each cohort was represented once in any meta-analysis. There was a 100% concordance between reviewers with respect to final inclusion and exclusion of studies reviewed based on the predefined inclusion and exclusion criteria. Diagnosis of HBV infection was made by detecting the presence of HBV surface antigen (HB-sAg) in serum; in a few reports20,21 diagnosis of HBV infection was done by ICD-9 codes. One report addressed the rate of HBV infection by assessing HBcAb serologic sta-tus,22 another survey identified HBV infection by patient medical history.29
Patient and study characteristicsTables 1-3 reports some salient demographic, and clinical characteristics of subjects enrolled in the included studies. The mean age of patient cohorts ranged from 18.9 ± 0.5 to 60.8 ± 11.5 years. The gender distribution ranged from 31.2% to 67.6% male. Five reports were Taiwan, four from China, one from Japan and Hong Kong, respectively. The average follow-up ranged between 3.5 and 6.5 years, among longitudinal studies. The quality scores ranged between 7 and 8 points (cohort/case-control studies), and between 8 and 9 (cross-sectional studies) (Supplementary file 2).
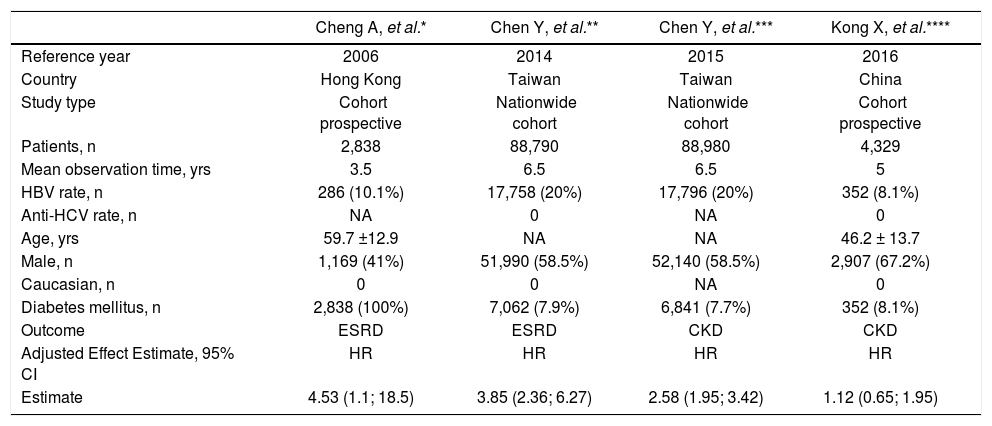
Longitudinal studies included in the meta-analysis (outcome: frequency of end-stage renal disease or chronic kidney disease).
| Cheng A, et al.* | Chen Y, et al.** | Chen Y, et al.*** | Kong X, et al.**** | |
|---|---|---|---|---|
| Reference year | 2006 | 2014 | 2015 | 2016 |
| Country | Hong Kong | Taiwan | Taiwan | China |
| Study type | Cohort prospective | Nationwide cohort | Nationwide cohort | Cohort prospective |
| Patients, n | 2,838 | 88,790 | 88,980 | 4,329 |
| Mean observation time, yrs | 3.5 | 6.5 | 6.5 | 5 |
| HBV rate, n | 286 (10.1%) | 17,758 (20%) | 17,796 (20%) | 352 (8.1%) |
| Anti-HCV rate, n | NA | 0 | NA | 0 |
| Age, yrs | 59.7 ±12.9 | NA | NA | 46.2 ± 13.7 |
| Male, n | 1,169 (41%) | 51,990 (58.5%) | 52,140 (58.5%) | 2,907 (67.2%) |
| Caucasian, n | 0 | 0 | NA | 0 |
| Diabetes mellitus, n | 2,838 (100%) | 7,062 (7.9%) | 6,841 (7.7%) | 352 (8.1%) |
| Outcome | ESRD | ESRD | CKD | CKD |
| Adjusted Effect Estimate, 95% CI | HR | HR | HR | HR |
| Estimate | 4.53 (1.1; 18.5) | 3.85 (2.36; 6.27) | 2.58 (1.95; 3.42) | 1.12 (0.65; 1.95) |
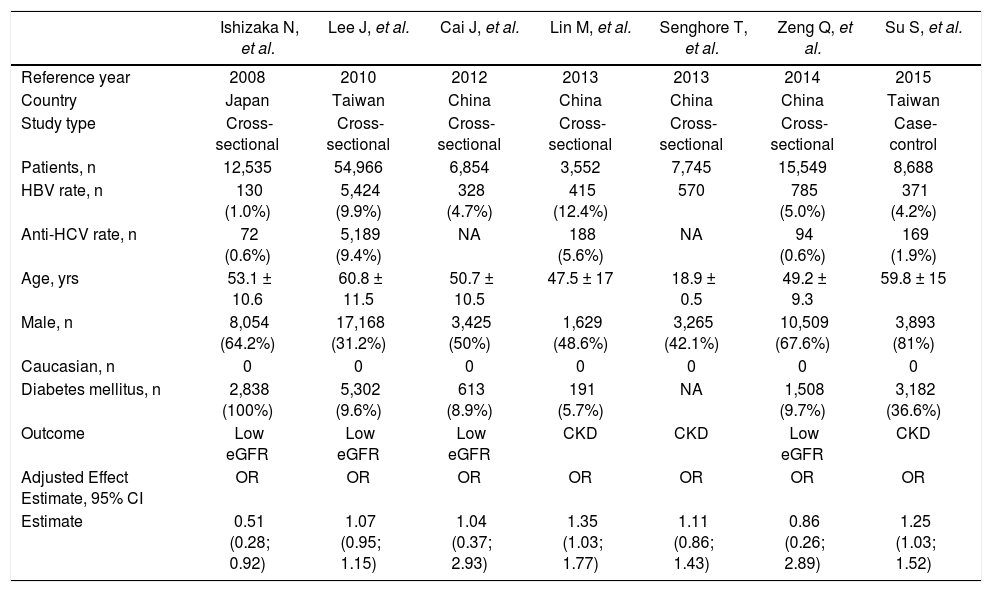
Cross-sectional studies included in the meta-analysis (outcome: frequency of chronic kidney disease, or low estimated glomerular filtration rate).
| Ishizaka N, et al. | Lee J, et al. | Cai J, et al. | Lin M, et al. | Senghore T, et al. | Zeng Q, et al. | Su S, et al. | |
|---|---|---|---|---|---|---|---|
| Reference year | 2008 | 2010 | 2012 | 2013 | 2013 | 2014 | 2015 |
| Country | Japan | Taiwan | China | China | China | China | Taiwan |
| Study type | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Case-control |
| Patients, n | 12,535 | 54,966 | 6,854 | 3,552 | 7,745 | 15,549 | 8,688 |
| HBV rate, n | 130 (1.0%) | 5,424 (9.9%) | 328 (4.7%) | 415 (12.4%) | 570 | 785 (5.0%) | 371 (4.2%) |
| Anti-HCV rate, n | 72 (0.6%) | 5,189 (9.4%) | NA | 188 (5.6%) | NA | 94 (0.6%) | 169 (1.9%) |
| Age, yrs | 53.1 ± 10.6 | 60.8 ± 11.5 | 50.7 ± 10.5 | 47.5 ± 17 | 18.9 ± 0.5 | 49.2 ± 9.3 | 59.8 ± 15 |
| Male, n | 8,054 (64.2%) | 17,168 (31.2%) | 3,425 (50%) | 1,629 (48.6%) | 3,265 (42.1%) | 10,509 (67.6%) | 3,893 (81%) |
| Caucasian, n | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diabetes mellitus, n | 2,838 (100%) | 5,302 (9.6%) | 613 (8.9%) | 191 (5.7%) | NA | 1,508 (9.7%) | 3,182 (36.6%) |
| Outcome | Low eGFR | Low eGFR | Low eGFR | CKD | CKD | Low eGFR | CKD |
| Adjusted Effect Estimate, 95% CI | OR | OR | OR | OR | OR | OR | OR |
| Estimate | 0.51 (0.28; 0.92) | 1.07 (0.95; 1.15) | 1.04 (0.37; 2.93) | 1.35 (1.03; 1.77) | 1.11 (0.86; 1.43) | 0.86 (0.26; 2.89) | 1.25 (1.03; 1.52) |
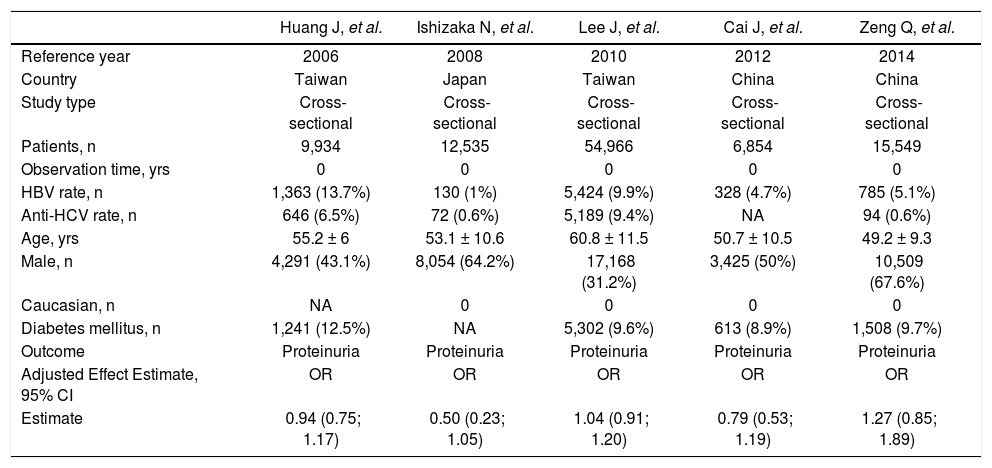
Studies included in the meta-analysis (outcome: frequency of proteinuria).
| Huang J, et al. | Ishizaka N, et al. | Lee J, et al. | Cai J, et al. | Zeng Q, et al. | |
|---|---|---|---|---|---|
| Reference year | 2006 | 2008 | 2010 | 2012 | 2014 |
| Country | Taiwan | Japan | Taiwan | China | China |
| Study type | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional |
| Patients, n | 9,934 | 12,535 | 54,966 | 6,854 | 15,549 |
| Observation time, yrs | 0 | 0 | 0 | 0 | 0 |
| HBV rate, n | 1,363 (13.7%) | 130 (1%) | 5,424 (9.9%) | 328 (4.7%) | 785 (5.1%) |
| Anti-HCV rate, n | 646 (6.5%) | 72 (0.6%) | 5,189 (9.4%) | NA | 94 (0.6%) |
| Age, yrs | 55.2 ± 6 | 53.1 ± 10.6 | 60.8 ± 11.5 | 50.7 ± 10.5 | 49.2 ± 9.3 |
| Male, n | 4,291 (43.1%) | 8,054 (64.2%) | 17,168 (31.2%) | 3,425 (50%) | 10,509 (67.6%) |
| Caucasian, n | NA | 0 | 0 | 0 | 0 |
| Diabetes mellitus, n | 1,241 (12.5%) | NA | 5,302 (9.6%) | 613 (8.9%) | 1,508 (9.7%) |
| Outcome | Proteinuria | Proteinuria | Proteinuria | Proteinuria | Proteinuria |
| Adjusted Effect Estimate, 95% CI | OR | OR | OR | OR | OR |
| Estimate | 0.94 (0.75; 1.17) | 0.50 (0.23; 1.05) | 1.04 (0.91; 1.20) | 0.79 (0.53; 1.19) | 1.27 (0.85; 1.89) |
- •
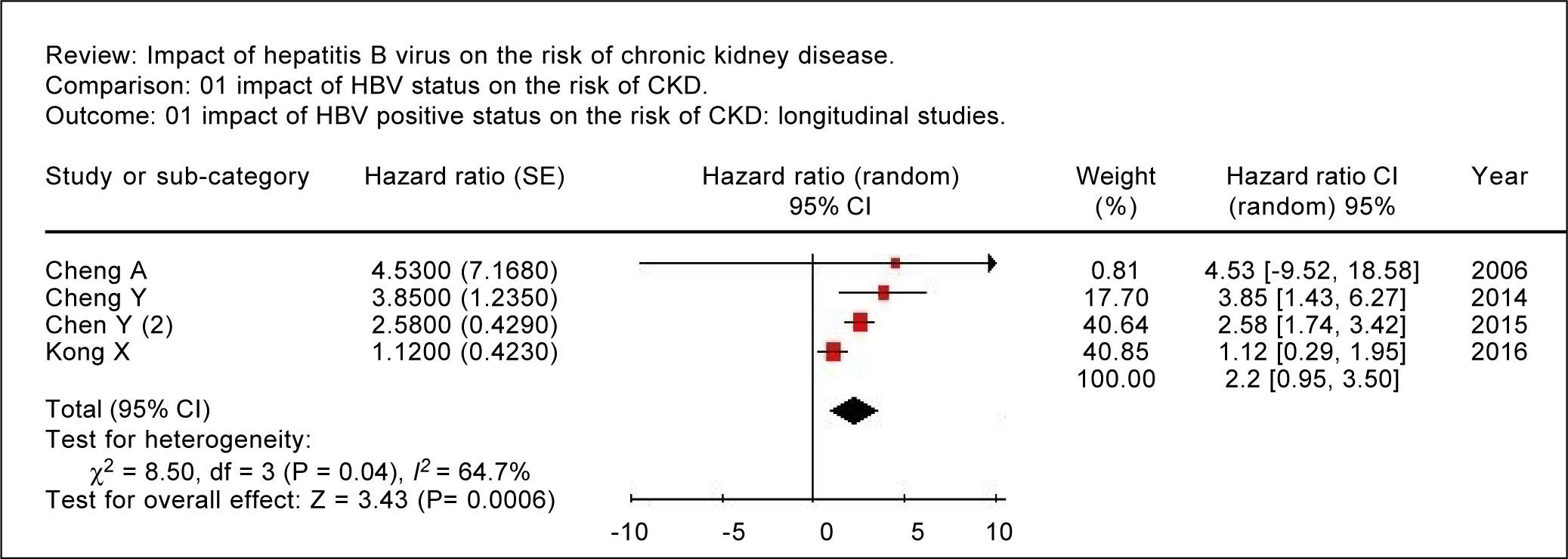
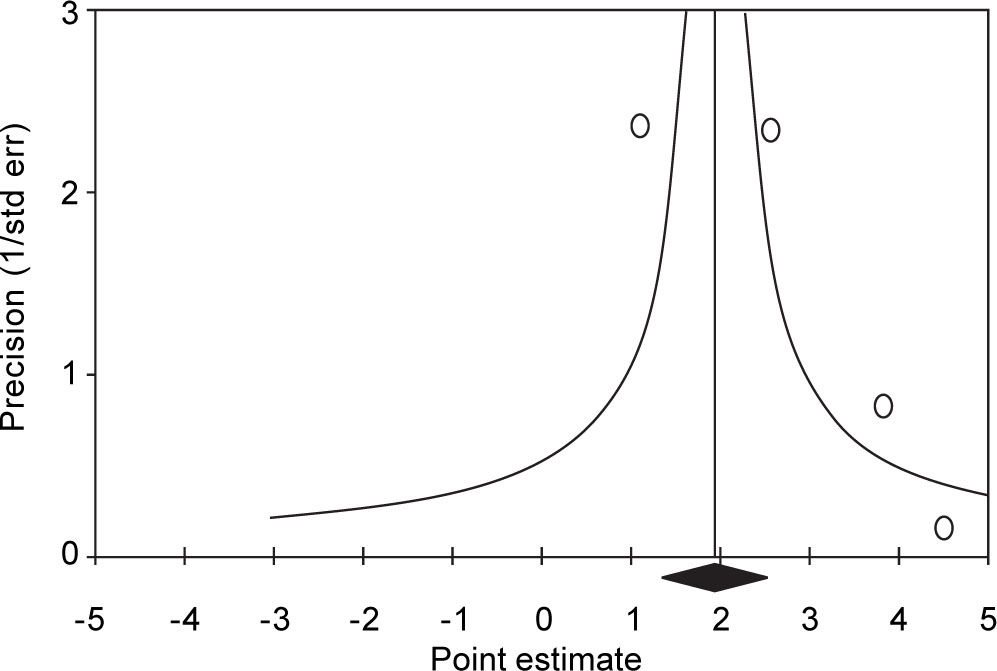
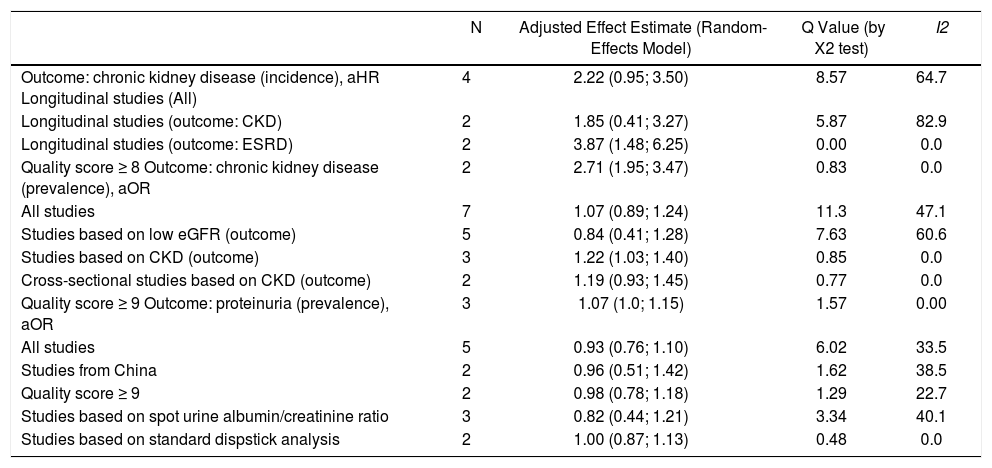
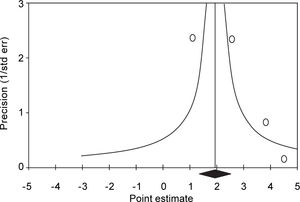
Summary estimate of outcome: Incidence of CKD (reduced eGFR or end-stage renal disease). Four longitudinal studies (n = 184,937 patients; 36,192 HBV positive and 148,745 HBV negative patients) gave information on the incidence of CKD (or ESRD) among HBV positive patients19-22 (Table 1). The relationship between positive HBV serologic status and increased incidence of CKD neared the statistical significance, adjusted HR with HBV across the surveys, 2.22 (95% CI, 0.95; 3.50, NS). There was some heterogeneity (I2 = 64.7%, P = 0.04) across the four studies (Figure 2). Publication bias was not found (Egger test, P = 0.61) (Figure 3). The subset of longitudinal studies addressing ESRD gave a pooled aHR 3.87 (95%CI, 1.48; 6.25, P < 0.0001) among HBV-infected patients and no heterogeneity was recorded (Table 4).
Table 4.Summary measure for adjusted effect estimate according to HBV serologic status among various subgroups of interest.
N Adjusted Effect Estimate (Random-Effects Model) Q Value (by X2 test) I2 Outcome: chronic kidney disease (incidence), aHR Longitudinal studies (All) 4 2.22 (0.95; 3.50) 8.57 64.7 Longitudinal studies (outcome: CKD) 2 1.85 (0.41; 3.27) 5.87 82.9 Longitudinal studies (outcome: ESRD) 2 3.87 (1.48; 6.25) 0.00 0.0 Quality score ≥ 8 Outcome: chronic kidney disease (prevalence), aOR 2 2.71 (1.95; 3.47) 0.83 0.0 All studies 7 1.07 (0.89; 1.24) 11.3 47.1 Studies based on low eGFR (outcome) 5 0.84 (0.41; 1.28) 7.63 60.6 Studies based on CKD (outcome) 3 1.22 (1.03; 1.40) 0.85 0.0 Cross-sectional studies based on CKD (outcome) 2 1.19 (0.93; 1.45) 0.77 0.0 Quality score ≥ 9 Outcome: proteinuria (prevalence), aOR 3 1.07 (1.0; 1.15) 1.57 0.00 All studies 5 0.93 (0.76; 1.10) 6.02 33.5 Studies from China 2 0.96 (0.51; 1.42) 1.62 38.5 Quality score ≥ 9 2 0.98 (0.78; 1.18) 1.29 22.7 Studies based on spot urine albumin/creatinine ratio 3 0.82 (0.44; 1.21) 3.34 40.1 Studies based on standard dispstick analysis 2 1.00 (0.87; 1.13) 0.48 0.0 Cheng, et al.:19 HR adjusted for age, age of onset of diabetes mellitus, gender, smoking use, systolic and diastolic blood pressure, body mass index, fasting plasma glucose, total cholesterol, triglyceride, white cell count, estimated glomerular filtration rate, and albumin:creatinine ratio. Chen, et al.:20 HR adjusted for age, gender, diabetes, hypertension, coronary artery disease, hyperlipidaemia, cirrhosis, use of herbs containing aristolochic acid, geographic region, urbanization level, enrollee category, number of medical visits, propensity score, and Charlson comorbidity index score. Chen, et al.:21 HR adjusted for gender, age, diabetes mellitus, hypertension, coronary artery disease, hyperlipidaemia, glomerulonephritis, chronic pyelonephritis, nephrolithiasis, renal and urinary tract tumor, cirrhosis, use of herbs containing aristolochic acid, geographic region, urbanization level, enrollee category, number of medical visits in one year before study entry, Charlson comorbidity index score, propensity score, and interactions terms. Kong, et al.:22 HR adjusted for age, gender, hypertension, diabetes, body mass index, uric acid, total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol. Ishizaka, et al.:23 OR adjusted for age, gender, fasting plasma glucose, systolic blood pressure, and anti-HCV seropositive status. Lee, et al.:24 OR adjusted for age, gender, educational status, body mass index, haemoglobin level, albumin level, cholesterol level, uric acid level, hypertension, and diabetes mellitus. Cai, et al.:25 OR adjusted for age, gender, hypertension, diabetes, waist circumference, HDL cholesterol levels, total cholesterol levels, and nephrolithiasis. Lin, et al.:26 OR adjusted for age, gender, annual income, hypertension, diabetes mellitus, cardiovascular disease, stroke, gout, liver disease, urinary tract disease, cancer, Chinese herbs use, oral analgesic use, analgesic injection, health supplements, cigarette smoking, betel-nut chewing, alcohol drinking, metabolic syndrome, hyperuricemia, haemoglobin, and positivity for anti-HCV. Senghore, et al.:27 OR adjusted for age, gender, body mass index, blood pressure, urine occult blood, blood urea nitrogen, uric acid, total cholesterol. Zeng, et al.:28 OR adjusted for age, gender, anti-HCV seropositive status, arterial hypertension, diabetes mellitus, body mass index, albumin, HDL cholesterol levels, LDL cholesterol levels, triglycerides, total cholesterol, and uric acid. Su, et al.:29 OR adjusted for gender, age, obsesity, income, HCV, hyperuricaemia, anaemia, hyperlipidaemia, smoking status, alcohol abuse, betel nut, exercise habits, groundwater using. Huang, et al.:30 OR adjusted for diabetes, hypertension, HCV serologic status, age, triglycerides, body mass index, ALT level, total cholesterol. - •
Summary estimate of outcome: Prevalence of CKD (reduced eGFR). Seven studies (n = 109,889 unique patients; 8,023 HBV positive and 101,866 HBV-negative patients) with cross-sectional (or case-control) design addressed the prevalence of CKD (or reduced GFR) in HBV-infected patients.23-29Table 2 shows some demographic, and clinical parameters of subjects enrolled in the included studies. We found no relationship between positive HBV serologic status and increased prevalence of CKD, adjusted OR with HBV across the studies, 1.069 (95% CI, 0.89; 1.248, P = NS). Tests for homogeneity of the aOR across the seven studies gave a Q-value (by χ2 test) of 11.3 (P = 0.007) (I2 = 47.13), that is, the homogeneity assumption was rejected (Table 4). No publication bias was found, according to the Egger test (P = 0.89).
- •
Summary estimate of outcome: prevalence of proteinuria. Five studies (n = 99,838 unique patients, 8,030 being HBV seropositive and 91,808 HBV nega-tive)23-25,28,30 evaluated the prevalence of proteinuria according to HBV positive serologic status. Two studies defined proteinuria by semiquantitative urine protein dispstick test24,30 and three measured albuminuria by spot urine albumin/creatinine ratio.23,25,28 The summary estimate for adjusted OR of proteinuria with HBV was 0.93 (95% CI, 0.76;1.10, P = NS) across the identified studies (Table 4). The homogeneity assumption was not rejected (Q = 6.02, P = 0.19). Publication bias did not occur (Egger test, P = 0.42).
- •
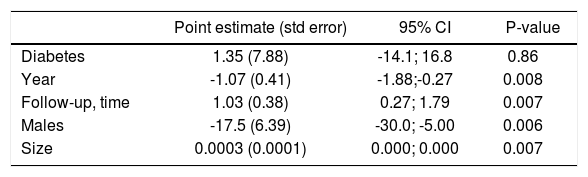
Stratified analysis and meta-regression. As shown in table 4, there was substantial difference in pooled effect estimates across designs (i.e., cross-sectional vs. longitudinal studies) and the homogeneity assumption was rejected in many subsets. As listed in table 5, meta-regression demonstrated an inverse relationship between the frequency of males (P = 0.006) and the outcome of interest (adjusted HR of incidence of CKD among HBV positive patients). In addition, a direct relationship between follow-up duration (P = 0.007) and the outcome of interest (adjusted HR of incidence of CKD among HBV positive patients) was noted.
Table 5.Meta-regression: impact of continuous covariates on the outcome of interest (incidence of CKD).
Point estimate (std error) 95% CI P-value Diabetes 1.35 (7.88) -14.1; 16.8 0.86 Year -1.07 (0.41) -1.88;-0.27 0.008 Follow-up, time 1.03 (0.38) 0.27; 1.79 0.007 Males -17.5 (6.39) -30.0; -5.00 0.006 Size 0.0003 (0.0001) 0.000; 0.000 0.007 There was no significant difference in outcomes according to the diagnosis of HBV infection (data not shown).
The association between HBV infection and chronic kidney disease in the general population is controversial even if the renal involvement of hepatitis B virus infection was first reported four decades ago.31 The relationship between hepatitis B virus infection and CKD occurs in several ways- some forms of renal disease are induced by HBV infection and patients with chronic kidney disease are at increased risk for acquiring HBV. In the current review, we have summarized the scientific evidence and carried out a meta-analysis on the exposure to HBV infection and the risk of chronic kidney disease and proteinuria in the adult general population. This meta-analysis (16 studies, n = 394,664 patients) should suggest an association between positive serologic status for HBV and an increased risk of chronic kidney disease, aHR being 2.22 (95% Confidence Interval, 0.95; 3.50) in HBV infected individuals compared with HBV negative. The subset of longitudinal studies addressing ESRD gave a pooled aHR 3.87 (95%CI, 1.48; 6.25, P < 0.0001) among HBV-infected patients, without heterogeneity.
Several pieces of evidence are in keeping with a detrimental role of HBV on the development of chronic kidney disease.32-39 In the 2-year cross-sectional HARPE study,32 renal abnormalities were highly prevalent in chronic HBV infection and occurred before the initiation of any antiviral therapy towards HBV. Around 64% of the patients enrolled in the HARPE study (n = 260) were found to have kidney disease according to the KDOQI/KDIGO classification. In their observational and longitudinal study, Mallet, et al.33 observed 214 patients with chronic HBV infection who were treated with various nucleos(t)ide analogues, the eGFR remained stable or increased over time in patients with chronic HBV mono-infection with a baseline eGFR of 90 mL/min/1.73 m2 or higher and treated with tenofovir disoproxil fumarate or entecavir. In the GLOBE study,34 a significant improvement in mean GFR was noted in patients treated with telbivudine for 2 years, but not in those on lamivudine. GLOBE extension studies demonstrated that the improvement was maintained throughout 4-6 years of continuous telbivudine therapy. The mean increase in eGFR was ± 14.9 mL/min/1.73 m2 at week 208 (P < 0.0001). In 74% (165 of 223) of the telbivudine-treated patients with baseline eGFR of 60-89 mL/min/1.73 m2 (CKD stage 2), renal function improved to ≥ 90 mL/min/1.73 m2 after 4 years of treatment. A prospective survey from Germany reported recently that GFR (calculated with the CKD-EPI equation), declined by approximately -2 mL/min/year in HBsAg-positive (n = 60) untreated patients over a median follow-up of 24 months.35
Chronic kidney disease is an important public-health problem which significantly increases the likelihood of adverse outcomes and high health-care costs; in addition to the conventional risk factors for chronic kidney disease in the general population, HBV may be an additional agent. Various mechanisms have been implicated in the adverse impact of HBV sero-positive status on chronic kidney disease, including an accelerated endothelial dysfunction at renal level. An atherogenic activity of HBV has been suggested to explain a five-fold increased risk of cardiovascular events in a selected cohort of HBsAg positive patients with type 2 diabetes and overt nephropathy over a median follow-up of 24 months.17 Steatosis is a typical feature of chronic HBV infection and could induce lipid peroxidation and increase plasma inflammatory biomarkers.39 The pathogenesis of HBV-associated nephropathy is still under investigation; however, the small number of patients who develop glomerulonephri-tis suggests that concomitant factors are needed for development of nephropathy (i.e., genetic susceptibility, abnormalities in cell-mediated immunity, and/or environmental conditions).5 These pieces of evidence are in apparent conflict with other findings from the general population- chronic HCV is an important factor for developing insulin resistance, type 2 diabetes mellitus and atherosclerosis.41 Such relationships in patients with chronic hepatitis B are not so straightforward.4042-43
The findings from our meta-analysis are subject to several limitations. First, many studies were cross-sectional, a design that does not allow for causal inference and can overestimate relative risks given its reliance on prevalence ratios. When restricted to cross-sectional studies, no significant relationship was found between hepatitis B serologic status and frequency of CKD and proteinuria. Therefore, there is some evidence that the findings may be impacted by study design. Second, we included in the current review only studies providing adjusted estimates of outcomes (risk of end-stage renal disease or chronic kidney disease, or pro-teinuria), but residual confounding (confounding remaining after adjustment) likely exists as full information has not been given on various covariates in all studies retrieved. As an example, information on HBV DNA or HBV genotypes, socioeconomic status, compliance with medical visits over follow-up, and substance abuse which are important potential confounders was incomplete. Finally, the occurrence of significant heterogeneity clearly precluded more definitive conclusions; our subgroup analysis with meta-regression was not able to capture all the sources of heterogeneity observed. As an example, all the studies enrolled in our systematic review came from Asia and we need studies from other continents.
In conclusion, this meta-analysis of observational studies should suggest a relationship between HBV infection and higher incidence of low eGFR and/or end-stage renal disease in the adult general population. We need additional studies with appropriate size and design (i.e., prospective longitudinal studies) to increase our knowledge on this issue and to explore potential mechanisms underlying such association. A heightened awareness of an increased chronic kidney disease risk should dictate more careful follow-up of renal defects among patients with hepatitis B virus infection.
Supporting Information- •
Supplementary file 1. PRISMA 2009 check list.
PRISMA’s items and their application within the paper.
- •
Supplementary file 2. Quality study.
Details on the quality study process (cohort and cross-sectional studies).
- •
Supplementary file 3. Excluded papers.
List of excluded papers sorted by publication year.
- •
ACR: albumin to creatinine ratio.
- •
APR: albumin to protein ratio.
- •
CC: case-control.
- •
CKD: chronic kidney disease.
- •
CI: confidence intervals.
- •
Co: cohort.
- •
CS: cross-sectional.
- •
eGFR: estimated glomerular filtration rate.
- •
ESRD: end-stage renal disease.
- •
HBV: hepatitis B virus.
- •
HBcAb: hepatitis B core antibody.
- •
HBsAg: hepatitis B surface antigen.
- •
HCV: hepatitis C virus.
- •
HR: hazard ratio.
- •
MDRD: Modification of Diet in Renal Disease.
- •
NOS: Newcastle/Ottawa Scale.
- •
OR: odds ratio
This work was supported in part by a ‘Project Glomer-ulonephritis’ grant, in memory of Pippo Neglia, by Asso-ciazione Amici del Croff-Onlus. The funders had no role in study design, data collection analysis, decision to publish, or preparation of the manuscript
DisclosuresNone.