Background and objective: Iron overload has been associated with HFE mutations (C282Y and H63D). We investigated the association between these mutations and high serum ferritin in a sample of healthy adult men.
Design and methods: We enrolled unrelated blood donors from three hospitals in Mexico City in a cross-sectional study. Serum ferritin (SF) was determined to define iron overload, and HFE gene mutations were identified by PCR–RFLP.
Results: We evaluated 2524 male blood donors and included 246 individuals for each group. We identified 108 individuals with HFE gene mutation, 20.5 % were heterozygote (wt/H63D or wt/C282Y) and the remaining homozygote (H63D/ H63D). The genotype wt/C282Y was observed in two cases, none cases with C282Y/C282Y. The allelic frequency of H63D and C282Y was 0.115 and 0.002, respectively. We observed different association for H63D allele with iron overload (OR 1.54, CI 95 %1.16-2.03) and none in allele C282Y. Although values averages were different, the extreme dispersion of serum ferritin not showed statistically significant differences between H63D and C282Y alleles and ferritin concentrations.
Conclusions: The male unrelated blood donors from Mexico City with iron overload prevalence of 13.8% hold similarities with other populations from Europe o America continent, respecting the allele frequency H63D. Nevertheless, allele frequency C282Y is lower than that observed in descendents from northern Europe. We have not observed statistic difference of SF or iron overload frequency by effect of both alleles.
The gene involved in hereditary hemochromatosis is located at 6p21.3 locus and was described in 1996 by Feder.1 The normal product is a protein of 343 amino acids located on the cell surface, with a sequence similar to MHC class I molecules. The HFE protein extends through the cell membrane to form a heterodimer with β2-microglobulin.2 The best-known variants of HFE gene are the C282Y and H63D mutants. A no directional G→A mutation occurs at nucleotide 845 (G845A), which changes a cysteine to tyrosine at residue 282 (C282Y). This cysteine is highly conserved and forms an intermolecular disulfide bridge. This loss impedes the interaction of HFE with β2-microglobulin and prevents the expression of the protein on cell surface, with the protein instead being retained within the Golgi complex.2 The H63D mutation is located in the alpha 1 chain of the protein and does not affect the expression of the protein or its interaction with β2-microglobulin, C→G change occurs at nucleotide 187 (C187G), which causes the substitution of aspartate for histidine at residue 63 (H63D) that alters the interaction between HFE protein and the transferrin receptor on cell surface.3
A large number of diseases related to defects in the absorption, distribution, or storage of iron have been identified,2 which in all cases result in a pathological increase in iron stores. Iron overload has attracted the attention of clinical researchers because it is associated with different chronic degenerative diseases, such as diabetes mellitus, hepatic cirrhosis, cardiomyopathy, cancer, reproductive failure, vascular damage, and other less specific conditions, such as chronic fatigue syndrome.9
The most common cause of hereditary iron overload is the presence of mutations in HFE gene. The prevalence of these mutations is influenced by ethnic origin.4 The C282Y mutation has been reported in up to 26% of a no selected population of individuals of Celtic ancestry, whereas it is practically absent from individuals of oriental or African origin.4,5 Conversely, the H63D mutation has a multicentric origin, with no ethnic associations. Initial evidence indicates that the prevalence of mutations of the HFE gene in the population of mixed Mexican origin (mestizos) is similar to that observed in Spanish people.6 The impact of mutations of HFE gene on iron overload is also determined by other environmental variables, such as the life style,7 regular blood donation, alcohol intake, and gender.8
The most common test used in the evaluation of iron store in clinical practice or epidemiological setting included the determination of serum ferritin (SF) and transferrin saturation (Ts), with different results in external or internal validation. The cut-off to define iron overload is SF > 300 ug/L or Ts > 55% form men and > 200 ug/L or Ts > 50% for women. The international literature reports that the prevalence of iron overload varies from 9.3 to 22.5%,4 whereas in Mexico, our group has identified a prevalence of up to 29% in selected healthy population.10 The occurrence of iron overload is variable due to the complex interaction of ethnic origin, life style and mutations of HFE gen. This antecedent forces to evaluate association of these three variables in each particular clinical setting.
With this perspective, the aim of this study was to investigate the association between HFE mutations and iron overload in a sample of male blood donors residing in Mexico City.
Design and methodsWe enrolled unrelated volunteer blood donors from three hospitals in Mexico City in a cross-sectional study, case-control design. We included male individuals, 18–64 years of age, who agreed to participate as blood donors were included in this study. Written informed consent was obtained from all participants before entry. All subjects were asked to complete a questionnaire that addressed demographic and medical variables. A physical examination was made, and erythrocyte index were determined according to established criteria for acceptation of blood donors in our country.11 The subjects diagnosed with post-transfusion iron overload, sideroblastic anemia, iron deficiency (SF < 30 ug/L), who had been transfused in the past five years, were excluded.
Analytical proceduresA blood sample was taken to determine the blood cell count and serum ferritin (SF) level, and for the extraction of DNA. Blood samples with hemolysis, lipemia, or insufficient or inadequate leukocyte DNA were eliminated. The values for hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red distribution width, were determined in an AcT 5 Diff Hematology Analyzer (Beckman Coulter Electronics, Miami, FL, USA). SF was determined in duplicate samples and expressed in μg/L, which allowed us to stratify the donors with iron overload or «Group I» (SF ≥ 300 μg/L), according to population values above the 90th percentile, as proposed by Koziol and Beutler,12 and Group II with normal SF (30-299 μg/L). A commercial reagent was used (Vidas® Ferritin, bioMerieux®, Lyon, France), based on an immunoenzymatic method with a final reading in fluorescence (enzyme-linked fluorescent assay), according to procedures validated in our laboratory.
The genomic DNA was extracted and the amplification protocol was based on the original proposals of Feder1 and Adams,13 with oligonucleotide sequence designed to allow the exploration of two mutations in HFE gene. The oligonucleotide sequences used in this experiment to amplify codon 282 were 5´-TGGC AAGGGTAAACAGATCC-3´ (forward) and 5´-CTCAGGCACTCCTCTCAACC-3´ (reverse); and for codon 63, 5´- ACATGGTTAAGGCCTGTTGC-3´ (forward) and 5´GCCACATCTGGCTTGAAATT-3´ (reverse). The amplicons were digested with Rsal (codon 282, exon 4) and BclI (codon 63, exon 2).
Statistical analysisTo establish the statistically significant risk of cases with iron overload and controls, with an exposed portion of 0.10, an alpha value of 0.05 (two tailed), and a beta value of 0.20, we estimated that an OR ≥ 2 was required. We compare the frequency of each HFE mutations in both groups with non parametric procedure of MannWhitney test. Descriptive statistics for the whole sample were obtained.
This protocol was approved by the Research and Bioethical Committees of Instituto Nacional de Perinatología. The confidentiality of both the information and the identity of the participants were maintained. To guarantee the anonymity of the individuals evaluated, the serum and DNA samples were identified by unique progressive numbers that allowed us to file, track, or repeat the necessary tests. This project was supported financially with public funds from the Instituto Nacional de Perinatología and Fundación Clínica Médica Sur.
ResultsWe studied 2,524 male blood donors and included 246 individuals in Group I and an equal number of controls were selected. The results for both groups were compared according to the variables age and erythrocyte index. The average age was 37 years (CI 95% 35-39) for Group I and 33 years (CI 95% CI, 32-35) for Group II, which are statistically different (p < 0.01) using the Mann-Whitney U test (Table I). The values for hemoglobin, hematocrit, mean corpuscular volume, mean concentration of hemoglobin, mean concentration of corpuscular hemoglobin, and red distribution width, showed independent distributions, but not statistical differences between two groups. The SF values were 466 (CI 95% 443490 ug/L) and 138 (129-147 ug/L) respectively.
Distribution of erythrocyte indices among groups of the study.
| Variable | Group I (n = 246) | Group II (n = 246) | P value* |
|---|---|---|---|
| Age (years) | 37 (35-39) | 33 (32-35) | < 0.010 |
| Haemoglobin (g/dL) | 16.8 (16.6-17.0) | 16.7 (16.6-16.9) | 0.219 |
| Hematocrit (%) | 48.9 (48.5-49.4) | 48.7 (48.2-49.1) | 0.293 |
| MCV (fL) | 91.6 (90.9-92.3) | 90.5 (89.9-91.2) | 0.052 |
| MCH (pg) | 31.5 (31.3-31.8) | 31.1 (30.9-31.4) | 0.056 |
| MCHC (g/pg) | 34.4 (34.1-34.5) | 34.4 (34.2-34.5) | 0.707 |
| RDW (%) | 12.9 (12.8-13.1) | 13.0 (12.9-13.1) | 0.534 |
| Serum Ferritin (μg/L) | 466 (443-490) | 138 (129-147) |
Average (95% confidence interval). MCV, mean corpuscular volume; MCH, mean concentration haemoglobin; RDW, red cell distribution width; MCHC, mean corpuscular haemoglobin concentration.
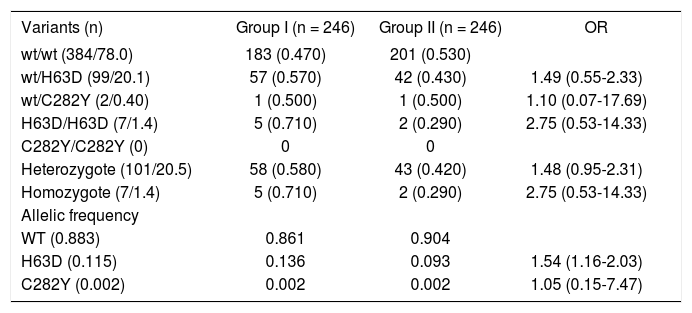
Mutations in HFE gene were determined in the 492 individuals included in the study (246 for each group). Using molecular analysis, we identified 384 individuals (78.0%) with the wild-type (wt) gene presentation, proportion 0.47 and 0.53 for each group (Table II). In 108 individuals with HFE gene mutation, 101 (20.5%) were identified as heterozygote (wt/H63D or wt/C282Y) and the remaining seven as homozygotes (1.4%, H63D/ H63D). We do not observed statistical association between two situations, OR 1.48 (CI 95%, 0.95-2.31) and 2.75 (CI 95% 0.53-14.33), respectively.
Distribution of the zygosity of the HFE alleles.
| Variants (n) | Group I (n = 246) | Group II (n = 246) | OR |
|---|---|---|---|
| wt/wt (384/78.0) | 183 (0.470) | 201 (0.530) | |
| wt/H63D (99/20.1) | 57 (0.570) | 42 (0.430) | 1.49 (0.55-2.33) |
| wt/C282Y (2/0.40) | 1 (0.500) | 1 (0.500) | 1.10 (0.07-17.69) |
| H63D/H63D (7/1.4) | 5 (0.710) | 2 (0.290) | 2.75 (0.53-14.33) |
| C282Y/C282Y (0) | 0 | 0 | |
| Heterozygote (101/20.5) | 58 (0.580) | 43 (0.420) | 1.48 (0.95-2.31) |
| Homozygote (7/1.4) | 5 (0.710) | 2 (0.290) | 2.75 (0.53-14.33) |
| Allelic frequency | |||
| WT (0.883) | 0.861 | 0.904 | |
| H63D (0.115) | 0.136 | 0.093 | 1.54 (1.16-2.03) |
| C282Y (0.002) | 0.002 | 0.002 | 1.05 (0.15-7.47) |
Number of subjects and proportion. OR (Odds Ratio).
The mutation wt/H63D were present in 99 subjects (20.1%), with proportion of 57 and 42 cases for each group, without statistical association (OR 1.49, CI 95% 0.55-2.33) and H63D/H63D in seven cases (proportion 0.71 and 0.29, for Group I and II, respectively), without statistical differences (OR 2.75, CI 95% 0.53-14.33). The variant wt/C282Y was observed in two cases (one for each group, OR 1.10, CI 95% 0.07-17.69). We do not identified cases with variant C282Y/C282Y.
The allelic frequency of wt was 0.883, H63D and C282Y was 0.115 and 0.002, respectively. The distribution of allelic frequency showed different association for H63D allele (OR 1.54, CI 95%1.16-2.03), with frequency 0.136 and 0.093 for Group I and II, respectively. There was not difference in allelic frequency for C282Y (OR 1.05, CI 95% 0.15-7.47).
To evaluate the global relationship between HFE gene mutations and iron overload, we grouped all cases with iron overload and all subjects with HFE mutation (either H63D or C282Y); in a «mutated» group with versus none mutated or wild-type group (Table III). There were 63 (58.3%) and 45 mutated subjects (41.7%) for group I and II, respectively, with border line statistical association (OR 1.54 CI 95%, 1.00-2.37). Although a greater (OR 1.54) association existed between individuals with iron overload and the presence of a mutations in the HFE gene, the value observed did not exceed the value calculated for OR in the design of the study (OR ≥ 2).
We evaluated the differences on the distribution of serum ferritin values between the individuals with the natural form of the gene (wild type), as well as with the mutations wt/H63D, H63D/H63D and wt/C282Y. Although values averages were different, the extreme dispersion of such concentrations not generates statistically significant differences. The same situation was documented with the allele’s wt, H63D and C282Y (data no showed).
DiscussionThe mutations of C282Y and H63D of HFE gene are documented widely in international literature.13-15 The selection bias in the type of target population is a determining factor to establish the frequency of these mutations and their correlation with the increase of iron store observed. Different reports have included newborns during neonatal screen,16 general population,8 subjects in healthy appraisal clinic or blood donors6,17 and relatives of patients with clinical hereditary haemochromatosis.5,18 The study of gene HFE mutations has been used in blood donors, although the frequent donation can be an important factor to modify the iron store, as to prevent its establishment the direct relation with mutations C282Y and H63D.17,19 However, the effect of frequent blood donations diminishes serum ferritin values and increases iron deficiency prevalence, without the protective effect from the HFE gene mutations.
The genotype and allele frequencies for C282Y and H63D in blood donors vary around the world (Table IV). The allele frequency C282Y is more prevalent in Europe with 0.011,13,15 particularly in Northern Ireland with 0.020 and South Wales with 0.012. In Spain, it varies widely from 0.020 to 0.007.20,21 The allele frequency of C282Y, compared between diverse population groups from North America, shows that white people from USA is more prevalent (0.115-0.196) than Afro-Americans (0.025)18,22 and practically null in Brazil,23 and native Amerindians.6,13,24,25 Among the Mexican American population, resident in the USA, it is three to five times greater,13,18,22 than reported in the present study for blood donors living in Mexico City, with 0.001, but less than Spanish blood donors.20,21 These results could be explained by the differences in the origin of the diverse American native populations, in addition to the possibility of the Mestizo Mexican population having a different proportion of mixed genes from European-Spanish origin,25 independently of the selection bias effect.
Genotype and allele frequencies for HFE gene mutations in diverse populations of blood donors.*
| Geographic area Population | Genotype frequency | Allele frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| C282Y/C282Y | wt/C282Y | wt/H63D | wt/wt | H63D/H63D | C282Y/H63D | C282Y | H63D | |
| Europe | 0.4 | 9.2 | 2 | 1.8 | 21.6 | 65.1 | 0.011 | 0.027 |
| Denmark | 1.4 | 11 | 1.5 | 2.5 | 20 | 65 | 0.016 | 0.026 |
| Germany | 0 | 3.3 | 2 | 2 | 19 | 73.9 | 0.005 | 0.025 |
| Italy | 0.8 | 8.2 | 6.2 | 3.7 | 28.6 | 50.4 | 0.068 | 0.224 |
| Northern Ireland | 1.2 | 14.8 | 1.5 | 2.5 | 22.8 | 57.2 | 0.02 | 0.028 |
| Norway | 0 | 12.8 | 2.1 | 0 | 18.1 | 67 | 0.013 | 0.022 |
| Spain | 0-0.2 | 4.1-4.5 | 4.1-4.3 | 1.4-1.7 | 32.3-33.8 | 57.1-66.5 | 0.02-0.007 | 0.16-0.044 |
| South Wales | 1 | 5.9 | 3 | 4 | 21.8 | 64.3 | 0.012 | 0.032 |
| England | 0.68 | 12.7 | 2.4 | 2.4 | 23.6 | 58.3 | 0.016 | 0.031 |
| America | 8.1-10.0?? | 0.3-7.8 | 2.1-5.0 | 1.7-5.3 | 21.5-24.2 | 55.7-65.3 | 0.020-0.046 | 0.051-0.069 |
| EUA White | 0.12-0.82 | 8.08-11.3 | 1.45-3.08 | 1.63-3.34 | 21.3-25.9 | 59.5-62 | 0.115-0.196 | 0.067-0.121 |
| San Diego, Cal. | 1 | 11.4 | 2.9 | 3.2 | 20.9 | 60.6 | 0.017 | 0.03 |
| USA (Blacks) | 0.06 | 2.33 | 0.32 | 0.06 | 5.55 | 91.69 | 0.025 | 0.062 |
| Brazil. | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 99.99 | 0 | 0 |
| Chile Indian | 0 | 1.28 | 0 | 0 | 5.13 | 93.59 | 0.001 | 0.005 |
| Chile White ethnicity | 0 | 2.56 | 1.28 | 0 | 18.59 | 77.57 | 0.01 | 0.087 |
| Caracas, Venezuela. | 0 | 3.7 | 0 | 0.41 | 18.2 | 77.69 | 0.004 | 0.019 |
| Mexican American | 0.02-0.4 | 1.6-3.9 | 0.6-2.0 | 0.02-0.9 | 17.4-23.3 | 71.1-76.0 | 0.002-0.006 | 0.09-0.028 |
| México City ** | 0 | 0.4 | 1.4 | 0 | 20.2 | 77.9 | 0.001 | 0.023 |
The H63D allele is highly prevalent in the world population, varying from 0 to 0.22513 for individuals not ethnically related to Celtic ancestors,27 which is consistent with the frequency of 0.023 reported in this issue. The fact that allele frequency of H63D in Mexican population is similar to that reported for other groups can be explained in two ways. The first is that this allele frequency was inherited during the Spanish racial intermixing and the second is that mutation H63D,26 contrary to the C282Y mutation, rose in more than one geographic area.14 This means that genetic frequency of C282Y is determined by the ethnic origin,13,15 whereas mutation H63D has a uniform world-wide distribution.13 Likewise, allele C282Y had a multicentric origin and did not depend initially on the flow of genes or genetic mixing.14 Finally, based on differences between allelic frequencies, it is assumed that H63D is more ancient than C282Y allele.27
The serum ferritin concentrations are related to bone marrow hemosiderin and modifiable iron. In the adult, 1 μg/L of ferritin corresponds to 7–8 mg of iron stored.28 The physiological variables related to changes in iron concentrations differ between men and women,10 due to the interactions of diverse biological and social factors. Physiological changes in the content of iron store occur slowly, varying with age.12 The balance in iron content, and the change from one state to another, occurs over a long period of 7–10 years.10,28 The prevalence of iron overload is similar in our report (12%) to that reported in other publications12,27,28 for blood donors (8.3%) or no selected populations (8.7–16.5%). We observed that individuals with alleles C282Y or H63D held higher median SF concentrations, lacking statistical differences (Figure 1).
Why is not there absolute statistical association between iron overload and HFE gene mutations? It is clear that iron-overload individuals tend to accumulate iron store during their course of life, but there is also an inevitable interaction with individual, environmental, and biological factors,10,18 including the gender,22 iron consumption on the diet,7 iron supplementation,8 and other social variables, such as consumption of alcohol28 or frequent blood donations.17,20 Our group has shown that there is an increase of over 20% in the average serum ferritin values between the 18–29 and 30–49 year age strata.10 The specific effect of each differs according to the context of each individual or population studied. 8,10,13,12,18
In conclusion, male blood donors from Mexico City with iron overload prevalence of 13.8%, hold similarities with other populations from Europe or America continent, respecting the allele frequency H63D. Nevertheless, allele frequency C282Y is lower than that observed in descendents from northern Europe. We have not observed, however, statistic difference of SF nor iron overload frequency by effect of both alleles. The global prevalence of HFE gene mutations is high (22%), suggesting a need to extend the study and interactions of these mutations in Amerindian Mexican population.