Background and aim: To what extent the serum levels of alanine aminotransferase (ALT) are related to histological characteristics of liver damage caused by hepatitis C virus (HCV) infection among patients with end-stage renal disease (ESRD) remains unclear.
Methods: Patients with a positive anti-HCV antibody titer confirmed by supplemental tests were evaluated by liver biopsy. We compared ALT levels in patients with and without renal damage, with similar histological grades and stages of inflammation and fibrosis. Patients were divided into two groups: patients with ESRD (n = 25) and patients without renal damage (n = 39).
Results: The ALT level was 42.1 ± 24.3 IU/L for the ESRD group, compared with 109.9 ± 55.8 IU/L for the non-ESRD group (P < 0.001). Liver inflammation (modified Knodell grade) was 4.0 ± 2.1 in the ESRD group versus 5.2 ± 2.4 in the non-ESRD group; fibrosis (6-point scale) was 1.1 ± 1.2 versus 1.7 ± 1.5, respectively.
Conclusions: Despite histological evidence of liver inflammation, ALT levels in the ESRD group were normal, while ALT levels were significantly higher in the non-ESRD group with similar levels of liver inflammation. In conclusion, ALT levels are not a useful indicator of HCV infection in patients with ESRD and liver biopsies should be recommended for kidney transplant candidates.
Abbreviations:
ALT, Alanine aminotransferase; ESRD, End-stage renal disease; Anti-HCV, hepatitis C virus antibody; ESRD-group, Patients with ESRD and hepatitis C; Non-ESRD-group, Patients with hepatitis C and normal renal function; CAPD, Chronic Ambulatory Peritoneal Dialysis; NA, Not applicable; NS, Not significant.
The histological evaluation of liver biopsies is the gold standard in determining the degree of inflammation in patients with liver disease related to hepatitis C virus (HCV) infection. Indeed, histological staging is still the only reliable predictor of prognosis and of the likelihood of disease progression.1 To date, few studies have evaluated the severity of HCV infection in patients with end-stage renal disease (ESRD) on dialysis and its correlation with serum alanine aminotransferase (ALT) levels. The prevalence of HCV infection is significantly elevated in patients with ESRD on dialysis (8% to 65%).2-6 However, the clinical evidence of HCV infection in patients with ESRD is limited and the manifestations of kidney disease predominate. In 75% of cases, ALT levels fall within a normal range according to the reference pattern of the nonuremic population, a situation that has led to an underestimation in the diagnosis of HCV infection.7-9 Furthermore, in histological terms, less inflammation and fibrosis occur in the liver of patients with ESRD and HCV infection when compared with patients without renal damage.10-13 The aim of this study was to compare the ALT levels in patients with and without renal damage and HCV infection, for whom liver biopsies displayed similar histological grades and stages.
MethodsStudy populationOver a 10-year period (1994-2003), patients with ESRD on a renal transplant program receiving peritoneal dialysis or hemodialysis for more than three months, and with positive anti-HCV antibody titers confirmed by supplemental tests, were evaluated at the Division of Nephrology and Transplants of the Centro Médico Nacional de Occidente. These patients were classified as the ESRD group. Patients with positive HCV antibody levels confirmed by supplemental tests and with normal kidney functions were evaluated in the Department of Gastroenterology of the Centro Médico Nacional de Occidente and the Department of Gastroenterology of Carmen’s Hospital; these patients were classified as the non-ESRD group. The protocol was directed toward patients with HCV infections who had abnormal ALT levels. Liver biopsy was performed on all patients in both groups to establish chronic hepatitis.
Patients with hepatitis B infection, primary biliary cirrhosis, sclerosing cholangitis, hemochromatosis or an incomplete clinical history were excluded from the study. The study protocol was approved by the Institutional Review Board and informed written consent was obtained from each patient.
Data collectionAll patients were Hispanic in origin and the demographic variables of each subject and the risk factors for HCV infection were recorded. In patients with a history of transfusions, the date of the first transfusion was noted. Surgical interventions were classified either as major surgery performed in a surgical operating room (e.g., hysterectomy or abdominal surgery) or as minor surgery, namely procedures performed at the bedside (e.g., peritoneal catheterization and arterial, venous, thoracic or lumbar punctures). Following the detection of an anti-HCV positive test, recombinant immunoblot assay, an HCV RNA test, or both, were used as supplemental tests. The ALT levels were recorded at least twice over six months. Elevation of ALT levels could be associated with factors other than HCV infection, such as hypovolemic shock, use of hepatotoxic medication and systemic infection. Abnormal ALT levels caused by these alterations were excluded from the analysis.
Laboratory methodsSerum ALT concentration was determined using a dry chemical assay (VITROS® 950 Chemistry System, Ortho-Clinical Diagnostics, Raritan, NJ, USA). ALT level reference values were 11-66 IU/L. Anti-HCV was assessed with an immunoenzymatic assay. Positive anti-HCV screening tests were confirmed using a recombinant immunoblot assay (Chiron RIBA® HCV 2.0 SIA versions 2.0 or 3.0, Chiron Corp., Emeryville, CA, USA), a qualitative HCV RNA assay, or both, performed using manual homemade or commercially available noncompetitive reverse transcription polymerase chain reaction assays.
Liver biopsyPercutaneous liver biopsies were obtained from all patients in both groups, fixed and embedded in paraffin wax. Sections were stained with hematoxylin and eosin. An experienced pathologist (GVC) evaluated all the samples and was blinded to each subject’s viral results. The Ishak (modified Knodell) numerical scoring system was used to describe the histology.14,15 Inflammation was classified as mild, 1-4 points; moderate, 5-8 points; and severe, > 9 points. The staging of fibrosis was determined as: mild, 1-2 points; moderate, 3-4 points; precirrhosis, 5 points; and cirrhosis, 6 points.
Statistical analysisMeans and proportions were used to compare groups. The statistical significance of differences in quantitative variables was estimated using the Mann-Whitney non-parametric U test and the Student’s t test. The chi-squared test was used for qualitative variables. The differences were considered statistically significant when p < 0.05. All the data were analyzed with SPSS software, version 13 (SPSS, Chicago, IL, USA).
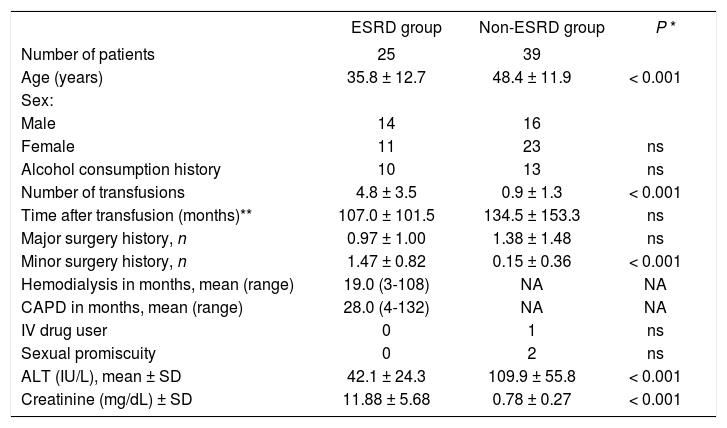
ResultsOf the 70 patients studied, six were excluded because of normal liver histology (five in the ESRD group and one in the non-ESRD group); 25 patients were included in the ESRD group and 39 patients in the non-ESRD group. Table I describes the characteristics of each group of patients. The mean age in the ESRD group (35.8 ± 12.7 years) was less than that of the non-ESRD group (48.4 ± 11.9, P < 0.001) and a higher proportion of male patients were in the ESRD group than in the non-ESRD group (56.0% vi 41.0%, not significant, ns). Transfusions were carried out more frequently in the ESRD group (4.8 ± 3.5) than in the non-ESRD group (0.9 ± 1.3, P < 0.001) and the tendency was towards a longer time between the first transfusion and the diagnosis of HCV infection in the non-ESRD group (107.0 ± 101.5 vi 134.54 ± 153.3 months; ns). The mean serum concentration of ALT of patients with ESRD was 42.1 ± 24.3 IU/L, lower than the 109.9 ± 55.8 IU/L in the non-ESRD group (P < 0.001). The HCV RNA results have not been included because the test was performed with different molecular techniques (manual and semiautomated) that precluded a valid statistical comparison.
Baseline demographics in patients with hepatitis C virus (HCV) infection and ESRD (ESRD group) compared with patients with HCV with normal renal function (non-ESRD group).
| ESRD group | Non-ESRD group | P * | |
|---|---|---|---|
| Number of patients | 25 | 39 | |
| Age (years) | 35.8 ± 12.7 | 48.4 ± 11.9 | < 0.001 |
| Sex: | |||
| Male | 14 | 16 | |
| Female | 11 | 23 | ns |
| Alcohol consumption history | 10 | 13 | ns |
| Number of transfusions | 4.8 ± 3.5 | 0.9 ± 1.3 | < 0.001 |
| Time after transfusion (months)** | 107.0 ± 101.5 | 134.5 ± 153.3 | ns |
| Major surgery history, n | 0.97 ± 1.00 | 1.38 ± 1.48 | ns |
| Minor surgery history, n | 1.47 ± 0.82 | 0.15 ± 0.36 | < 0.001 |
| Hemodialysis in months, mean (range) | 19.0 (3-108) | NA | NA |
| CAPD in months, mean (range) | 28.0 (4-132) | NA | NA |
| IV drug user | 0 | 1 | ns |
| Sexual promiscuity | 0 | 2 | ns |
| ALT (IU/L), mean ± SD | 42.1 ± 24.3 | 109.9 ± 55.8 | < 0.001 |
| Creatinine (mg/dL) ± SD | 11.88 ± 5.68 | 0.78 ± 0.27 | < 0.001 |
Key: non-ESRD, patients with HCV infection and normal kidney function; NA, not applicable; ns, not significant; CAPD, chronic ambulatory peritoneal dialysis; ALT, alanine aminotransferase.
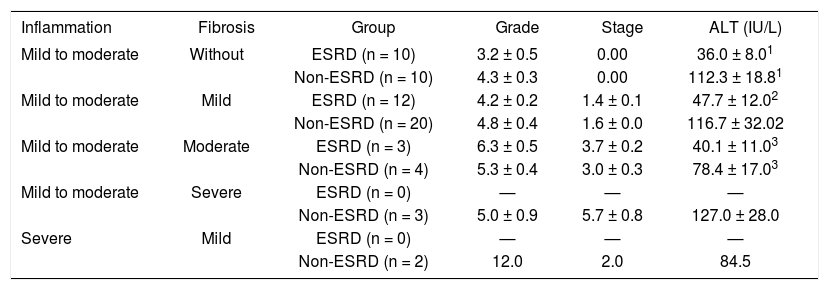
Liver inflammation was graded at a mean of 4.0 ± 2.1 in the ESRD group, versus 5.2 ± 2.4 in the non-ESRD patients; fibrosis was graded at 1.1 ± 1.2 versus 1.7 ± 1.5, respectively. We classified liver biopsy results into five subgroups to analyze ALT values in patients with similar characteristics in terms of the histological grade and stage of their liver biopsy in both groups (Table II). Differences in ALT levels were found between the ESRD (n = 10) and non-ESRD (n = 10) groups of patients with mild to moderate chronic hepatitis without fibrosis. These patients were ascribed similar histological liver scores; the degree of inflammation was 3.2 ± 0.5 in the ESRD group and 4.3 ± 0.3 in the non-ESRD group (ns). Despite the histological evidence of liver inflammation, ALT levels in patients with ESRD were normal (36.0 ± 8.0 IU/L), while the mean ALT level in the non-ESRD group of patients that displayed a similar inflammation grade was significantly different (112.3 ± 18.0 IU/L, P < 0.08). Differences in ALT levels were observed between the subgroup with mild to moderate chronic hepatitis and mild fibrosis. In this subgroup, among the 12 patients with ESRD with an inflammation score of 4.2 ± 0.2 and fibrosis of 1.4 ± 0.1, ALT levels were 47.7 ± 12.0 IU/L. In contrast, the 20 non-ESRD patients with an inflammation grade of 4.8 ± 0.4 and fibrosis of 1.6 ± 0.0 (P = ns) displayed elevated ALT levels (116.6 ± 32.0 IU/L, P < 0.03). The ALT levels obtained from ESRD and non-ESRD patients in the subgroup with mild inflammation and moderate fibrosis also differed (40.1 ± 11.0 vs 78.4 ± 17.0), although the numbers of patients were too small to determine statistical significance. Severe inflammation and fibrosis were found only in the non-ESRD group.
| Inflammation | Fibrosis | Group | Grade | Stage | ALT (IU/L) |
|---|---|---|---|---|---|
| Mild to moderate | Without | ESRD (n = 10) | 3.2 ± 0.5 | 0.00 | 36.0 ± 8.01 |
| Non-ESRD (n = 10) | 4.3 ± 0.3 | 0.00 | 112.3 ± 18.81 | ||
| Mild to moderate | Mild | ESRD (n = 12) | 4.2 ± 0.2 | 1.4 ± 0.1 | 47.7 ± 12.02 |
| Non-ESRD (n = 20) | 4.8 ± 0.4 | 1.6 ± 0.0 | 116.7 ± 32.02 | ||
| Mild to moderate | Moderate | ESRD (n = 3) | 6.3 ± 0.5 | 3.7 ± 0.2 | 40.1 ± 11.03 |
| Non-ESRD (n = 4) | 5.3 ± 0.4 | 3.0 ± 0.3 | 78.4 ± 17.03 | ||
| Mild to moderate | Severe | ESRD (n = 0) | — | — | — |
| Non-ESRD (n = 3) | 5.0 ± 0.9 | 5.7 ± 0.8 | 127.0 ± 28.0 | ||
| Severe | Mild | ESRD (n = 0) | — | — | — |
| Non-ESRD (n = 2) | 12.0 | 2.0 | 84.5 |
Key: ALT, alanine aminotransferase; ESRD, patients with end-stage renal disease; Non-ESRD, patients with normal kidney function.
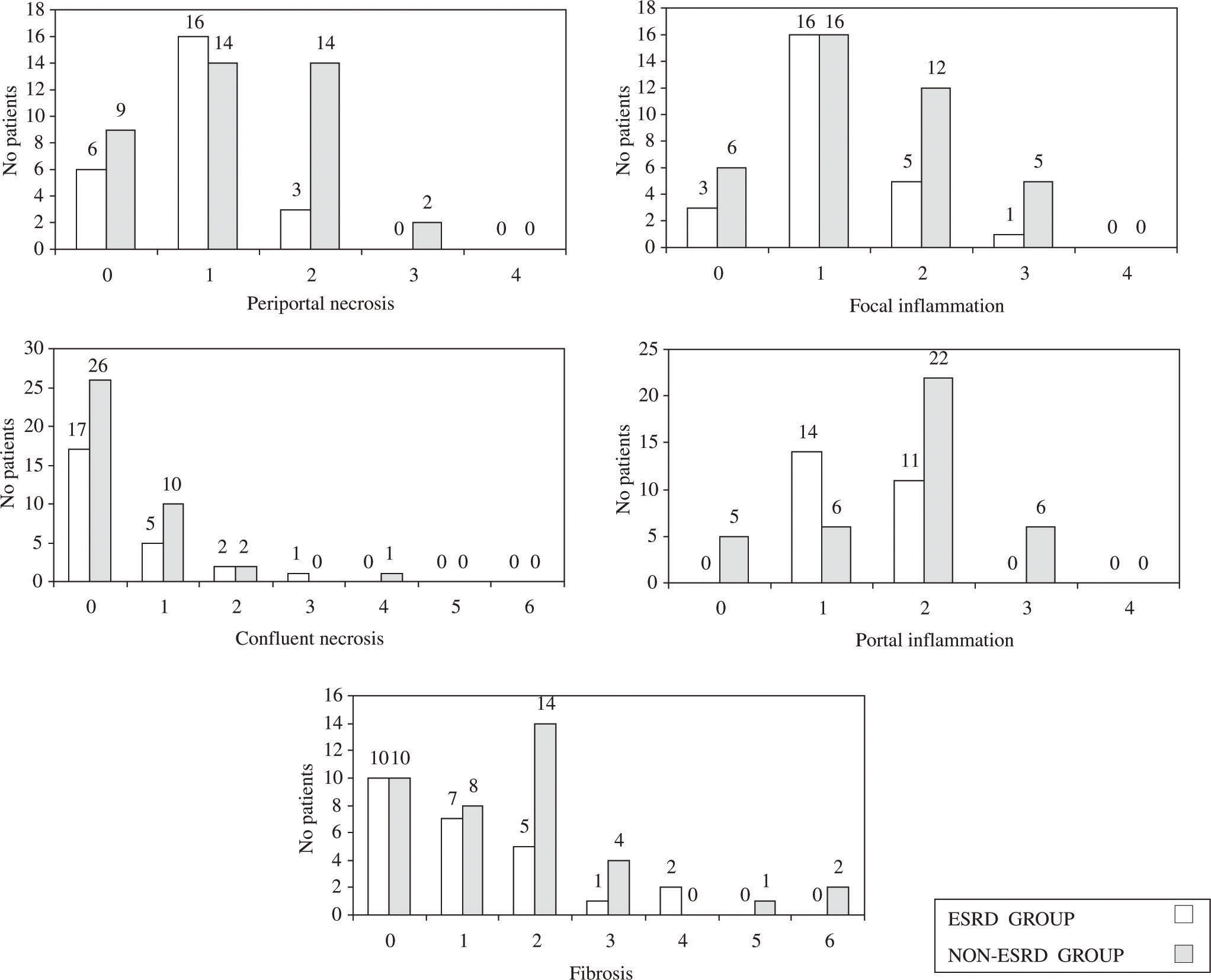
The histological parameters analyzed, according to the Ishak (modified Knodell) numerical scoring system, included periportal necrosis, focal inflammation, confluent necrosis, portal inflammation and fibrosis (Figure 1). Whereas mild to moderate periportal necrosis was observed in 16 out of 39 (41%) patients of the non-ESRD group, it was only detected in 3 out of 25 patients (12%, P = 0.001) with ESRD. The tendency was towards more severe focal inflammation in the non-ESRD group (17 out of 39, 46%) than in the ESRD group (6 out of 25, 24%: P = ns). Portal inflammation was observed in 28 out of 39 patients (72%) of the non-ESRD group compared with 11 out of 25 patients (44%, P < 0.05) of the ESRD group. Likewise, liver fibrosis appeared to be more frequent in the non-ESRD group (21 out of 39, 53%) than in the ESRD group (8 out of 25, 32%: P = ns). A simplified dichotomized analysis was made for mild and severe inflammation/fibrosis. Mild inflammation was found in 16 out of 25 patients (64%) of the ESRD group compared with 16 out of 39 patients (41%) of the non-ESRD group; severe inflammation was found in 9 out of 25 patients (36%) and 23 out of 39 patients (59%), respectively (ns). Mild fibrosis was observed in 22 out of 25 patients (88%) of the ESRD group and 32 out of 39 patients (82%) of the non-ESRD group and severe fibrosis in 3 out of 25 patients (12%) and 7 out of 39 patients (18%), respectively (ns).
In 8 out of 25 patients (32%) of the ESRD group, mild postbiopsy bleeding was observed as manifested by blood discoloration in the peritoneal dialysis liquid, which resolved within 48-72 h. Only one patient (4%) required a laparotomy for secondary shock after liver biopsy bleeding. In the non-ESRD group, no postbiopsy complications were reported.
DiscussionPatients with ESRD and HCV infection displayed normal ALT levels. Indeed, the ALT levels in these patients were significantly lower than those found in patients infected with HCV without renal damage but with similar histological grades and stages of liver alterations. To our knowledge, this is the first such study carried out in a completely Hispanic population.
ALT levels have been utilized in the diagnosis and follow up of patients with HCV infection.16,17 However, this approach appears inadequate in such patients with ESRD. ALT levels are not directly correlated with the severity of chronic liver disease and they are within normal limits or are only slightly elevated in patients with ESRD.8,9,18-20 In one study from 39 centers including 2,440 patients with ESRD on hemodialysis, elevation of ALT levels did not prove to be useful in identifying acute and chronic HCV infections. In that study, ALT levels gave a sensitivity of 83% and 21% and a positive predictive value of 4% and 16% for acute and chronic HCV infections, respectively.10 Because liver biopsies were not performed in either group of patients, that study concluded that more rigorous methods were necessary to diagnose HCV infections in patients with ESRD. Our findings confirm that ALT levels are not useful in diagnosing HCV infection in patients with ESRD and that a liver biopsy is necessary to determine chronic liver disease.
The patients studied here were classified into subgroups with similar histological grade and stage of their liver biopsies. We found that periportal necrosis and portal inflammation were more frequently associated with the non-ESRD group of patients (P < 0.05). These results are similar to those reported when 46 patients with ESRD and HCV infection were compared with 46 infected patients lacking without renal damage who had liver biopsies taken.11 In accordance with the data presented here, fewer liver lesions were observed in patients with HCV infection with renal damage, particularly in terms of the parameters of portal inflammation, periportal necrosis and fibrosis. In Cotler’s study,11 patients with ESRD and HCV infection had significantly lower ALT levels (41 IU/mL, range 3051) than infected patients lacking without renal damage (62 IU/mL, range 49-82, p < 0.001). They also reported that the HCV RNA levels were similar between the groups and concluded that ALT levels do not predict the grade and stage of hepatic alterations in patients with ESRD.
Our study did not include a group of non-ESRD patients with normal ALT levels because the protocol for liver biopsy during the study period was directed toward patients with HCV infection and abnormal ALT levels. However, an earlier study was carried out comparing patients infected with HCV with normal and abnormal ALT levels and patients with ESRD.12 This study included 50 patients infected with HCV and showing ESRD who were on a renal transplant protocol, and 86 patients infected with HCV without renal damage. The latter group was subclassified into two groups: one with normal ALT levels (43 patients) and the other with abnormal ALT levels (43 patients). In 47 out of 50 patients (94%) with ESRD and infected with HCV, ALT levels were normal. No significant differences existed in the degrees of inflammation and fibrosis between the patients with ESRD and the patients with normal ALT levels without renal damage, although these parameters were higher in the patients with abnormal ALT levels but without renal damage. ALT levels were similar between patients with ESRD (32.8 ± 17.6) and patients without renal damage with normal ALT levels (37.9 ± 9.9). Our study showed grades of inflammation (4.0 ± 2.1) and fibrosis (1.1 ± 1.2) in the ESRD group similar to those in patients with normal ALT levels in the aforementioned study (inflammation 4.33 ± 1.8, fibrosis 0.73 ± 1.15). On the other hand, the non-ESRD group of patients in our study displayed degrees of inflammation (5.2 ± 2.4) and fibrosis (1.7 ± 1.5) similar to those for patients with abnormal ALT levels in Sterling’s study (inflammation 6.53 ± 2.24, fibrosis 1.8 ± 1.5).12 Thus, patients with ESRD infected with HCV appear similar to patients infected with HCV but without renal damage and with normal ALT levels, in terms of both ALT levels and histological liver abnormalities.
The pathophysiological mechanisms involved in regulating the ALT levels in patients with ESRD following HCV infection are still unclear. It has been proposed that the increase in growth factor levels in hepatocytes of HCV-infected patients with ESRD who are on chronic dialysis produces a hepatoprotective effect, which translates into less severe histopathology.21 However, further studies are necessary to confirm this and to clarify the underlying mechanisms. The persistence of elevated ALT levels is indicative of progressive liver damage. However, progression of fibrosis and cirrhosis has been demonstrated in patients with consistently normal ALT levels.13,22,23 Other hypotheses are that the difference in the ALT levels is caused by greater excretion of ALT in patients with ESRD, or from the presence of an aminotrans-ferase inhibitory factor that interferes with the determination of the levels of this enzyme in patients with ESRD and HCV infection.21
We found that the patients with ESRD were significantly younger (35.8 ± 12.7 years) than the patients in the non-ESRD group (48.4 ± 11.9 years), which could reflect a shorter evolution of HCV infection in the ESRD group. However, considering that in our country transfusion history is the principal risk factor,24 we found no significant difference between the two groups in the time that elapsed after the first transfusion. A comparison has been made between 30 patients with ESRD on hemodialysis and 30 patients with HCV infection without renal damage but with the same disease progression after acquiring the infection (130.5 ± 9.7 months vs 130.9 ± 11.5 months, respectively).13 The patients with ESRD had less inflammation and fibrosis than the patients with HCV infection without renal damage (inflammation grade 0.92 ± 0.2 vs 1.39 ± 0.1 and fibrosis grade 1.03 ± 0.3 vs 1.70 ± 0.3, respectively). These results provide the most convincing evidence to date for a slower progress of liver disease in patients with ESRD and HCV infection.25
In our study, one patient with ESRD experienced a severe complication secondary to percutaneous liver biopsy. Few studies have determined the risks or benefits of performing a liver biopsy in patients with ESRD and HCV infection. Nevertheless, consensus exists regarding the importance of determining the grade and stage of chronic liver disease for patients for whom a renal transplant is planned.11,12,26 The main objective in the pre-transplant evaluation of patients with ESRD and HCV infection is to identify advanced liver disease.27,28 Recently, transjugular liver biopsy was reported as effective and safe in obtaining liver tissue in patients with ESRD and has a lower complication rate than percutaneous liver biopsy.29,30 On the other hand, antiviral treatment for HCV infection with peginterferon alpha and ribavirin is effective, although for patients with ESRD and HCV infection, monotherapy with peginterferon alpha alone is recommended.31-33
A limitation of the present study is its retrospective nature. Thus, relating the HCV RNA level to other measures was not possible because the same technique was not used during the study period. However, as viral load is not related to ALT alterations and liver histology,34 this should not affect our interpretation of the results. Moreover, virological tests have no prognostic value. Indeed, current virological markers (including HCV RNA level and HCV geno-typing) do not correlate with the severity of liver injury or fibrosis and they cannot be used to predict the natural course or outcome of the infection.35
In conclusion, the ALT levels in the patients with ESRD infected with HCV were normal, irrespective of the histological alterations found in the liver biopsies. Thus, ALT levels are not a useful indicator of HCV infection in patients with ESRD; rather, a liver biopsy should be recommended for kidney transplant candidates. Other noninvasive tests have been proposed as serum markers in the diagnosis of liver damage for patients with HCV infection;36,37 however, more studies are warranted.
AcknowledgmentsThe authors are grateful to MVP Romero Flores, Rodolfo Ochoa-Jiménez and María R. Flores for their critical reading of the manuscript.