Hepatitis A virus (HAV) is the most common cause of acute viral hepatitis worldwide. The virus is mainly transmitted via the fecaloral route and, the incidence of infection is closely related to low socioeconomic conditions and poor sanitation. Mexico, previously categorized an area of high endemicity for HAV infection, is undergoing epidemiological transition. However, a limited number of HAV-related scientific reports regarding to virus burden is available. According to the local government health agency (Secretarla de Salud, SSA in Spanish), from 1994 to 2017 a reduction in the incidence of hepatitis related to HAV has been reported. However, HAV is still the most common cause of viral hepatitis in the country, and the pediatric population is the most prone to be infected with this virus. The analysis of the SSA data reveals that most of the reported cases from 1994 to 2017 were found in highly industrialized states. This information contradicts the documented relationship between the highest prevalence of infection and the lowest socio-economic status, and supports the necessity of viral detection and notification of HAV cases. Moreover, in spite that four HAV vaccines are available in Mexico and universal vaccination has been shown to be beneficial in developing countries in terms of declining endemicity, HAV vaccination is not mandatory in Mexico. In this review, preventive strategies including appropriate diagnosis, vaccination and public health policies on the basis of the epidemiologic status of HAV in Mexico are discussed.
Hepatitis A virus (HAV), first identified in 1973, is a small, positive-strand RNA virus and is the causative agent of acute hepatitis in humans. It affects approximately 10 million people annually worldwide. Currently, three HAV-genotypes (I, II, III) with two subtypes (A and B), and one serotype have been known to infect humans.1 HAV belongs to the Picornaviridae family, is mainly transmitted via the fecal-oral route by contaminated drinking water and is endemic in many countries, particularly those with poor sanitation. HAV is also considered a foodborne pathogen2 based on the documented outbreaks of infection caused by the consumption of frozen fruits in developed and developing countries.3
The clinical manifestations of HAV range from asymptomatic infections to acute liver failure (ALF), but infection does not progress to chronic disease.However, some patients may show atypical manifestations including prolonged cholestasis, relapsing hepatitis, extrahepatic manifestations or ALF associated with autoimmune hepatitis.4 It has been accepted that clinical outcomes vary with age, and older individuals are prone to develop more severe forms of the disease. While HAV-induced ALF is not common, ALF development results in intensive care and requires a decision regarding liver transplantation.5 Acute HAV infection resolves spontaneously in > 99% of infected individuals, and relapsing hepatitis A with subsequent complete resolution has been reported in 3-20% of patients with clinical hepatitis. Fulminant hepatitis is rare, with a wide range of estimated rates in immunocompetent individuals. However, reports from Korea and South-America, including Mexico, have raised concern that the current incidence of fulminant hepatitis A may be rising. Immune-suppressed patients and patients with chronic liver disease are at an increased risk of developing severe hepatitis A.6’7
Acute hepatitis A is diagnosed by serologic testing to detect HAV-specific IgM antibodies. The presence of anti-HAV IgG antibodies in the serum denotes previous infection. Furthermore’ viral detection through molecular methods is not common for diagnosis. Prior to 1960, the seroprevalence of IgG antibodies against the virus approached 100% worldwide, indicating that almost every person in the world was infected with HAV at that time.8 Since then, improved hygiene and vaccination have reduced the infection rate by approximately 5%-10% in industrialized countries, and universal mass vaccination (UMV) for children aged > 1 year has been shown to be beneficial in developing countries in Latin America in terms of declining endemicity.9 In Mexico, previously considered a high endemic region for HAV infection, UMV is not mandatory and sanitary improvements have resulted in a reduction in acute HAV cases. Thus, it has been proposed that Mexico is currently in transition for infection. However, diversity in environmental and economic conditions in distinct geographical regions of the country should be taken into account to better characterize HAV burden, particularly in regions associated with the lowest levels of sanitation, where poverty and a shortage in health services prevail.
Hav endemicity and risk groupsThe global spread of HAV infection has been assessed by monitoring overall and age-specific prevalence which enables an indirect measurement of incidence rates. Overall prevalence has been classified as high (> 50% of population), intermediate (15-50%) and low levels of endemicity (< 15%) based on the detection of anti-HAV IgG antibodies. High endemicity of HAV is found in countries with poor sanitary and socioeconomic conditions, where infection typically occurs early childhood. Intermediate endemicity of HAV is typically found in countries transitioning from a low socioeconomic status to improved housing and hygienic conditions and in segments of the middle-class population. In such countries, the pediatric population may escape HAV infection in early childhood. As a result, older children and young adults become susceptible to HAV infections during outbreaks. In countries with low HAV endemicity, the risk of acquiring HAV infection is low. Currently, a new classification of endemicity is emerging worldwide based on the reported incidence of confirmed acute HAV cases. Thus, endemicity to HAV may also be classified as very low, with an estimated incidence of 5 cases/105: low, 5-15 cases/105; intermediate, 15-150 cases/105 and high > 150 cases/105.10
Epidemiological risk groups include populations of low socioeconomic status living under crowed conditions; households that come into contact with infected individuals; children visiting daycare centers; men who have sex with men (MSM); intravenous drugs users; patients with liver disease; food handlers; and patients with bloodclotting disorders. However, the source of HAV infection remains unidentified in > 50% of cases.11 International travel from countries with low endemicity to areas with intermediate or high endemicity has been extensively documented as a risk factor for infection. For instance, a higher frequency of HAV cases documented in the United States (USA) has been found in those states that are adjacent to Mexico. From 2000 to 2009, a total of 1,437 cases of acute hepatitis A were reported from sites on the border between Mexico and USA, and cross-border travel during the incubation period is common among acute viral hepatitis cases in both countries.12
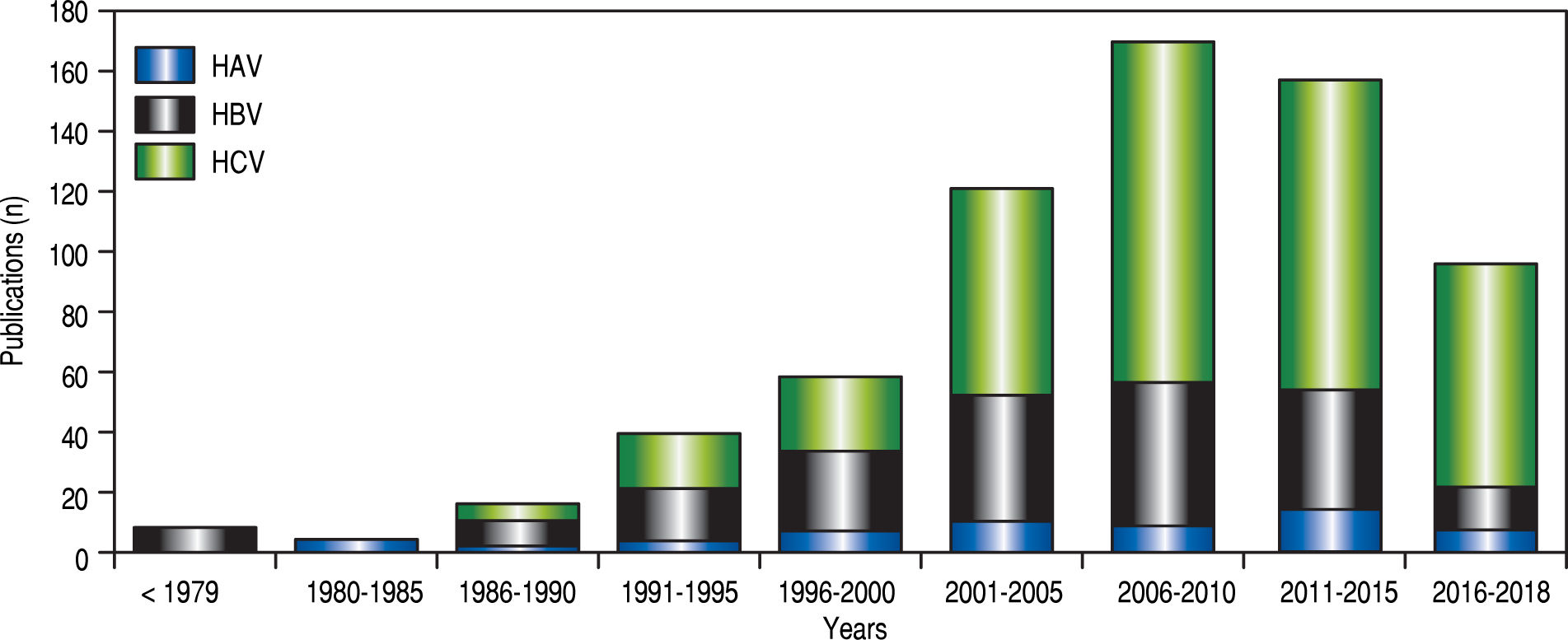
HAV burden in Mexico: comparison between government and public sourcesIn contrast to the potential for developing a chronic disease from hepatitis B and C viral infections (HBV, HCV), HAV infections are self-limiting in nature. Over the past three decades, progress in the study of HAV infection has been scarce. This is illustrated by the limited number of HAV-related scientific reports regarding to the number of HBV and HCV studies in Mexico (Figure 1). The first scientific HAV-related description in Mexico was published in 1982 and revealed a high seroprevalence of antibodies anti-HAV in samples from children from the 1970’s.13 Since then, information about the epidemiological status of HAV in Mexico from a scientific perspective has been limited. Notably, the increased number of HAV-related reports found during 2011 to 2015 denotes interest in the study of the immune-pathogenesis associated with the infection (Figure 1). However, information relative to risk groups, viral transmission and HAV current burden is still scarce.
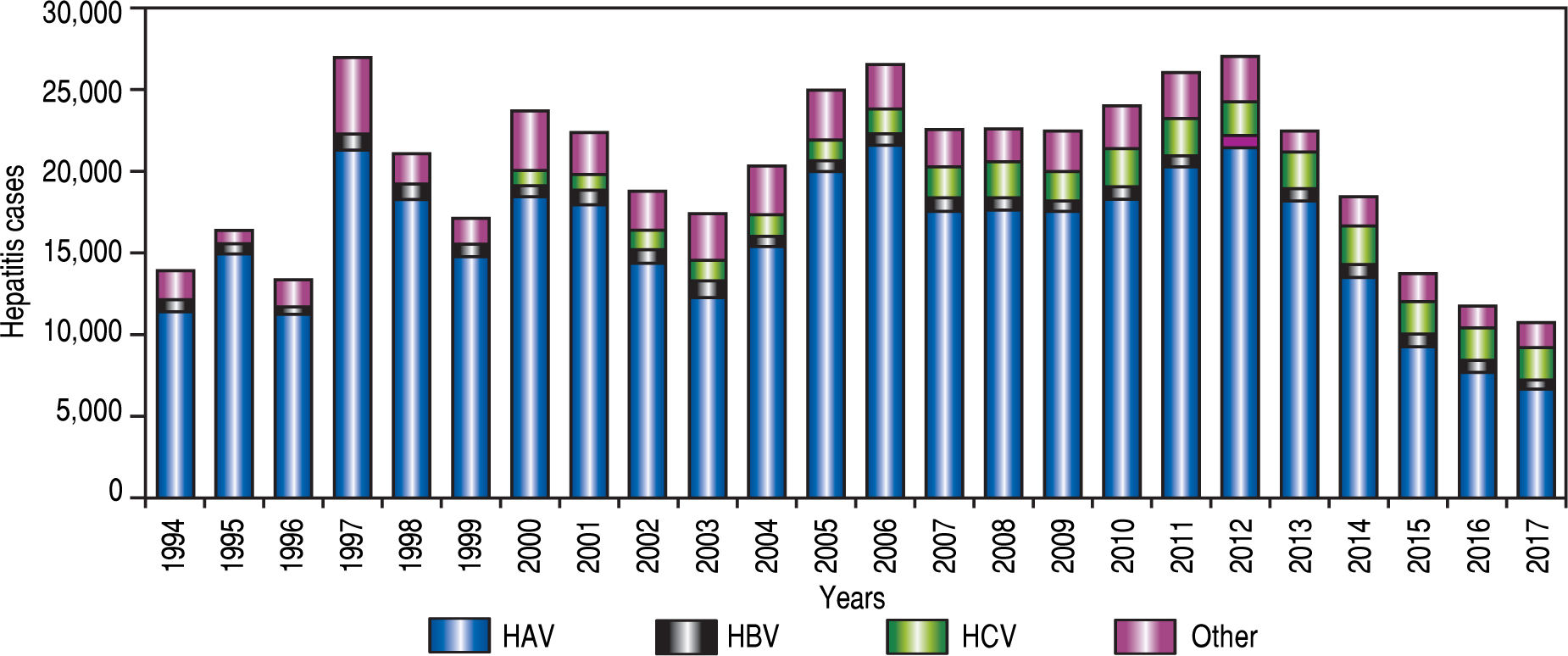
The local government health agency (Secretarfa de Salud, SSA in Spanish), the agency responsible for statistics of infectious disease in Mexico started to report cases of hepatitis associated with HAV infection in 1994 (Figure 2). According to the National System for Epidemiological Surveilleance (Sistema Unico para la Investigation Epidemiologica, SUIVE in Spanish) by the SSA, from 1994 to 2017 a total of483,907 viral hepatitis cases were registered. From those, a 78.4% (379,261 cases) corresponded to HAV infection, followed by 6.5% (31,370 cases) HCV infections, 3.7% (17 857 cases) HBV infections and 11.4% (55,419 cases) viral hepatitis without etiology detected14,15 (Figure 2). In the early 1980’s, according to the frequency of HAV infection reported by scientific publications, Mexico was considered a region of high endemicity for type A viral hepatitis. In Mexico, 90% of children between 1-5 years old were positive for anti-HAV IgG antibodies, indicating high exposure to the virus early on.13 This is in agreement with a study in 2000, where a seroepidemiological analysis conducted in five countries (Venezuela, Mexico, Dominican Republic, Chile and Brazil) from Latin America revealed that the seroprevalence of antibodies to HAV was highest in Mexico and the Dominican Republic.16 However, a reduction in the seroprevalence in children at age of 8 years old was reported in Mexico in 2008 showing 51% of IgG and 13% IgM antibodies anti-HAV.17-18
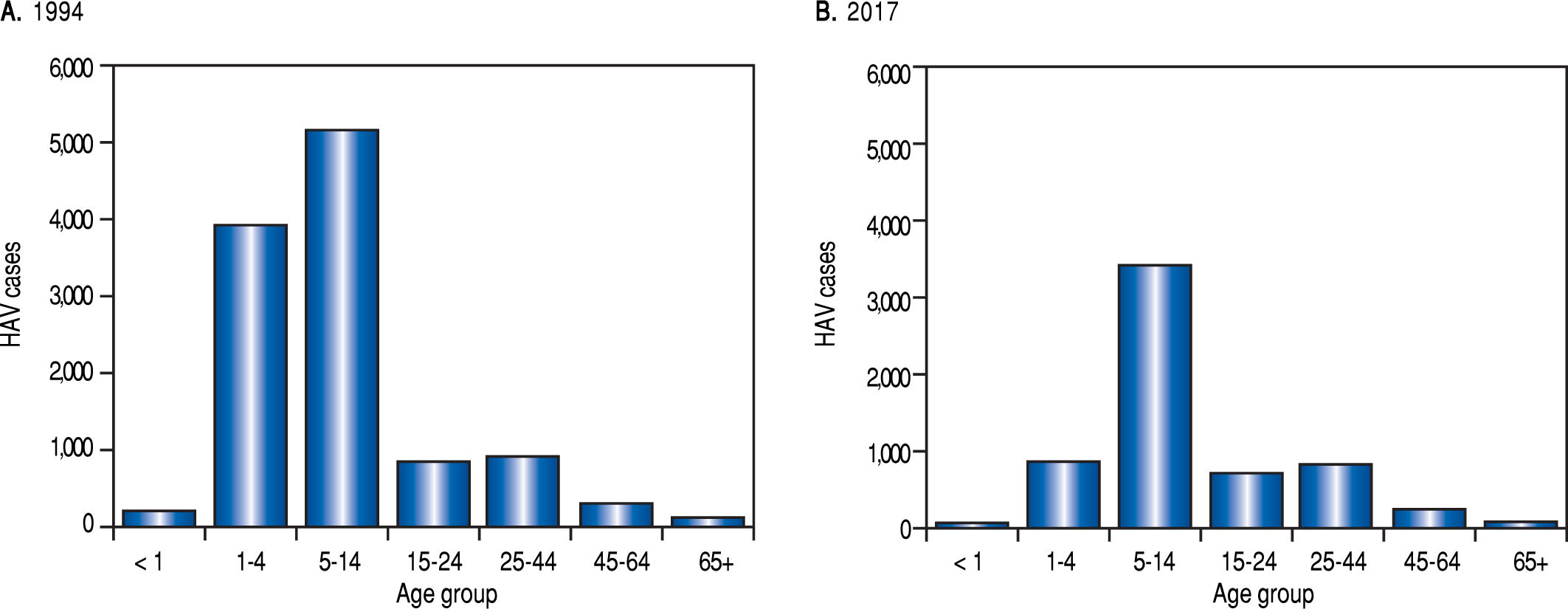
Although data from the SSA reveal that HAV epidemiology varies through the years. For instance a high incidence of HAV was found in 1997 and 2005 probably as a consequence of Pauline and Wilma hurricanes. In general, a reduction in the incidence of hepatitis related to type A hepatitis virus has been reported by the SSA from 1994 to 2017 (Figure 2). Moreover, the SSA data reveal a trend towards a reduction in HAV incidence and infection as a cause of hepatitis in younger individuals. However, the data indicate that the pediatric population in Mexico is still the most prone to be infected with this virus (Figure 3).
Viral hepatitis in the Mexican population (1994 vs. 2017): age groups. Total HAV infection data from the National System for Epidemiological Surveilleance (SUIVE) by the SSA, between 1994 to 2017 were collected and classified by age group. A. Represents HAV cases classified by age group in 1994. B. Represents to HAV cases classified by age group in 2017.
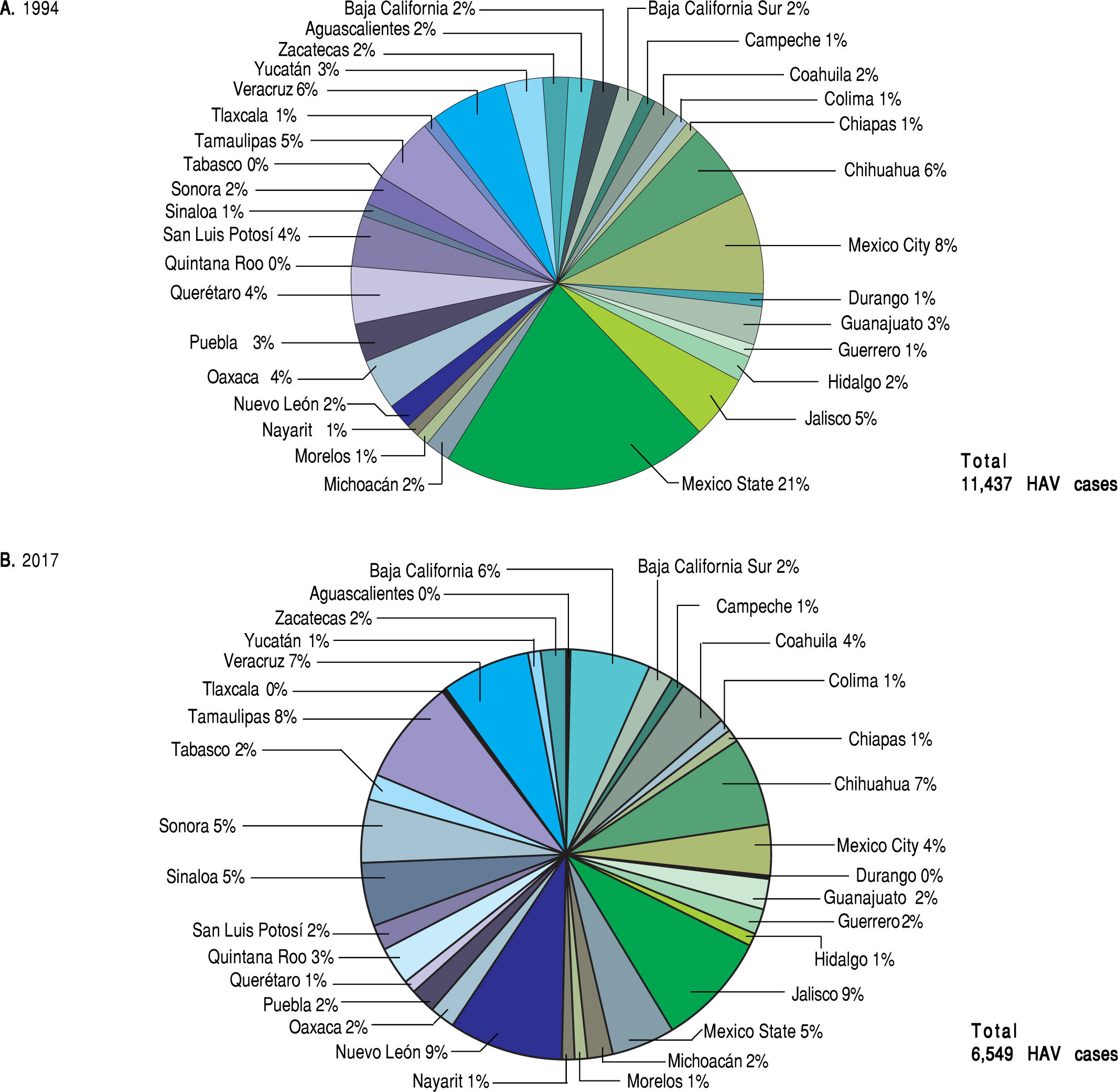
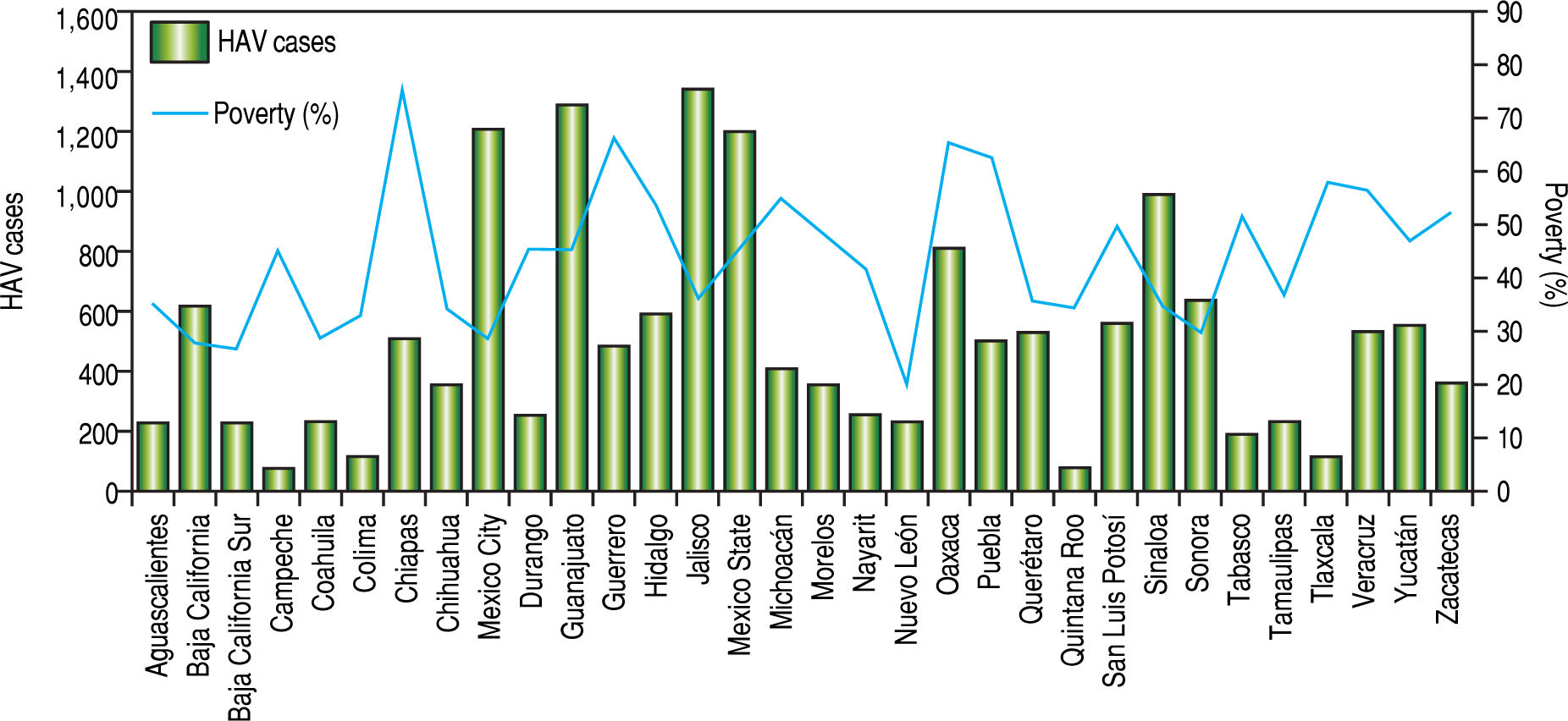
The analysis of the SSA data conducted on the basis of distinct geographic regions in Mexico contradicts the documented relationship between the highest prevalence of HAV-infection and the lowest socio-economic status. In 1994 and 2017, most of the reported HAV-cases by the SSA were found in highly industrialized states in Mexico, including Mexico state and Mexico City in 1994 and Nuevo Leon and Jalisco in 2017. In contrast, those regions associated with low economic status, including Oaxaca and Chiapas located in south Mexico, showed the lowest frequency of hepatitis related to HAV infection (Figure 4). Despite the lower number of HAV cases found in the most marginalized states of Mexico, as denoted in figure 5,14.15.19 it is plausible that deficient health services still prevail in these regions. This may be a limitation in terms of detection and notification of HAV cases. Additionally, is important to take into account that many infected children are asymptomatic and usually do not have jaundice. Thus, they can only be identified with viral detection and liver function tests. Moreover, we recently reported an unexpected high frequency of anti-HAV and anti-hepatitis E virus (HEV) IgM antibodies in children from west Mexico exhibiting acute hepatitis.20 The detection rate of HEV-RNA was 17% in coinfected samples,21 and supports the need to screen for type A and E viral content since hepatitis A is often indistinguishable from liver disease caused by HEV.
Prevalence of HAVinfections in the states from Mexico (1994 vs. 2017). Total HAV infections data from the National System for Epidemiological Surveilleance (SUIVE) by the SSA in 1994 and 2017 were collected and classified in HAV-infections by each state from Mexico, A. Represents HAV infection distribution by state in 1994; a total of 9700 HAV cases were reported. B. Represents HAV infection distribution by state in 2017; a total of 4636 HAV cases were reported.
Prevalence of HAV infections relative to poverty level in states from Mexico. Iota! HAV infections data from the National System for Epidemiological Surveilleance (SUIVE) by the SSA from 2008 to 2016 were collected and classified in HAV infections in each state from Mexico. The bars represent the total average of HAV infections reported every two years from 2008 to 2016 by state. The percentages of poverty from National Council for the Evaluation of Social Development Policy (Consejo National de Evaluation de la Polftica de Desarrollo Social, CONEVAL in Spanish) by state, taking into account the total population by state from 2008 to 2016, were collected. The line represents the percentage of poverty.
It has been accepted that because infection is mostly asymptomatic in children, low-income areas with high incidence rates usually have a low burden of disease. Thus, HAV infection may be underestimated in the country. This is in agreement with scientific reports showing a high frequency of HAV mainly in children.22-28 Furthermore, a study of4907 sera to detect anti-HAV antibodies estimated that in 2007, approximately 78.7 million Mexicans were infected; the risk factors associated with the infection in that time included childhood, poverty associated with limited access to sanitary services (clean water) and living in rural communities of south Mexico.29 Since then, sanitary improvements and vaccine campains are some of the strategies implemented to resist infection. Unfortunately, in the most marginalized areas of Mexico, deficient sanity and limited health systems prevent efficient vaccine distribution. Therefore, the infection still prevails in these geographical areas.
Hepatitis A virus has been described as one of the water pollutants in coastal areas of Baja California, Mexico, and California, USA.30 To date, the only molecular characterization of HAV in Mexico (genotype I) resulted from its detection in contaminated water from Mazatlan and Altata, Mexico.31 In spite of virus dissemination through water, virus transmission has been modeled as a function of setting specific access to safe water, and projections of HAV outcome in Mexico to 2050 have been conducted. From this analysis, assuming improvement in water quality and no introduction of a universal vaccination program over the project period, Mexico is expected to experience a decrease in HAV incidence rates without a substantial decrease in the incidence of symptomatic HAV infections. The prediction supports an increase in the mean age of HAV symptomatic cases that shift from childhood to early adulthood.32 This information should be taken into account in public health policies.
Universal massive vaccination against HAV: the resultsThe vaccine against HAV was introduced in 1995 in the USA.33 Since then, several HAV vaccines containing attenuated HAV have been developed, and two types of HAV vaccines are currently used worldwide (formalde-hyde-inactivated and live-attenuated HAV vaccines) and result in a nearly 100% of people develop protective levels of antibodies to the virus within 1 month after injection of a single dose of vaccine. World Health Organization (WHO) recommends UMV against HAV in national immunization schedules for children aged > 1 year, if justified on the basis of acute HAV incidence, declining endemicity from high to intermediate and cost-effectiveness.10
The implementation of this WHO recommendation has resulted in a decline in acute hepatitis associated with HAV in several countries. Studies in Argentina, Belgium, China, Israel, Panama and USA have provided data on the incidence of acute hepatitis A before the introduction of UMV showing percentages of reduction of HAV incidence from 88% to 96% and persistence of antibodies against the virus 5 years later.10 This reduction in viral incidence was independent of the brand of the HAV vaccine used in the national programs, the number of given doses and the target age at first vaccination. In addition, indirect effects of vaccine coverage including: reduction in the number of cases of fulminant hepatitis, declining in the number of outbreak-related acute hepatitis A cases reported, reduction in the age-adjusted hepatitis A-mortality rate and reduction in the hospitalization rates associated with the infection were observed.9 In Argentina, for instance, no case of fulminant hepatitis associated with HAV infection was reported after UMV, and a decline in acute hepatitis A incidence was seen in all age groups, including in children too young to be vaccinated when the UMV programs were introduced in 2005.34
The same decline was found in Panama after the introduction of a UMV national program in 2007,35 and similar reduction in hepatitis A-associated hospitalization rates was observed in nonvaccinated age groups in the USA.36 Moreover, single-dose universal vaccination for children aged two years was introduced in Brazil in 2014, which resulted in a high rate of anti-virus positivity in the short term.37
HAV vaccination in MexicoIn Mexico, four HAV vaccines are available: HAVRIX (GlaxoSmithKline), VAQTA (Schering Plow), AVAXIM (Sanofi Aventis) and TWINRIX (GlaxoSmithKline). They are approved by the Federal Commission for Protection Against Health Risks (Comision Federal para la Proteccion contra Riesgos Sanitarios, COFEPRIS in Spanish).38 However, the HAV vaccine is only available in a few specialized vaccinations centers supported for the Mexican government to be administered for free or can be acquired in private consultations.39
The HAV vaccine in Mexico was recommended to be used in risk populations in 2008; however, it was not until 2013 that single-dosage HAV vaccine was authorized to be used in children > 1 year old in childcare centers.40 This action resulted in a decreased frequency of HAV infections in the following years (Figure 2). Indeed, this recommendation is in agreement with the WHO declaration that the vaccination against hepatitis A should be part of a comprehensive plan for the prevention and control of viral hepatitis and should be included in regular childhood immunizations programs. However, HAV vaccination is not mandatory in Mexico.
National immunization programs should consider the inclusion of HAV vaccines in the immunization schedules. This option seems to be comparable in terms of prevention and effectiveness and is supported by a model study of dynamic transmission that estimated the potential impact of universal infant HAV vaccination in Mexico using two doses of HAVRIX on the incidence of all HAV infections (symptomatic and asymptomatic). The results of this model indicate that universal HAV vaccination in children reduces the cumulative incidence of all HAV cases over a 25-year time window compared with no vaccination and shows a 70% first-dose coverage and 85% second-dose coverage.41 It is important to take into account that a deficient UMV against HAV may also result in a growing number of susceptible adults as reported in the USA, where outbreaks continue to occur as result of lower hepatitis A immunization rates than other vaccines.42 Thus, health policies should ensure that no child outgrows the pediatric practice without being vaccinated.
The worldwide immunization efforts on HAV control have continued. In June 2016, 16 countries (including 6 countries in the American region, 3 in the eastern Mediterranean region, 4 in the European region and 3 in the western Pacific region) included hepatitis A vaccine in the routine immunization of children. Currently, the WHO reports that 10 countries in the American region (Argentina, Brazil, Chile, Colombia, Honduras, Panama, Paraguay, Uruguay, USA and Mexico) use HAV vaccine in children and risk groups.43 However, there are limited guidelines and regulatory mechanisms for the study of highly dynamic diseases that impact public health, such as HAV. The use of HAV vaccine in each country has special indications and standard guidelines should be implemented with the aim of standardizing vaccination globally.
RemarksThe WHO recommends the eradication of hepatitis by 2030. Thus, joined efforts are required to assess and better understand the disease burden due to HAV, particularly in those regions with the lowest sanitary conditions and where people are at a higher risk for infection. With the mission of improving the diagnosis and general management of HAV infections in Mexico and the ultimate goal of limiting the spread of this virus, the following recommendations can be followed:
- •
Serological diagnosis in identified high-risk populations for contracting the infection is priority. Viral detection is needed and allows to discriminate acute cases related to HAV relative to those associated with other viruses (for instance HEV).
- •
Careful scrutiny of the virus distribution is required to support handling strategies of the disease, to define treatment and to prevent potential outbreaks. A detailed guideline for following cases in endemic regions needs to be developed to contain the virus in emergency situations.
- •
The origin and transmission of HAV infections in Mexico need to be identified. The systemic identification of risk represented by water, particularly in areas considered to have poor health conditions and deficient treatment of water, is required. The consumption of contaminated fresh produce represents a risk of public health. Appropriate surveillance systems are required to adopt risk management practices for reducing the likelihood of contamination.
- •
Close monitoring of the infection and periodically undertaking cost-effectiveness analyses of immunization strategies are required, considering that the creation of a complete immunization schedule can reduce the medical and social costs in terms of new infections and outbreaks.
- •
It is imperative to increase awareness for HAV-associated diseases as part of health teaching programs for the general population, particularly in those regions where the infection prevails.
In Mexico, HAV infection is the first causative agent for viral hepatitis. Although improvement in health conditions in the country has reduced the incidence of HAV infection, Mexico is a transitional region for this infection. A detailed analysis of the HAV epidemiologic status, particularly in those regions of the country with the highest levels of poverty and deficient access to public health services is required.
Abbreviations- •
ALF: Acute liver failure.
- •
COFEPRIS: Comision Federal para la Protection contra Riesgos Sanitarios.
- •
CONEVAL: Consej o Nacional de Evaluacion de la Polftica de Desarrollo Social.
- •
HAV: Hepatitis A virus.
- •
HBV: Hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
HEV: Hepatitis E virus.
- •
MSM: Men who have sex with men.
- •
SSA: Secretarfa de Salud.
- •
SUIVE: Sistema Unico para la Investigation Epidemiologica.
- •
UMV: Universal mass vaccination.
- •
USA: United States.
- •
WHO: World Health Organization.
No competing financial interests exist.
AcknowledgementsThis work was funded by a grant from the Consejo Nacional de Ciencia y Tecnologfa (CONACYT) No. 239470 to NAF. OVS was supported by a Ph.D. Scholarship from the CONACYT.