The effect of interferon alfa against hepatitis C virus has been well documented. However, clinical efficacy is low due to the short interferon residence in the body. To prolong half-life, interferon molecules have been bound to the biologically inert polymer, polyethyleneglycol. Pegylated interferons exhibit a longer residence time with an improved clinical efficacy, although the rate of therapeutic failure is still important. Addition of ribavirin to interferon, either pegylated or not, significantly increases efficacy. Therefore, the combination of a pegylated interferon with ribavirin has become the standard treatment of chronic hepatitis C. As the efficacy and safety of such combinations are not yet optimal, different drugs, including other types of long-acting interferon’s and ribavirin analogs, are presently been investigated.
In 1957, Issacs and Lindenmann1 reported the existence of a factor that interfered with the replication of the influenza virus, coined “interferon”. This factor was not fully purified until 1978.2 It is now known that interferon’s (IFNs) are a family of natural proteins produced by the immune system in response to a challenge by a foreign agent. IFNs are classified according to their type of cell surface receptors. Type 1 IFNs bind to the α/β receptor, type 2 IFNs bind to the γ receptor, and type 3 IFNs bind to a λ receptor. Type 1 IFNs are subdivided into 4 subtypes: IFN α, IFN β, IFN ω, and IFN τ. There are approximately 12 functional subtypes of IFN α. Type 1 IFNs, are produced in direct response to viral infections.3-5
IFN α binds to a receptor expressed on the surface of the target cell, which leads to the activation of the Jak-STAT signaling pathway, leading to the formation of interferon-stimulated gene factor 3 (ISGF3). ISGF3 then binds to the IFN-stimulated response element (ISRE) of cellular genes, known as IFN-stimulated genes (ISGs). This leads to the expression and synthesis of ISG products that produce the primary antiviral action. Thus, IFN does not exhibit a direct antiviral action, but induces gene products which, in turn, act against the virus.4,6
IFN a contains 166 amino acids. Eighty-five of these amino acids are conserved in the known IFNα subtypes. There are three approved forms of INFα for the treatment of chronic hepatitis C (CHC). IFN α-2a and IFN a-2b are structurally very similar to each other, differing in only a single amino acid. Consensus interferon (CIFN), which has been genetically modified to enhance its efficacy, differs in 18 amino acids with regards to IFN α-2a, and in 19 amino acid differences with respect to IFN α-2b.7,8
Treatment of what was called chronic non A, non B (NANB) hepatitis with IFN α started in 1986,9 with results focusing mainly in alanine transamina-se (ALT) reduction. Later on, when hepatitis C was identified, further trials evaluated the efficacy of re-combinant IFN α, showing an important rate of ALT normalization, but also high relapse rates. When testing for RNA-HCV levels, these were undetectable only in 15-20% of IFN-treated patients.10-12 Addition of ribavirin to IFN therapy allowed levels of response to reach 38% of treated patients.13,14
It should be mentioned that the standard goal of antiviral therapy is considered as undetectable levels of RNA-HCV six months after end of treatment. This end-point is known as sustained virological response (SVR).12-14
Pegylated InterferonsIFN treatment yields a low level of response because, being a protein, it is rapidly cleaved by pro-teolitic enzymes. One of the strategies to prolong protein residence in the organism is to protect them by binding to a polyethylene glycol (PEG) chain.15,16 This process is known as pegylation, PEG being a biologically inactive polymer. Depending on the type of PEG chain’ linear or branched’ the protein phar-macokinetic profile can be modulated. The choice of the PEG chain is critical. Small PEG chains produce little change in pharmacokinetics, whereas very large PEG chains result in lack of efficacy, due to an allosteric impediment which prevents binding to cell receptors. Hence, pegylation is not an easy process.
Currently, they are two licensed pegylated interferons to treat hepatitis C.17-20 Their properties are listed in Table 1.
The difference between IFN α2a and IFN α2b is just one amino acid. Notwithstanding, the technology used to prepare the pegylated versions of these molecules exhibits important differences21-23 (Table 1). Pegylated Interferon α2b exhibits a linear PEG moiety with a molecular weight of 12 Kd and an unstable urethane bond. On the other hand, pegylated Interferon α2a has a heavy branched PEG chain of 40 Kd of molecular weight, with a stable amide bond. As summarized in Table 2, these variations in pegylation technology are translated in different pharmacokinetic properties.
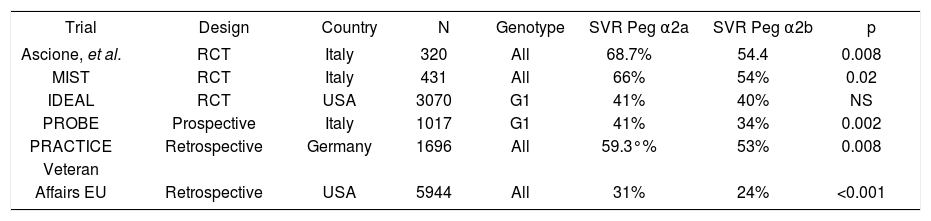
Pegylated IFNs exhibit a significantly higher efficacy compared to non-pegylated IFN, as a result of a prolonged half-life.21-27 Initial comparative showed that both types of PEG IFNs were equally effective. However, further trials suggest a higher efficacy for PEG-IFN α2a,28-33 although there is a high variability in the reported outcomes (Table 3).
| Trial | Design | Country | N | Genotype | SVR Peg α2a | SVR Peg α2b | p |
|---|---|---|---|---|---|---|---|
| Ascione, et al. | RCT | Italy | 320 | All | 68.7% | 54.4 | 0.008 |
| MIST | RCT | Italy | 431 | All | 66% | 54% | 0.02 |
| IDEAL | RCT | USA | 3070 | G1 | 41% | 40% | NS |
| PROBE | Prospective | Italy | 1017 | G1 | 41% | 34% | 0.002 |
| PRACTICE | Retrospective | Germany | 1696 | All | 59.3°% | 53% | 0.008 |
| Veteran | |||||||
| Affairs EU | Retrospective | USA | 5944 | All | 31% | 24% | <0.001 |
PEG IFNs improve efficacy, but the rate of therapeutic failure is still high.30,33 Therefore, new long-acting IFNs have been developed for the treatment of chronic hepatitis C. Albinterferon α2b is a single polypeptide molecule that combines the therapeutic activity of interferon alfa with the long half-life of human serum albumin. It can be anticipated that this new molecule will be soon approved in the United States for the treatment of hepatitis C.34,36
Ribavirin and Ribavirin AnalogsThe mechanism of action of ribavirin has not yet been completely elucidated, although it is clear that it improves interferon efficacy. Proposed mechanisms of actions includes direct antiviral activity, inhibition of inosine-monophosphate-dehydrogenase (IMPDH) leading to depletion of intracellular guanosine triphos-phate (GTP) pools, immunomodulation and mutations of the viral genome.37 Clinical trials have definitely demonstrated that ribavirin improves antiviral efficacy of both, pegylated and non-pegylated IFNs at all stages of the treatment of hepatitis C. Unfortunately, ribavirin limiting factor has been its toxicity, mainly its haematological effects.38-39
Ribavirin-like drugs have been developed in order to diminish anaemia, but the results have not been encouraging. The fist analog to be investigated was levovirin, which appeared to be significantly less effective compared with ribavirin.38 Taribavirin, previously known as viramidin, has also been studied. It showed less haematological toxicity, but also a lower efficacy when compared with ribavirin. However, it appears that taribarin optimal dose has not yet been determined. Hence, current trials are focusing in adjusting the dose of this new ribavirin-like drug to determine its actual therapeutic poten-tial.40,41 At present, ribavirin is still the drug of choice to combine with all sorts of IFNs.42
Place in Therapy of the Different InterferonsSince PEG IFN began being used in the treatment of viral hepatitis C, conventional IFN α utilization has importantly decreased. Currently, non-pegylated IFN α is indicated only for some hepatitis C genotype 2 and 3 cases. Consensus in-terferon also has limited indications at present, showing some promising results in treating previous non responders as well as those patients who do not tolerate PEG IFN therapy.43-44 Increasing PEG IFNs doses had being assayed to improve efficacy. However, the available results have not been conclusive, and thus the actual benefit of higher doses has not been established. Treatment duration and different response predictors are presently been extensively studied, as current guidelines point to a more personalized approach.33 Ribavirin dose can be increased to improve SVR, but hematological toxicity is often limiting. Present guidelines with PEG IFNs and ribavirin are based on genotypes, viral load and early predictors of response, allowing clinicians to establish treatment duration and dosing according to patient characteristics. The rate of therapeutic failure, however, still remains significant.30,32
Due to the still important number of non-responders to the PEG IFN ribavirin combination strategy, further advances in hepatitis C therapy rely on new molecules and combinations. NS3/4A protease and NS5B polymerase inhibitors are presently been studied intensively. New long-acting interferon’s, such as albinterferon α2b, are also been extensively studied. However, information on its efficacy and safety is not yet complete. Thus, in the short run, PEG IFN and ribavirin will continue to be the cornerstone for treating hepatitis C. Notwithstanding, the new perspectives are already at sight. We are just at the beginning of a new road to travel.