Hepatitis C Virus (HCV) is a blood-borne, hepatotropic RNA virus causing both acute and chronic infection. Chronic HCV infection predisposes individuals to liver fibrosis, cirrhosis and hepatocellular carcinoma. Staging of fibrosis prior to treatment to determine either treatment choice or required follow up, is standard practice. However, this often acts as a barrier to treatment initiation. We sought to validate the hypothesis that those individuals; mono-infected with HCV, ≤35 years of age; with no additional hepatic insult were unlikely to have significant fibrosis.
MethodsWe performed a retrospective analysis of a Hepatitis C Virus database; with collation of relevant basic demographics including age, sex and baseline Transient Elastography measurements pre-treatment. Additionally, we compared the reliability of biochemical fibrosis scores with corresponding transient elastography scores.
ResultsOur results support the hypothesis that those individuals with chronic HCV ≤35 years old, with no additional risk for fibrogenesis did not have significant liver fibrosis within our cohort.
ConclusionPatients ≤35 years old likely do not necessitate fibrosis assessment prior to Direct Acting Antiviral (DAA) treatment in the absence of other significant risk factors for fibrosis. Given the emerging evidence that DAA treatment results in a significant decrease in all-cause mortality and hepatocellular carcinoma development, treatment of those with chronic HCV represents a global priority.
The importance of identification and treatment of hepatitis C populations cannot be overstated, given it is a leading cause of liver disease worldwide [1,2].
According to the World Health Organisation (WHO) global hepatitis report [3], there are 71 million people with chronic HCV. Owing to its inherent viral heterogeneity and frequent mutations; development of an HCV vaccine has remained elusive. However highly effective therapy now opens the possibility cure for all from HCV if the therapy can be delivered.
In the UK, transmission of HCV is predominantly through injection drug use.1 Therefore, those most affected by HCV infection are persons in socially excluded groups including people who inject drugs (PWID). PWIDs are amongst the most stigmatised populations and find it difficult to access healthcare due to services which discriminate against them or don't meet their specialised needs. This makes diagnosis, referral and treatment challenging for them and those providing services.
In patients with progressive hepatitis, approximately 10-20% of patients will develop cirrhosis within 20-30 years, and once cirrhosis develops there is a 1–5% annual risk of HCC and a 3–6% annual risk of hepatic decompensation [1]. When determining1 the rate of disease progression, factors that increase fibrogenesis are alcohol consumption, advancing age at infection (>40years at time of infection), male gender and co-infection with HIV or other hepatotropic viruses and BMI >25 [2,4,5].
Since the common cause of HCV transmission in the UK is injection drug use, younger infected individuals are less likely to have significant liver fibrosis due to the inherent lag in fibrogenesis development. By extrapolation, those under 35 should not have significant liver fibrosis given the short duration from time of infection acquisition. This rationale is dependent on the absence of additional hepatic insults. Within the developed world, both alcohol and metabolic liver disease are important co-factor disease which should be considered. Alcohol consumption appears to be a potent inducer of fibrosis in those with HCV, particularly when drinking to hazardous quantities i.e. >40g/day, with a significant risk of cirrhosis [6].
HCV treatment pathways may be considered as a cascade of care, from diagnosis through to treatment endpoints [4]. The cascade of care maps how infected persons move along a treatment pathway from diagnosis, to engagement in care, treatment and finally, cure. Intrinsic within these cascades are further steps such as referral to HCV care, pre-treatment assessment, treatment monitoring and acquisition of blood samples to confirm sustained viral response. Each of these points represents a potential step at which a patient may disconnect from services [7].
Worryingly, within these pathways a significant proportion of patients often fail to even initiate therapy. Previous research by ourselves, and others has shown that simplifying the treatment pathways can be beneficial in engaging and retaining people in care [8–10]. Absolute consensus surrounding pre-treatment investigations is lacking and there are several steps undertaken prior to commencement of treatment. Guidelines recommend baseline liver fibrosis/cirrhosis assessment but the absolutes of these vary significantly [11–13].
Baseline liver biochemistry is almost universally undertaken comprising of; ALT, AST, GGT, alkaline phosphatase, bilirubin, INR, albumin, gamma-globulins and full blood counts [5]. Some aspects of the care cascade and pre-treatment assessment is a hangover from when interferon containing regimes were the mainstay of treatment. Using current technology, it is possible to make an HCV diagnosis using capillary blood, obviating the need for venepuncture which may be challenging in those most likely to be infected with HCV [14]. However, current strategies for fibrosis staging necessitates venepuncture or an alternative imaging modality, which creates another step in the cascade of care.
Given the changing treatment landscape of HCV with the introduction of direct acting antivirals including pan-genotypic regimes and reduced treatment durations there is an opportunity to re-evaluate the steps in our care cascades and remove defunct steps. Thus, by removing the barrier of fibrosis assessment at point of diagnosis the expectation would be that increased engagement with treatment strategies would ensue. Such evolution would be most welcome in primary care, where, recent work by Whiteley [15] identified a principle concern within primary-care based HCV treatment pathways related to fibrosis assessment. Therefore, clear strategies obviating this risk are necessary.
The simplification of the care pathway is expected to lead to increased flow through the cascade of care, increasing individual health benefits and reducing the pool of infectious people as vectors of onward transmission. Several modelling studies have shown that increased HCV treatment among PWID will reduce prevalence and onward transmission, especially when combined with opioid substitution therapy and high coverage of needle and syringe programmes which has been subject to a Cochrane review [16]. Successful eradication programmes are dependent on a multifaceted approach to this cohort of patients.
We sought to validate the hypothesis that patients who are aged 35 and under; and do not have additional risk factors for liver disease would not have significant fibrosis and do not routinely require fibrosis assessment as part of their treatment strategy. Therefore, it would be possible to treat patients in this category immediately after confirming the diagnosis of chronic HCV infection.
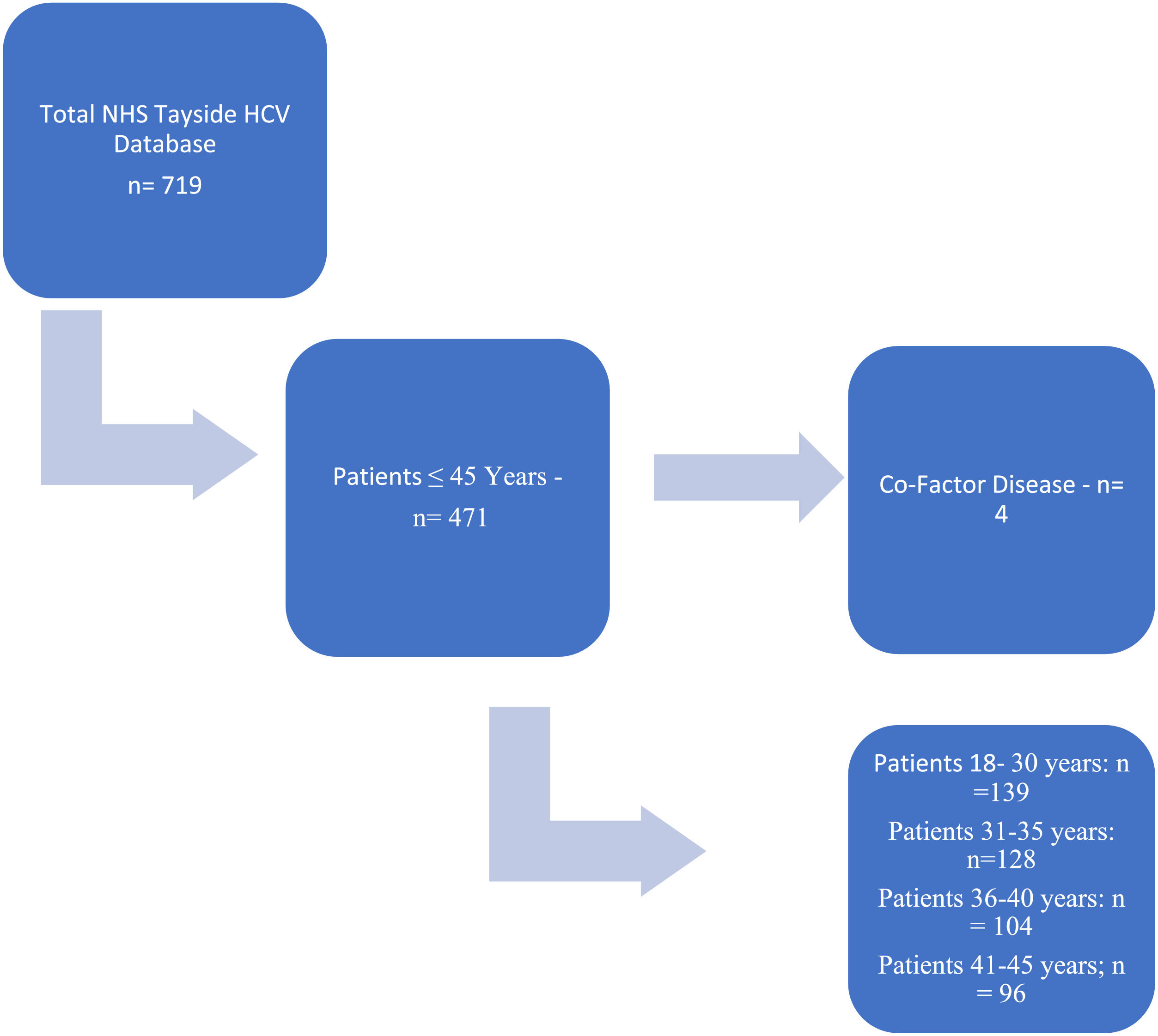
2MethodsThe Tayside HCV clinical database records those patients diagnosed with HCV within NHS Tayside which covers; Dundee, Perth and Kinross, North Fife and Angus (circa ∼450,000 catchment population) from 1998. Fig. 1 outlines the process by which the cohort was determined and subsequently analysed.
A retrospective analysis of the Tayside Hepatitis C dataset was performed with collation of relevant demographics including age, gender, and baseline transient elastography (TE) measurements for those patients to age 45 (see Table 1), spanning from years 2009-2018. Patients were age-stratified according to 4 categories, ≤30 years, 31-35, 36-40, and 41-45 years old and were treatment naïve at time of assessment.
In determining the transient elastography measurement, all patients underwent a Fibroscan © (Echosens, Paris, France) in the supine position, having been fasted for > 4hours. Only the M probe was used for LS measurement, with at least 10 validated measurements with an interquartile range of ≤30% and a success rate of >70% were included. No patients in the cohort were excluded on this basis as all had reproducible results.
While the reproducibility of transient elastography and equivalent METAVIR fibrosis stages has been demonstrated by previous studies, variable cut-off values for each stage of fibrosis have been proposed [12,13]. In this study the following classifications of fibrosis were utilised: F0-1: <7, F2: 7-8.9, F3: 9-11.9 and F4: ≥12 kPa. Previous validation of transient elastography (TE) in HCV has suggested that a cut off of less than 12 kPa excludes significant fibrosis [17].
FIB4 and APRI were assessed using validated cut-off scores to determine the presence of fibrosis (within maximum 90 days of Transient Elastography measurements). Validated low (with high sensitivity) and high (with high specificity) cut-off values used by WHO for detection of fibrosis were 0.5 and 1.5 for APRI and 1.45 and 3.25 for FIB4. A value above the high cut-off suggests a high probability of advanced fibrosis and below the lower cut-off interval intimating a low probability of advanced fibrosis. Overall, for those above the relative cut-off values, we assessed for confounding risk factors; particularly that of hazardous alcohol ingestion, NAFLD (features of metabolic syndrome, significant radiological steatosis or histology where available) and co-infection with other hepatotropic infections.
Elastography results are presented descriptively with mean and relevant ranges. In determining age-related fibrosis association, we used single factor ANOVA. All relevant calculations and statistics were performed using Microsoft Open 365 Office Excel © (Microsoft Corporation).
3Results3.1Transient elastographyUsing the Tayside Hepatitis C database, there were a total of 719 patients who had been tested positive for HCV and undergone TE. The TE results were taken prior to treatment. Initially, we screened patients below the age of 45 and stratified them by age across 5-year intervals. Of a total 719 patients with HCV, 471 of them were below the age of 45 (see Fig. 1). This cohort was the subject of the investigation.
The numbers and distribution between the subgroups of the cohort are described in Table 1. Subgroup analysis demonstrated that 98% of patients below the age of 30 had a Fibroscan score less than 9kPa, with no patients having elastography >12kPa. Within the cohort aged between 31-35 years; 15 patients (11.7%) had Fibroscan results above 9kPa; with only 10 (8%) patients of these patients had transient elastography >12kPa. Examination of these individuals risk factors identified 2 patients in the >9–<12kPa cohort exhibited hazardous alcohol consumption, and 3 further patients with risk factors for NAFLD.
Further, 5 persons within the >12kPa subgroup had a history of hazardous alcohol consumption. Finally, there are 5 persons (1.8%) of the total 267 cohort ≤35 (or 3.9% of those 31-35 years) with significant fibrosis in absence of additional hepatic risk factor ≤35 years old. On an individual basis, only 2 of these 5 individuals had a transient elastography measurement >18kPa.
For completeness, we assessed those persons up to 45 years old. Single factor ANOVA assessment across the groups (following exclusion of those with significant additional fibrosis factors) demonstrates clear age-related elastography increments (F = 7.988457, p = 3.36E-05).
3.2Biochemical fibrosis scoresSeparately, as an additional insight, we looked to determine the utility of biochemical fibrosis markers commonly utilised in HCV assessment in our cohort of individuals who had sequential transient elastography. The distribution of these scores is demonstrated in Fig. 2.
Within the original cohort of patients with HCV under the age of 35 (n=267); there were 134 patients who did not have bloods performed within the required timeframe or had an incomplete dataset.
Of the remaining 133 patients with complete data; 127 patients had an APRI score of under 1.5 (95.5%) and 86 patients had a score under 0.5 (64.7%). 131 patients had a FIB4 score of under 3.25 (98.5%) and 125 patients had a FIB4 score of under 1.45 (94%).
Of the 41 patients with an APRI between 0.5 and 1.5, 4 had a FIB4 score over 1.45. Of the 6 patients with an APRI score over 1.5, 3 had a FIB4 score over 1.45 (50%). A single patient had both an APRI over 1.5 and FIB4 over 3.25; however, they had TE under 9kPa. Further, 7 patients had both a FIB4 over 1.45 and APRI over 0.5 and therefore would require further assessment with elastography. These 7 patients subsequently all had TE of less than 9kPa, as demonstrated in Fig. 3.
4DiscussionGiven the challenge set by the WHO eradication targets, novel approaches in the identification and treatment of HCV are paramount. As suggested, a large proportion of those with HCV are poorly served by fragmented, complex care systems which impacts significantly on engagement and successful treatment. One aspect of improving compliance is to reduce the requisite steps to access and initiate treatment from time of diagnosis, minimizing patient attendances across multiple locations and timepoints [7].
Given the advent of capillary blood for HCV diagnosis, venepuncture is now relatively unnecessary, other than for staging of disease. While routinely utilised in clinical practice, biochemical fibrosis scores have clear limitations. APRI and FIB4 have previously been shown to provide reliable, non-invasive fibrosis scoring in those with hepatitis C, particularly when elastography is not available. Our results show that of 133 patients under 35 years old with HCV and no other co-factors, 7 would have required further fibrosis assessment following biochemical scoring. These 7 patients all had a TE scores excluding significant fibrosis. TE represents an alternative to blood-based fibrosis assessment; however, there is little merit in offering both methods.
Overall, these results support the hypothesis that; patients with chronic HCV less than 35 years old in the absence of co-infectivity with hepatotropic viruses, hazardous alcohol consumption or other liver insult have a negligible risk of significant fibrosis. The chances of missing someone with significant fibrosis is less than 1 in 20, which is markedly lower than the risk of losing someone from a treatment pathway through the addition of requisitel steps, thereby losing any apparent benefit of treatment. No one within the cohort had sufficiently injurious liver fibrosis to impact on choice of treatment, given the safety of DAA regimes even in patients with compensated cirrhosis [18]. Furthermore, the relative benefit of DAA treatment in reducing all-cause mortality and hepatocellular carcinoma development in chronic HCV represents a clear benefit: risk compromise in such an approach to treatment [18]. In extending our assessments to include those patients up to age 45 years, our data supports previous findings that prolonged duration of infection contributes to temporally mediated fibrogenesis in HCV infection.
Therefore, from our results we suggest a modified approach to community HCV treatment. This proposed pathway suggests that following positive screening for anti-HCV antibody; confirmatory testing and concurrent history is acquired by a healthcare professional. This screening would include additional hepatotoxic risk factors including alcohol and metabolic features. This would then allow immediate dispensing arrangements for DAA. Of course, this approach can be modified somewhat to allow for local/national agreements, healthcare infrastructure and relative logistics.
This approach has clear perceived benefit, whereby, the immediate offering of DAAs should improve uptake and completion of treatment given the emerging real-world data. Radley and colleagues [19] demonstrated how incremental SVR could be achieved through such reconciliation of complex pathways. This work identified that those patients randomized to traditional medical care model were significantly less likely to achieve SVR, with progressive drop out at each step within the cascade of care. Depending of the intervention, observed dropout was between 5-59%.
Clearly whilst the numbers demonstrate an increase in overall numbers with increasing fibrosis propensity, most patients do not have advanced disease.
Clearly our study has some limitations in its design, particularly as it is retrospective and there are several individuals whose medical records are incomplete. It is also accepted that we use TE as an de-facto gold-standard for fibrosis assessment, it is however, helpful in the context of those who have had both biochemical and TE performed, given the utility of TE in HCV fibrosis assessment is well described. We do recognise the limitations of transient elastography, however, we have attempted to mitigate this in so far as possible, particularly operator dependence and reproducibility. In those patients without overt risk factors clearly documented, we looked to include them in the statistical assessment, therefore we may indeed underestimate co-factors in these individuals.
Overall, however, the data here represents real-life information in a challenging area of practice, albeit, in one where real change is possible. A similar smaller study was performed in a needle-exchange centre in Stockholm [20], with similar outcomes, however, it would be worthwhile validating these findings across other populations in other geographical regions.
5ConclusionCurrently, standard recommendations advocate liver fibrosis assessment prior to initiation of antiretroviral therapy. We demonstrate that individuals who are mono-infected with HCV, of the age ≤35 years old and do not have additional hepatic insult have a negligible likelihood of having significant fibrosis. Therefore, these patients do not need fibrosis staging and should rather proceed direct to treatment.
Simplification of treatment stratagems as proposed herein should readily facilitate and improve treatment engagement, and consequent completion rates through minimising steps in the HCV cascade of care. It is through novel, innovative approaches to age-old challenges that the realisation of global HCV elimination by 2030 may be achieved.
Authors contributionsPNB, PC, EMR and AA were involved in basic data acquisition and analysis. PNB, PC and EMR drafted manuscript and undertook critical appraisal of document. JFD was responsible for original research design and provided input in critical manuscript writing and editing. All authors approve final draft of manuscript.
Prof John F Dillon is guarantor of this article