Four regimens are recommended for treating hepatitis C (HCV) genotype 1 infection. Study aims were to (1) compare frequencies of contraindicated drug interactions (XDDIs) when each HCV regimen is added to medication profiles of HCV-monoinfected patients, (2) quantify the proportion of patients with XDDIs to all four regimens and (3) determine covariates independently associated with having a XDDI to all four regimens.
Materials and methodsA cross-sectional study was performed within Upstate New York Veterans Healthcare Administration. Inclusion criteria: (1) age ≥18 years, (2) HCV monoinfection and (3) available medication list. Data extracted were: demographics, comorbidities, and medication list. Primary outcome was XDDIs involving patient's home medications and each HCV regimen. University of Liverpool drug interaction website was used to define XDDIs. Two-way comparisons of regimens were performed using McNemar's test where p<0.0083 was considered statistically significant. Multivariate regression analyses were performed to determine predictors.
ResultsOf the 4047 subjects, mean±standard deviation age was 59.8±7.6. Median (interquartile range) number of medications used was 7 [4–11]. Frequencies of XDDIs after the addition of each regimen ranged from 2.8% to 17.8% and were mostly statistically different from one another. There were 95 (2.3%) patients with XDDIs to all four regimens. Predictors of having XDDIs to all four regimens were ≥6 medications and HCV infection ≥10 years.
ConclusionThe frequencies of XDDIs varied between HCV regimens. Number of medications and duration of HCV infection were predictors of having XDDIs to all four regimens.
Currently, there are several therapeutic options available to treat chronic genotype 1 hepatitis C virus (HCV) infection. These include glecaprevir/pibrentasvir (GLE/PIB), grazoprevir/elbasvir (GZR/EBR), ledipasvir/sofosbuvir (LDV/SOF) and sofosbuvir/velpatasvir (SOF/VEL) [1–5]. Each has demonstrated impressive rates of sustained virologic response in treatment recipients with low toxicity [6–10]. While the safety and efficacy appear to be similar, some characteristics distinguishing these four regimens are not entirely clear, potentially complicating treatment selection by clinicians. One potential difference is the occurrence of contraindicated drug–drug interactions (XDDIs) with patients’ medications used to treat other medical conditions. In general, patients with chronic HCV infection are generally advanced in age and may have developed certain comorbidities necessitating medication therapy [11]. As a result, use of multiple medications simultaneously, otherwise known as polypharmacy, may create a therapeutic dilemma [12,13]. Polypharmacy significantly elevates the risk of experiencing XDDIs and may impact clinical outcomes [14–16].
Limited comparative data exist evaluating the potential risk of XDDIs between GLE/PIB, GZR/EBR, LDV/SOF and SOV/VEL. It is unclear which regimen is safest to administer to HCV-monoinfected patients who have a high probability of XDDIs due to age-related polypharmacy [14]. These issues also have important cost implications. The presence of XDDIs can potentially lead to dangerous health effects [15–17]. In extreme scenarios, mitigation of XDDIs may require utilization of costly additional healthcare resources [18]. Third party payors can attempt to minimize patient harm and contain the potentially unnecessary expenditures by limiting formularies to anti-HCV medications with a low probability of XDDIs.
Additionally, the proportion of patients who have XDDI to all HCV treatment regimens has not been defined. Understanding which patients have XDDIs to all anti-HCV regimens is crucial so appropriate modifications can be made to concomitant medications to facilitate prompt HCV treatment and prevent the consequences of untreated HCV infection [1].
The objectives of this study were to: (1) compare prevalence of contraindicated drug interactions (XDDIs) between GLE/PIB, GZR/EBR, LDV/SOF and SOF/VEL when added to the home medication profiles of patients with HCV monoinfection; (2) quantify the proportion of patients with XDDIs to all four HCV treatment regimens; and (3) determine clinical/demographic risk factors of having XDDIs to all four regimens.
2Materials and methodsThe Institutional Review Board at the Stratton Veterans’ Affairs Medical Center approved this study with a waiver of informed consent.
2.1Study design and populationWe performed a cross-sectional study among HCV monoinfected Veterans receiving care in Upstate New York Veterans’ Healthcare Administration between January 1, 2000 and December 31, 2013.
Inclusion criteria were: (1) age ≥18 years; (2) HCV infection (International Classification of Diseases 9th Revision codes 070.41, 070.44, 070.51, 070.54, 070.70, and 070.71), confirmed by laboratory tests for either HCV RNA or anti-HCV; and (3) availability of medication list. The focus of this study was HCV monoinfection and therefore patients with hepatitis B or HIV coinfection were excluded.
2.2Data collectionData were obtained from computerized medical records. Information abstracted from patients’ medical records included demographics, comorbidities, and most recent medication lists. The full list of covariates extracted from patients’ medical records is described elsewhere [19]. Home medications were initially categorized according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification system [20]. The ATC-categorized medications were subsequently collapsed into therapeutic classes based on distribution of medication use.
2.3Outcome assessmentThe primary outcome was the occurrence of contraindicated drug–drug interactions (XDDIs) with the patient's home medication profile after the addition of each of the four HCV regimens evaluated: GLE/PIB, GZR/EBR, LDV/SOF, and SOF/VEL. The HCV treatment guidelines specifically list the University of Liverpool drug interaction website as a resource to evaluate drug–drug interactions [1,21]. In the present study, this website was used to classify the type of drug–drug interaction (do not coadminister, potential interaction, no interaction expected, no clear data). Drug–drug interactions classified as “do not coadminister” were the only ones recorded and deemed contraindicated. The University of Liverpool drug interaction website only assesses drug–drug interactions where at least one of the medications used in the drug–drug combination is used to treat HCV infection [21], and this study was limited to such interactions. Various XDDIs between non-HCV medications assessed by other drug interaction programs are described elsewhere [12].
2.4Statistical analysesTwo-way comparisons were performed using McNemar's test to assess the frequencies of XDDIs between each of the HCV regimens. Because of the multiple comparisons being made between each of the HCV regimen's prevalence of XDDIs in the bivariate analyses, a p-value of 0.0083, adjusted for multiple comparisons, was used to denote statistical significance (Kappa, κ, =6 possible combinations of comparisons between anti-HCV regimens divided by α of 0.05).
To determine the predictors of having XDDIs to all four HCV treatment regimens, bivariate analyses were performed comparing presence of XDDIs to all regimens with clinical/demographic characteristics. Continuous variables were compared using student's t or Mann–Whitney U tests and categorical variables were compared using chi-square or Fisher's exact tests. Classification and regression tree (CART) analyses were performed to identify thresholds in continuous predictor variables associated with a significantly increased likelihood of having an XDDI to all four HCV treatment regimens when dichotomized around the threshold (i.e. CART identifies 6 as number of medications associated with XDDIs to all four regimens; patients are dichotomized into two groups – those using ≥6 or <6 medications; the percentage of patients with XDDIs to all four regimens differs significantly between those using ≥6 and <6 medications). Variables in the bivariate analyses with an associated p-value <0.25 were eligible for model entry into the multivariable logistic regression analyses. Using a backward, stepwise approach, the most parsimonious model contained variables that were significantly associated with XDDIs to all regimens. The only confounders retained in the model were those that changed the measure of association for the final predictor variables by more than 10%. The exponentiated beta coefficients from the final multivariable model were used to compute the predicted probabilities of having XDDIs to all four HCV treatment regimens when certain covariates were present or absent.
3ResultsOf the 4047 patients evaluated, the majority were male (96.4%). Mean±standard deviation (SD) age was 59.8±7.6 years. Patients were using a median (interquartile range, IQR) of 7 (4–10) medications. The most commonly used medication classes were antihypertensives (77.1%), vitamins/supplements (70.1%), antidepressants (67.9%), and antiplatelet medications (62.9%).
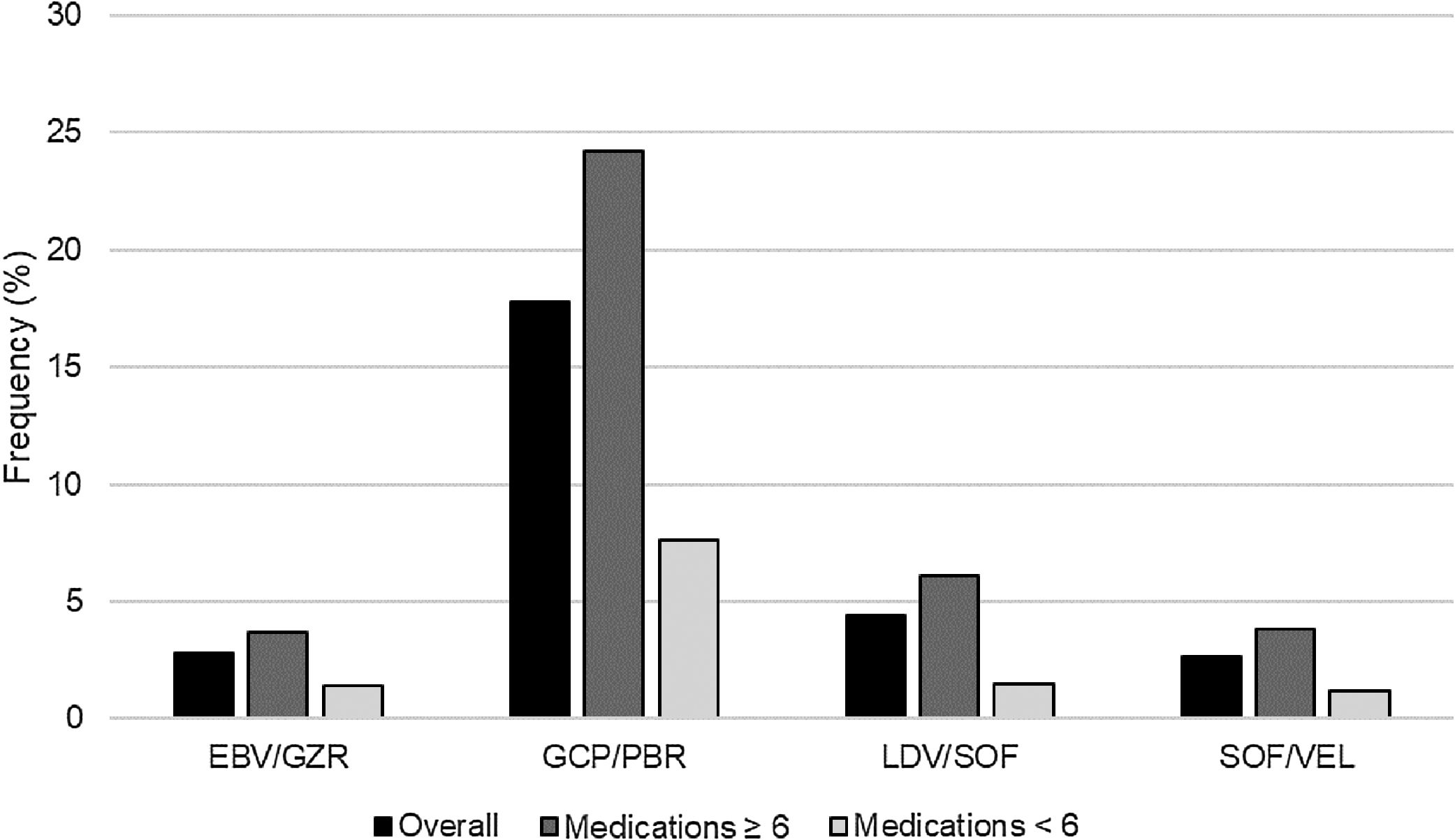
After adding each HCV regimen to the patients’ home medication profiles, there were 814 (20.1%) patients with XDDIs to at least one HCV regimen. The frequencies of XDDIs after the addition of each HCV regimen are in displayed Fig. 1. The prevalence of XDDIs was highest for GLE/PIB (17.8%), followed by LDV/SOF (4.4%), SOF/VEL (2.8%), and EBV/GZR (2.8%). There were significant (p<0.0083) differences in two-way comparisons of the frequency of XDDIs between most pairings of HCV treatment regimens. The only exception to this was the comparison between SOF/VEL and EBV/GZR (p=0.85).
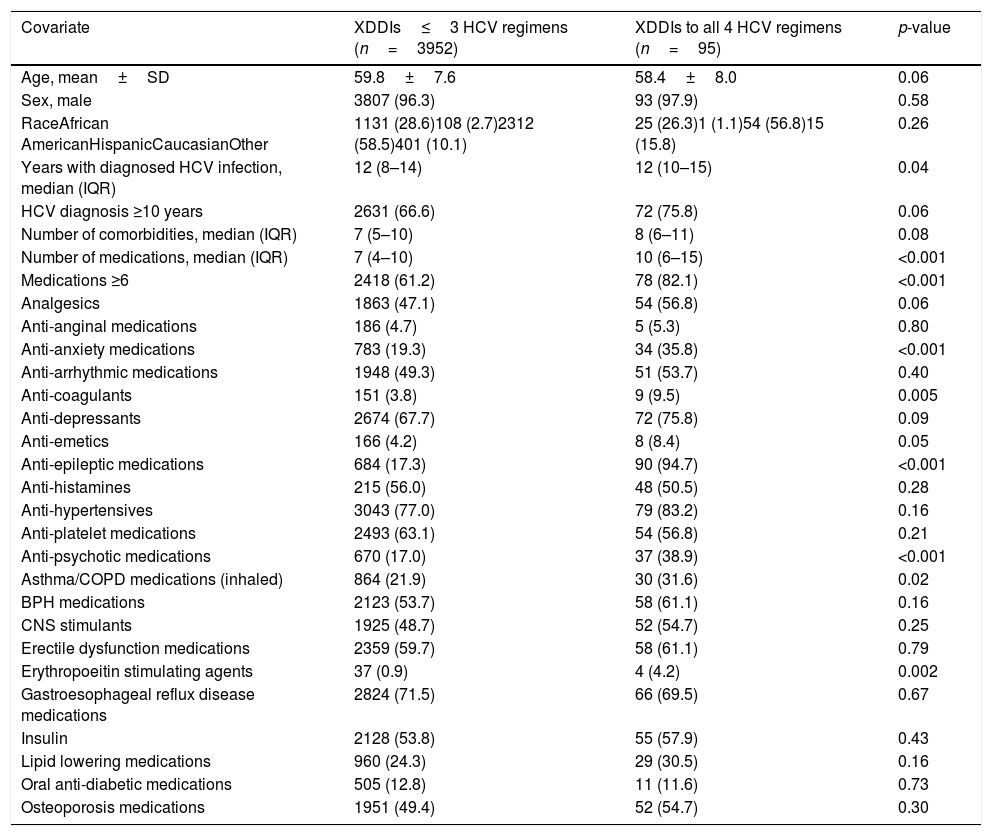
There were 95 (2.3%) patients with XDDIs to all four HCV regimens. Bivariate analyses comparing clinical/demographic characteristics between patients with and without XDDIs to all four HCV regimens is displayed in Table 1. Variables significantly associated with XDDIs to all four HCV regimens were number of medications, use of anti-anxiety medications, anti-coagulants, anti-emetics, anti-epileptic medications, anti-psychotics, asthma/chronic obstructive pulmonary disorder medications, erythropoietin stimulating agents (ESAs) and years with HCV diagnosis. A CART-derived breakpoint was identified for number of concomitant medications associated with an increased risk of having an XDDI to all four regimens. This breakpoint was 6 medications. Patients using ≥6 concomitant medications had a significantly higher prevalence of XDDIs to all four regimens than patients below this threshold (3.1% versus 1.1%, p<0.001). The prevalence of XDDIs after the addition of each HCV regimen to the patients’ home medication profiles, stratified by use of <6 or ≥6 medications, is displayed in Fig. 1. The other CART-derived breakpoint identified was the number of years with chronic HCV infection. The prevalence of XDDIs was significantly higher for patients with an HCV diagnosis of at least 10 years compared to those with a more recent diagnosis <10 years prior (2.7% versus 1.7%, p=0.05).
Bivariate relationship between clinical/demographic covariates and presence of contraindicated drug–drug interactions to all four hepatitis C treatment regimens.
| Covariate | XDDIs≤3 HCV regimens (n=3952) | XDDIs to all 4 HCV regimens (n=95) | p-value |
|---|---|---|---|
| Age, mean±SD | 59.8±7.6 | 58.4±8.0 | 0.06 |
| Sex, male | 3807 (96.3) | 93 (97.9) | 0.58 |
| RaceAfrican AmericanHispanicCaucasianOther | 1131 (28.6)108 (2.7)2312 (58.5)401 (10.1) | 25 (26.3)1 (1.1)54 (56.8)15 (15.8) | 0.26 |
| Years with diagnosed HCV infection, median (IQR) | 12 (8–14) | 12 (10–15) | 0.04 |
| HCV diagnosis ≥10 years | 2631 (66.6) | 72 (75.8) | 0.06 |
| Number of comorbidities, median (IQR) | 7 (5–10) | 8 (6–11) | 0.08 |
| Number of medications, median (IQR) | 7 (4–10) | 10 (6–15) | <0.001 |
| Medications ≥6 | 2418 (61.2) | 78 (82.1) | <0.001 |
| Analgesics | 1863 (47.1) | 54 (56.8) | 0.06 |
| Anti-anginal medications | 186 (4.7) | 5 (5.3) | 0.80 |
| Anti-anxiety medications | 783 (19.3) | 34 (35.8) | <0.001 |
| Anti-arrhythmic medications | 1948 (49.3) | 51 (53.7) | 0.40 |
| Anti-coagulants | 151 (3.8) | 9 (9.5) | 0.005 |
| Anti-depressants | 2674 (67.7) | 72 (75.8) | 0.09 |
| Anti-emetics | 166 (4.2) | 8 (8.4) | 0.05 |
| Anti-epileptic medications | 684 (17.3) | 90 (94.7) | <0.001 |
| Anti-histamines | 215 (56.0) | 48 (50.5) | 0.28 |
| Anti-hypertensives | 3043 (77.0) | 79 (83.2) | 0.16 |
| Anti-platelet medications | 2493 (63.1) | 54 (56.8) | 0.21 |
| Anti-psychotic medications | 670 (17.0) | 37 (38.9) | <0.001 |
| Asthma/COPD medications (inhaled) | 864 (21.9) | 30 (31.6) | 0.02 |
| BPH medications | 2123 (53.7) | 58 (61.1) | 0.16 |
| CNS stimulants | 1925 (48.7) | 52 (54.7) | 0.25 |
| Erectile dysfunction medications | 2359 (59.7) | 58 (61.1) | 0.79 |
| Erythropoeitin stimulating agents | 37 (0.9) | 4 (4.2) | 0.002 |
| Gastroesophageal reflux disease medications | 2824 (71.5) | 66 (69.5) | 0.67 |
| Insulin | 2128 (53.8) | 55 (57.9) | 0.43 |
| Lipid lowering medications | 960 (24.3) | 29 (30.5) | 0.16 |
| Oral anti-diabetic medications | 505 (12.8) | 11 (11.6) | 0.73 |
| Osteoporosis medications | 1951 (49.4) | 52 (54.7) | 0.30 |
All data presented as n (%) unless otherwise indicated.
Legend: BPH – benign prostatic hyperplasia, COPD – chronic obstructive pulmonary disorder, CNS – central nervous system, HCV – hepatits C infection, IQR – interquartile range, SD – standard deviation.
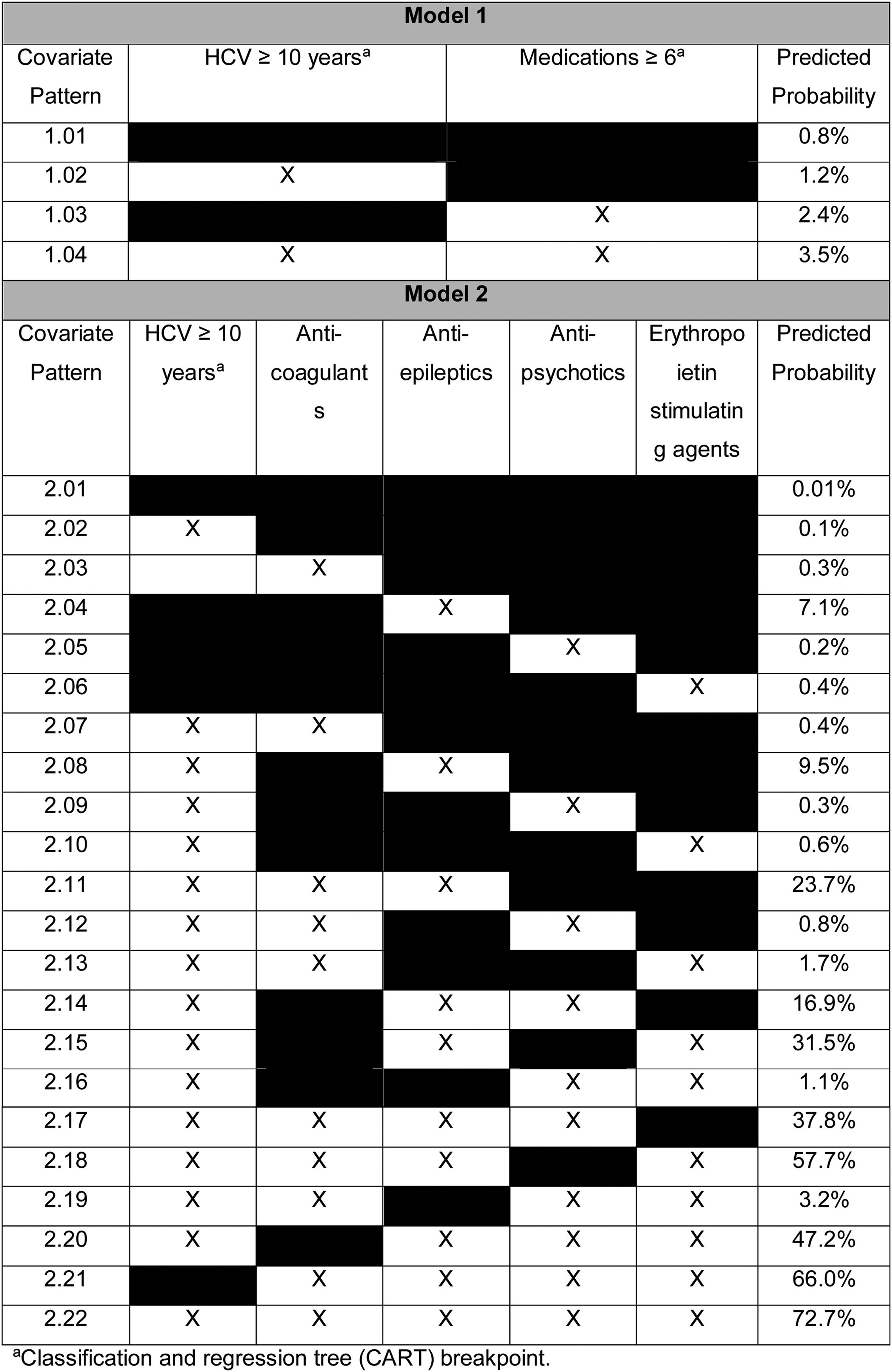
In multivariable analyses, variables independently associated with XDDIs involving all four HCV treatment regimens were use of ≥6 medications (odds ratio, OR: 2.85, 95% confidence interval, CI: 1.68–4.84, p<0.001) and having an HCV diagnosis ≥10 years (OR: 1.49, 95% CI: 0.93–2.40, p=0.10). The results of the multivariable analyses were used to determine the predicted probabilities of experiencing an XDDI to all four HCV treatment regimens given certain covariate patterns. The predicted probabilities of XDDIs to all four HCV treatment regimens is displayed in Table 2. The reference probability was 0.8% when neither covariate was present (covariate pattern 1.01), and rose to 3.5% when both covariates were present (covariate pattern 1.04).
A second multivariable model was assessed where use of ≥6 medications was replaced with individual medication classes. In the second model, variables independently associated with XDDIs to all four HCV treatment regimens were: anti-coagulants (OR: 2.98, 95% CI: 1.35–6.54, p=0.007), anti-epileptic medications (OR: 80.26, 95% CI: 32.37–199.02, p<0.001), anti-psychotic medications (OR: 1.95, 95% CI: 1.24–3.06, p=0.004), ESAs (OR: 4.39, 95% CI: 1.26–15.36, p=0.02) and HCV infection ≥10 years (OR: 1.37, 95% CI: 0.83–2.268, p=0.22). The predicted probability of having an XDDI to all four HCV regimens when none of the covariates were present was 0.001% (Table 2, covariate pattern 2.01). Conversely, the predicted probability of having an XDDI to all four HCV regimens when all of the covariates were present was 72.7% (Table 2, covariate pattern 2.22).
4DiscussionSeveral treatment options are available for patients with HCV infection [1–5]. One potential distinguishing feature is the potential for XDDIs. Our study demonstrated differences in the frequency of XDDIs between the various HCV regimens (Fig. 1). The prevalence of XDDIs was highest for GLE/PIB (17.8%) followed distantly by LDV/SOF (4.4%) and the remaining two regimens (2.8%). The high frequency of XDDIs involving GLE/PIB was driven by statins and was not surprising given the ubiquity of statin use in the Veterans Affairs population. While most of the two-way comparisons between HCV treatment regimens were statistically significant, it is important to note that the absolute differences for some comparisons were as little as 1.6% (comparison of LDV/SOF versus SOF/VEL) and the number needed to avert one XDDI would be >60 patients. Conversely, there were comparisons with absolute differences as large as 15% (GLE/PIB versus SOF/VEL) and the number needed to avert one XDDI would be <7 patients.
Another unique feature of our study was an examination of risk factors for having a XDDI to all four HCV treatment regimens. Understanding these risk factors is important because these patients would be ineligible for HCV treatment without modifying concomitant medication(s) used to treat comorbidities. Duration of HCV infection ≥10 years and use of ≥6 medications were variables that independently predicted having an XDDI to all four HCV treatment regimens. This is of important clinical significance because patients with HCV infection are often afflicted by a variety of age-related comorbidities and use of concomitant medications is common. It is also important because there are multiple clinicians involved in the care of patients with HCV infection. An awareness of the factors leading to XDDIs to all HCV regimens by both specialists and non-specialists can help facilitate patient care. We attempted to improve the granularity of the model by replacing use of 6 medications with individual medication classes. The medication classes that remained in the final model were anti-coagulants, anti-epileptic medications, anti-psychotic medications and ESAs. With the exception of anti-epileptic medications, these classes are not generally associated with XDDIs to the HCV regimens evaluated. However, their use appears to be more common among patients with an elevated probability of XDDIs to all four HCV regimens. Based on the results of this analysis, patients using these medication classes should be screened more aggressively for XDDIs when considering HCV treatment.
Some limitations of the present study should be considered. First, the four HCV treatment regimens were added to the home medication profiles of patients with chronic HCV infection in a simulated fashion. The theoretical nature of the study allows for detection of XDDIs under different exposure scenarios while keeping all other covariates constant. If this investigation examined a population of individuals receiving each of these regimens, selective forces that led individuals to use one regimen over another would need to be controlled. Second, several drug interaction software programs exist. We chose to use University of Liverpool website since it is specifically mentioned as a resource in the HCV treatment guidelines [1]. It may not be intuitive to use this website for non-specialty clinicians screening for drug interactions since other commercially available programs exist. The use of a second interaction screening tool will almost always be necessary since the University of Liverpool website only detects interactions between drug–drug combinations where at least one agent is used to treat HCV infection [21]. It does not identify interactions that may exist between two non-HCV medications within a patient's home medication list. A formal comparison should be conducted between the University of Liverpool website and drug interaction software programs to ensure that sensitivity/specificity of detecting XDDIs is reasonable. Third, drug interaction programs are routinely updated and the severity of some drug interactions are downgraded from contraindicated to use with caution (e.g., ledipasvir and proton pump inhibitors when initially approved) [2]. The converse may occur where the severity of drug interactions is upgraded in response to emerging clinical data (e.g., sofosbuvir and amiodarone) [17]. Time since market entry could affect the prevalence of XDDIs detected, and future studies should attempt to control this factor. Fourth, the predictors of having XDDIs to all HCV treatment regimens may be surrogates for other factors which were not studied; future analyses should consider the role these other unmeasured factors might play. For instance, duration of HCV ≥10 years may be a proxy measure for age or advanced stage of disease and may reflect higher medication use. It could also indicate patients should be considered for treatment as early as possible to avoid issues related to managing XDDIs. Fifth, this study used the statistical tool CART to identify breakpoints in continuous variables associated with XDDIs to all four HCV regimens. It should be noted that CART identifies breakpoints that are specific to a population and the same breakpoint may not be observed in other populations with different distributions within continuous variables or frequency of outcome. Finally, this study was performed among predominantly male Veterans. A varied frequency of XDDIs may be expected in other populations with a different distribution of medication use. This is particularly important for females that may be using hormonal therapies, such as ethinyl estradiol, which is contraindicated with GLE/PIB.
In summary, there were significant differences in the frequency of XDDIs between various HCV treatment regimens. Of the HCV regimens evaluated, XDDIs were significantly more frequent with GLE/PIB compared to other regimens. A very low percentage of patients had XDDIs to all four HCV regimens. This was driven primarily by a high volume of medication use and prolonged duration of chronic HCV infection. Clinicians prescribing HCV treatment should include drug interactions in the multiple considerations that are involved in selecting an HCV treatment regimen.
AbbreviationsATC Anatomical Therapeutic Chemical classification and regression tree confidence interval scontraindicated drug–drug interactions erythropoietin stimulating agents glecaprevir/pibrentasvir grazoprevir/elbasvir hepatitis C interquartile range ledipasvir/sofosbuvir odds ratio sofosbuvir/velpatasvir standard deviation
The work presented in this manuscript was partially funded by an investigator-initiated research grant from Gilead Sciences.
Conflict of interestNP has received investigator-initiated research grant support from Gilead Sciences and Merck & Co. NP has received consultant honoraria from Gilead Sciences. None of the other authors have conflicts of interest to declare.
This material is based upon work partially supported by the Office of Research and Development, Department of Veterans Affairs. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.