The aim of this paper was to evaluate the association of hepatic encephalopathy with survival of patients with liver failure.
Materials and methodsWe retrieved the relevant articles from the PubMed, Embase and Cochrane Library, up to May 2017. The pooled odds ratio (OR) as well as their 95% confidence intervals (CI) was calculated by the software of R package version 3.12.
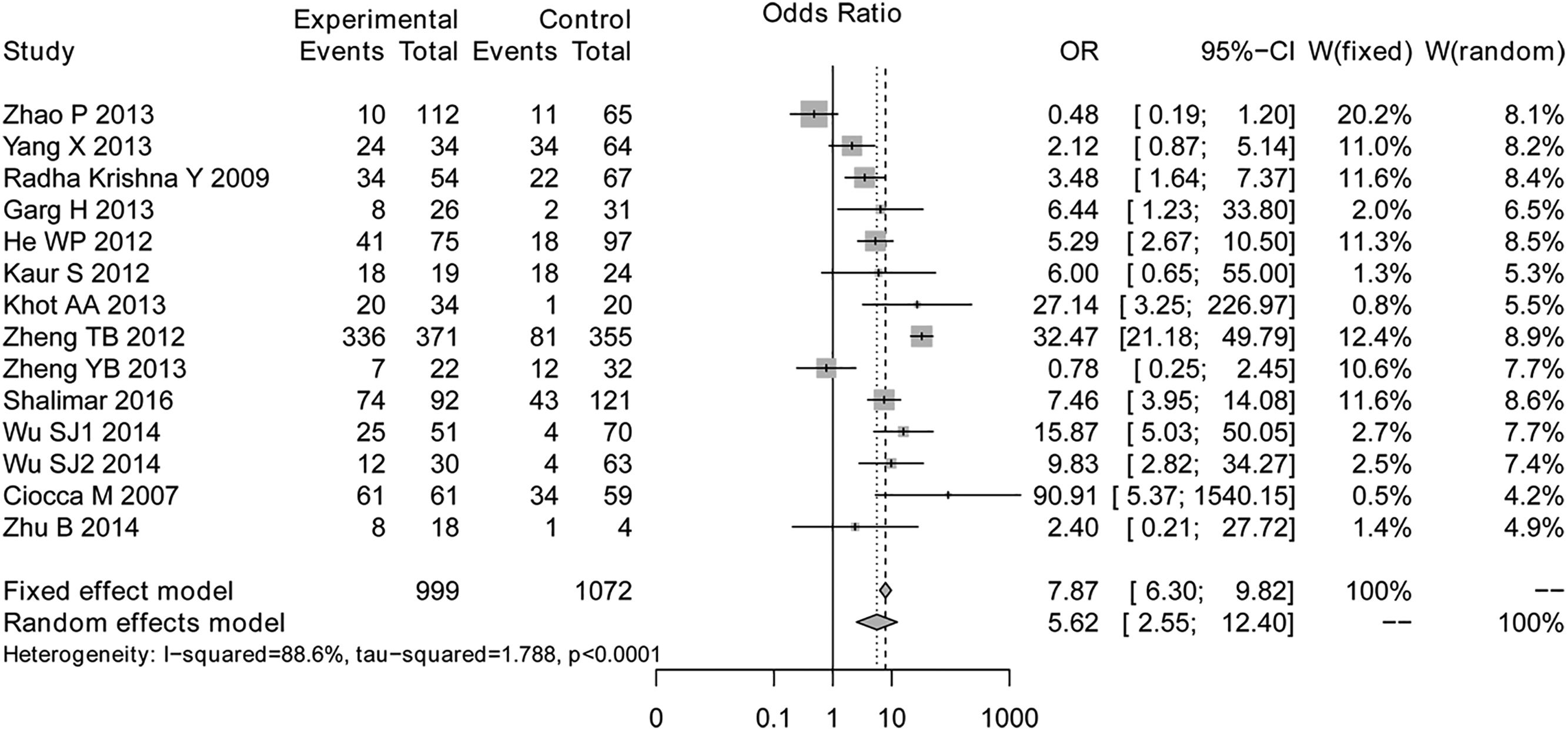
ResultsTotal 13 studies with 2071 liver failure patients were included and reanalyzed in this meta-analysis. The results proved the prognostic value of hepatic encephalopathy for survival of patients with liver failure (OR=5.62, 95%CI=6.30–9.82, P<0.001). The subgroup analyses showed that the type of liver failure and the follow up duration may be the factor influencing the association between hepatic encephalopathy and survival of patients with liver failure.
ConclusionsThe results proved that hepatic encephalopathy was a prognostic factor of survival in patients with liver failure.
Liver failure, which is present as acute liver failure, acute-on-chronic (AOC) liver failure, is characterized by massive necrosis of hepatocytes which is caused by a variety of acute or chronic injuries that are induced by autoimmune attack of hepatocytes, hepatotoxic drugs, alcohol consumption, or infection with viruses (hepatitis B/C virus) [1,2]. Acute liver failure often occurs without existence of any preexisting liver disease [3] and with the development of some life-threatening symptoms, such as hepatic encephalopathy, coagulopathy, and hepatorenal syndrome [4]. AOC liver failure, as a chronic liver disease, is characterized by an acute deterioration of liver cirrhosis [5]. Hepatic encephalopathy is also one of the common symptoms of AOC liver failure, and has been reported to be associated with a reduced survival in AOC liver failure [6,7].
Currently, many studies have evaluated the prognostic value of hepatic encephalopathy in liver failure patients. Although some studies proved the association between hepatic encephalopathy and survival of patients with liver failure [8–10], there were some evidences disavowing this association [11,12]. These controversies may be caused by some corresponding factors. Thus, we performed this meta-analysis with relevant studies to further comprehensively evaluate the prognostic value of hepatic encephalopathy for survival of patients with liver failure. Meanwhile, the subgroup analysis was performed based on study location, type of liver failure and follow up duration to investigate the influence of these factors on the results.
2Materials and methods2.1Data sourcesWe retrieved the relevant articles from PubMed, Embase and Cochrane Library, up to May 2017 with the language limitation in English. The key words of “hepatic failure” OR “liver failure”, “hepatic encephalopathy” and “prognosis” OR “prognostic” were used for literature searching. Meanwhile, references from relevant reviews were reviewed for searching additional studies.
2.2Inclusion and exclusion standards of studiesThe included studies should meet the following criteria: (1) the participant was patient with liver failure; (2) the hepatic encephalopathy as a prognosis factor for survival of patient with liver failure was investigated.
In addition, we excluded the studies based on the following criteria: (1) studies were reviews, letters or comments; (2) if the studies were duplicated publications, only the one including most complete data was included; (3) there was no available data.
2.3Data extractionTwo investigators identified studies and extracted data independently using a standardized form after training. The following data should be recorded: general information for studies (including the name of first author, study region, year of study publication and study design), characteristics of participants (including, as age, gender, sample size, diagnosis of liver failure and type of liver failure), follow up and clinical outcomes. Inconsistence should be resolved by discussion within our team.
2.4Quality assessmentThe quality of these included studies was independently evaluated by two authors with Newcastle-Ottawa Scale (NOS). This scoring system including three items: selection (4 questions), comparability (1 question) and explore (3 questions). Two scores were assigned for the only one question in the item of comparability and one score was assigned for each question in the item of selection and explore (3 questions). Thus, the total scores were nine and the study with less than 5 scores was deemed to have a low quality.
2.5Meta-analysis methodsThis meta-analysis was conducted using the R package version 3.12. The pooled estimates of odds ratios (ORs) as well as their 95% confidence intervals (CIs) were calculated. We assessed the heterogeneity among studies based on the Cochran's Q-statistic test [13] and I2-statistic (I2=100%×(Q−df)/Q) test [14]. A P<0.10 or I2>50% indicated significant heterogeneity and then the random effects model was used for pooling data. Otherwise, the fixed effects model was applied. The Z-test was performed to evaluate the significance of the pooled OR with a P value of <0.05. Sensitivity analyses were performed to test the stability of the results in overall analysis by excluding individual studies one at a time. The publication biases of the included studies were evaluated using the Egger's linear regression test with P<0.05 indicating significant publication bias [15].
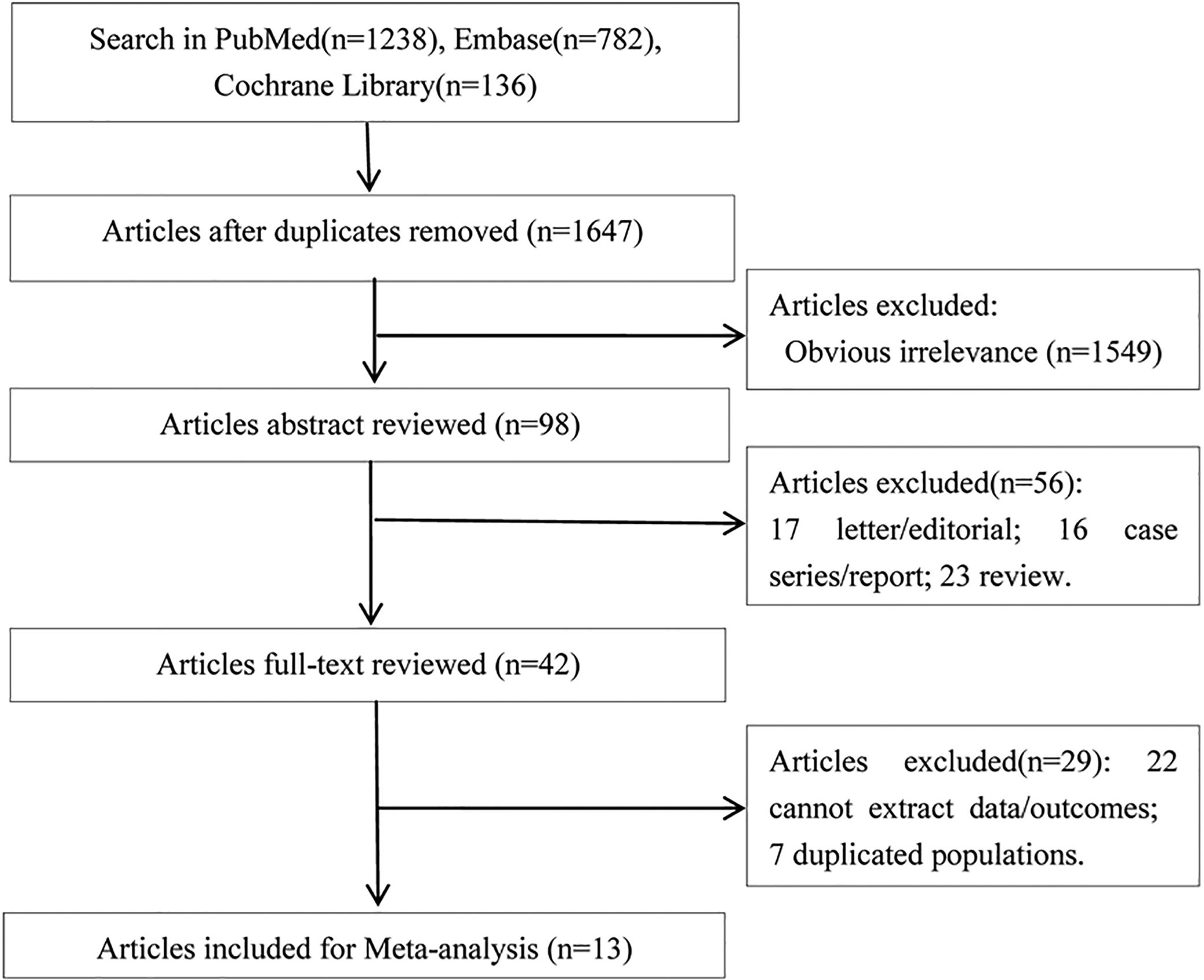
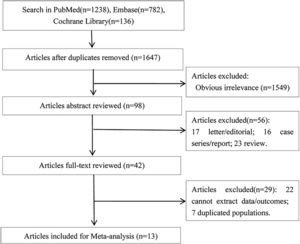
3Results3.1Literature retrievalThere were 1238 articles in PubMed, 782 articles in Embase, and 136 in Cochrane Library potentially relevant to the search terms. The study selection and literature search process was shown in Fig. 1. After removing duplicates or obviously irrelevant studies, a total of 98 potentially relevant studies were remained. Then through reading the abstracts, 56 of these articles were excluded (23 reviews, 17 letters or editorials, and 16 case series or reports). Then full-text of the remaining 42 studies were reviewed, 29 articles (including 22 articles without available data and 7 duplicated publications) were excluded according to the exclusion and inclusion criteria. Thus, 13 articles [8–12,16–23] were included in this meta-analysis.
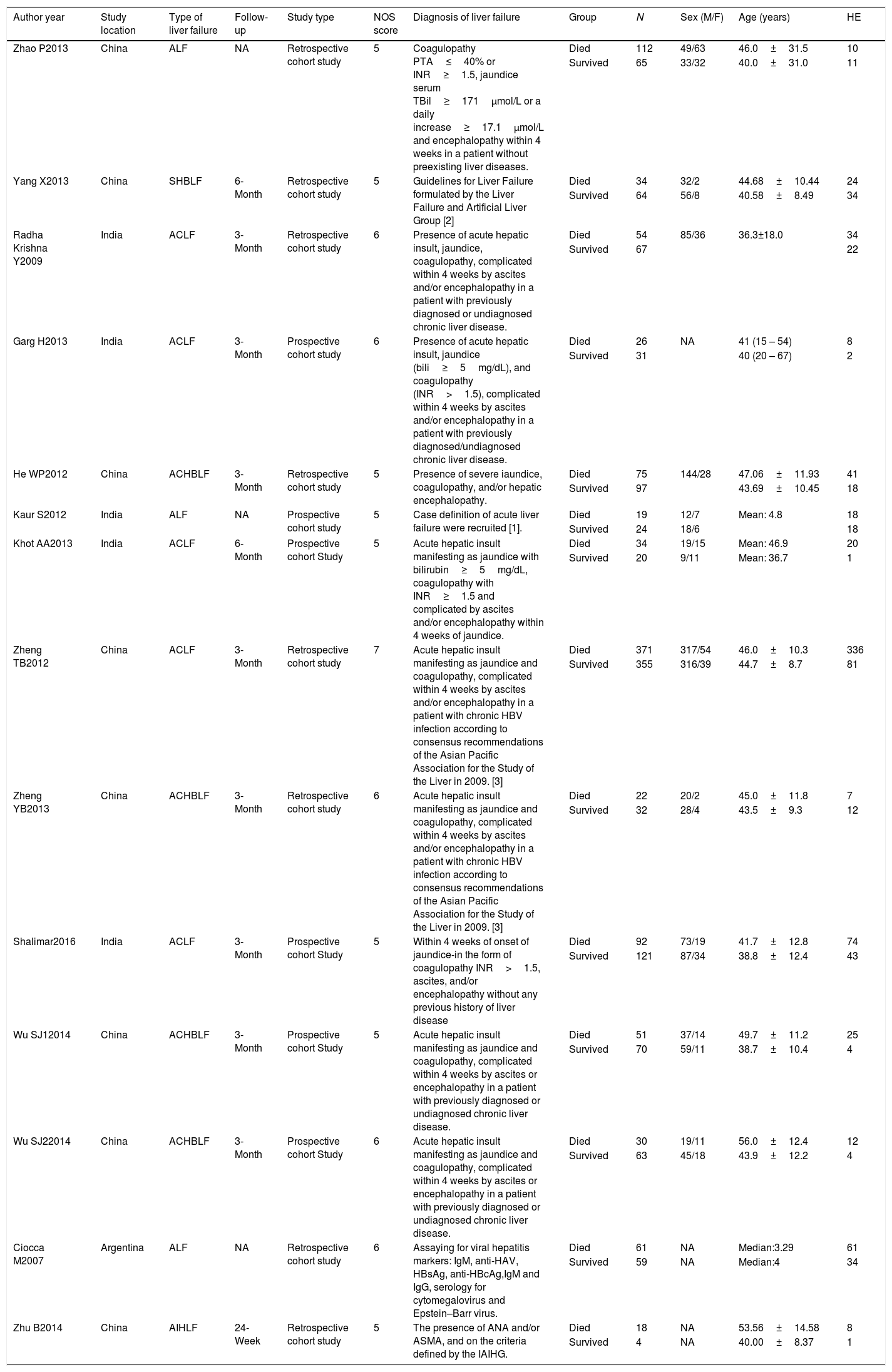
3.2Characteristics of included studiesThe characteristics of included studies in the meta-analysis were presented clearly in Table 1. The included studies were published from 2007 to 2016. All these studies were cohort studies including 8 retrospective studies [8–11,16,18,22,23] and 5 prospective studies [12,17,19–21]. A total of 2071 liver failure patients were included and reanalyzed in this meta-analysis, including patients with acute liver failure, severe hepatitis B-induced liver failure, autoimmune-hepatitis-induced liver failure, AOC liver failure, and AOC hepatitis B liver failure. These studies were performed in China, India or Argentina. Only one study reported the data on children aged lower than 18 years old [16]. All these studies were high quality studies with 5 or more than 5 NOS scores.
Characteristics of studies included in the meta-analysis.
| Author year | Study location | Type of liver failure | Follow-up | Study type | NOS score | Diagnosis of liver failure | Group | N | Sex (M/F) | Age (years) | HE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao P2013 | China | ALF | NA | Retrospective cohort study | 5 | Coagulopathy PTA≤40% or INR≥1.5, jaundice serum TBil≥171μmol/L or a daily increase≥17.1μmol/L and encephalopathy within 4 weeks in a patient without preexisting liver diseases. | Died | 112 | 49/63 | 46.0±31.5 | 10 |
| Survived | 65 | 33/32 | 40.0±31.0 | 11 | |||||||
| Yang X2013 | China | SHBLF | 6-Month | Retrospective cohort study | 5 | Guidelines for Liver Failure formulated by the Liver Failure and Artificial Liver Group [2] | Died | 34 | 32/2 | 44.68±10.44 | 24 |
| Survived | 64 | 56/8 | 40.58±8.49 | 34 | |||||||
| Radha Krishna Y2009 | India | ACLF | 3-Month | Retrospective cohort study | 6 | Presence of acute hepatic insult, jaundice, coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. | Died | 54 | 85/36 | 36.3±18.0 | 34 |
| Survived | 67 | 22 | |||||||||
| Garg H2013 | India | ACLF | 3-Month | Prospective cohort study | 6 | Presence of acute hepatic insult, jaundice (bili≥5mg/dL), and coagulopathy (INR>1.5), complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed/undiagnosed chronic liver disease. | Died | 26 | NA | 41 (15 – 54) | 8 |
| Survived | 31 | 40 (20 – 67) | 2 | ||||||||
| He WP2012 | China | ACHBLF | 3-Month | Retrospective cohort study | 5 | Presence of severe iaundice, coagulopathy, and/or hepatic encephalopathy. | Died | 75 | 144/28 | 47.06±11.93 | 41 |
| Survived | 97 | 43.69±10.45 | 18 | ||||||||
| Kaur S2012 | India | ALF | NA | Prospective cohort study | 5 | Case definition of acute liver failure were recruited [1]. | Died | 19 | 12/7 | Mean: 4.8 | 18 |
| Survived | 24 | 18/6 | 18 | ||||||||
| Khot AA2013 | India | ACLF | 6-Month | Prospective cohort Study | 5 | Acute hepatic insult manifesting as jaundice with bilirubin≥5mg/dL, coagulopathy with INR≥1.5 and complicated by ascites and/or encephalopathy within 4 weeks of jaundice. | Died | 34 | 19/15 | Mean: 46.9 | 20 |
| Survived | 20 | 9/11 | Mean: 36.7 | 1 | |||||||
| Zheng TB2012 | China | ACLF | 3-Month | Retrospective cohort study | 7 | Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with chronic HBV infection according to consensus recommendations of the Asian Pacific Association for the Study of the Liver in 2009. [3] | Died | 371 | 317/54 | 46.0±10.3 | 336 |
| Survived | 355 | 316/39 | 44.7±8.7 | 81 | |||||||
| Zheng YB2013 | China | ACHBLF | 3-Month | Retrospective cohort study | 6 | Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with chronic HBV infection according to consensus recommendations of the Asian Pacific Association for the Study of the Liver in 2009. [3] | Died | 22 | 20/2 | 45.0±11.8 | 7 |
| Survived | 32 | 28/4 | 43.5±9.3 | 12 | |||||||
| Shalimar2016 | India | ACLF | 3-Month | Prospective cohort Study | 5 | Within 4 weeks of onset of jaundice-in the form of coagulopathy INR>1.5, ascites, and/or encephalopathy without any previous history of liver disease | Died | 92 | 73/19 | 41.7±12.8 | 74 |
| Survived | 121 | 87/34 | 38.8±12.4 | 43 | |||||||
| Wu SJ12014 | China | ACHBLF | 3-Month | Prospective cohort Study | 5 | Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. | Died | 51 | 37/14 | 49.7±11.2 | 25 |
| Survived | 70 | 59/11 | 38.7±10.4 | 4 | |||||||
| Wu SJ22014 | China | ACHBLF | 3-Month | Prospective cohort Study | 6 | Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. | Died | 30 | 19/11 | 56.0±12.4 | 12 |
| Survived | 63 | 45/18 | 43.9±12.2 | 4 | |||||||
| Ciocca M2007 | Argentina | ALF | NA | Retrospective cohort study | 6 | Assaying for viral hepatitis markers: IgM, anti-HAV, HBsAg, anti-HBcAg,IgM and IgG, serology for cytomegalovirus and Epstein–Barr virus. | Died | 61 | NA | Median:3.29 | 61 |
| Survived | 59 | NA | Median:4 | 34 | |||||||
| Zhu B2014 | China | AIHLF | 24-Week | Retrospective cohort study | 5 | The presence of ANA and/or ASMA, and on the criteria defined by the IAIHG. | Died | 18 | NA | 53.56±14.58 | 8 |
| Survived | 4 | NA | 40.00±8.37 | 1 |
HE: hepatic encephalopathy; *: diagnostic data; male/female; ALF: acute liver failure; SHBLF: severe hepatitis B-induced liver failure; ACLF: acute-on-chronic liver failure; ACHBLF: acute-on-chronic hepatitis B liver failure; AIHLF: autoimmune-hepatitis-induced liver failure; 1: Training Cohort; 2: Validation Cohort; HAV: hepatitis A virus; HbsAg: hepatitis B surface antigen; HBcAg: hepatitis B core antigen; INR: international normalized ratio; PTA: Prothrombin Activity; TBil: total bilirubin; ANA: anti-nuclear antibody; ASMA: anti-smooth muscle antibody; IAIHG: International Autoimmune Hepatitis Group; 1: Bucuvalas J, Yazigi N, Squires R. Acute liver failure in children. Clin Liver Dis. 2006; 10: 149–68; 2: The Liver Failure and Artificial Liver Group, the Chinese Society of Infectious Diseases; the Severe Liver Diseases and Artificial Liver Group, the Chinese Society of Hepatology, and the Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi 2006;14:643. 3: Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:269–82.
The overall meta-analysis was shown in Fig. 2. The results of test for heterogeneity (I2=0%, P<0.001) showed significant heterogeneities among studies, and then the random effects model was selected. The overall OR was 5.62 (95%CI=6.30–9.82, P<0.001), indicating that there was significant association between hepatic encephalopathy and survival of liver failure patients.
In the sensitivity analyses, no inconsistent result was found. Importantly, after excluding the study of Ciocca et al. (which was the only one study investigating the survival of children with liver failure), the meta-analysis still indicated the significant prognostic value of hepatic encephalopathy for survival in liver failure patients (OR=4.97, 95% CI=2.22–11.12, P<0.001).
In addition, we also performed the subgroup analyses to further evaluate the stability of results abstained by overall analysis and explore the sources of heterogeneity.
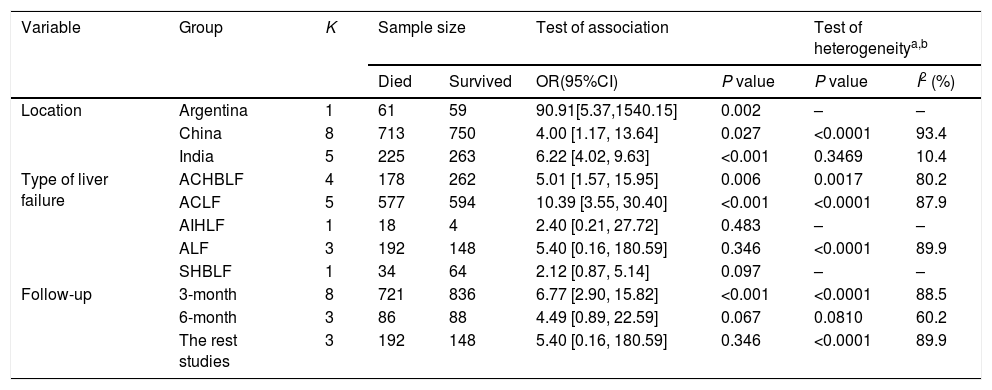
The results of subgroup analyses were listed in Table 2. Inconsistence results were found when the meta-analysis was performed based on autoimmune-hepatitis-induced liver failure patients (OR=2.40, 95% CI=0.21–27.72, P=0.483), acute liver failure patients (OR=5.40, 95% CI=0.16–180.59, P=0.346), severe hepatitis B-induced liver failure patients (OR=2.12, 95% CI=0.87–5.14, P=0.097), studies with 6 months follow up (OR=4.49, 0.89–22.59, P=0.069). In addition, the meta-analysis on the rest study (after excluding the studies with 3 or 6 months follow up) also indicated no significant association between hepatic encephalopathy and survival of patients with liver failure (OR=5.40, 95% CI=0.16–180.59, P=0.346). These results indicated that the type of liver failure and follow up time could significantly influence the association between hepatic encephalopathy and survival of patients with liver failure.
Results of subgroup meta-analysis.
| Variable | Group | K | Sample size | Test of association | Test of heterogeneitya,b | |||
|---|---|---|---|---|---|---|---|---|
| Died | Survived | OR(95%CI) | P value | P value | I2 (%) | |||
| Location | Argentina | 1 | 61 | 59 | 90.91[5.37,1540.15] | 0.002 | – | – |
| China | 8 | 713 | 750 | 4.00 [1.17, 13.64] | 0.027 | <0.0001 | 93.4 | |
| India | 5 | 225 | 263 | 6.22 [4.02, 9.63] | <0.001 | 0.3469 | 10.4 | |
| Type of liver failure | ACHBLF | 4 | 178 | 262 | 5.01 [1.57, 15.95] | 0.006 | 0.0017 | 80.2 |
| ACLF | 5 | 577 | 594 | 10.39 [3.55, 30.40] | <0.001 | <0.0001 | 87.9 | |
| AIHLF | 1 | 18 | 4 | 2.40 [0.21, 27.72] | 0.483 | – | – | |
| ALF | 3 | 192 | 148 | 5.40 [0.16, 180.59] | 0.346 | <0.0001 | 89.9 | |
| SHBLF | 1 | 34 | 64 | 2.12 [0.87, 5.14] | 0.097 | – | – | |
| Follow-up | 3-month | 8 | 721 | 836 | 6.77 [2.90, 15.82] | <0.001 | <0.0001 | 88.5 |
| 6-month | 3 | 86 | 88 | 4.49 [0.89, 22.59] | 0.067 | 0.0810 | 60.2 | |
| The rest studies | 3 | 192 | 148 | 5.40 [0.16, 180.59] | 0.346 | <0.0001 | 89.9 | |
Random-effects model was used when the P-value for heterogeneity test<0.05, otherwise the fixed-effect model was used.
P-value<0.05 is considered statistically significant for Q statistics; K: number of included studies. ALF: acute liver failure; SHBLF: severe hepatitis B-induced liver failure; ACLF: acute-on-chronic liver failure; ACHBLF: acute-on-chronic hepatitis B liver failure; AIHLF: autoimmune-hepatitis-induced liver failure.
Subgroup analyses also showed that the significant heterogeneity among studies disappeared (P=0.35, I2=10.4%) in the analysis based on population in India, indicating that the study location was one of the sources of heterogeneity.
The publication bias was evaluated based on the included studies in overall analysis and the P-value were 0.02, which revealed significance publication bias. Thus, trim and fill method was performed and consistent result with the overall analysis was found (OR=11.7153, 95% CI=5.02–27.32, P<0.001).
4DiscussionThe results of this meta-analysis have confirmed the prognostic value of hepatic encephalopathy in patients with liver failure. Moreover, subgroup analyses indicated that the type of liver failure and follow up duration can significantly influence the association between hepatic encephalopathy and survival of patients with liver failure. Besides, although the subgroup did not found considerable influence of study location on that association, the study location may also be a factor influencing that association because it was found to be a source of heterogeneity among studies. Further studies should be performed to give more evidences.
Based on the results of this meta-analysis, the hepatic encephalopathy can be used as a prognostic factor of survival in patients with AOC liver failure (including AOC hepatitis B liver failure), but not for patients with severe hepatitis B-induced liver failure, acute liver failure or autoimmune-hepatitis-induced liver failure. As known for us, the presence of hepatic encephalopathy is one criteria for diagnosing the AOC liver failure [24]. Moreover, it has reported that the presence of hepatic encephalopathy can increase the mortality of patients with AOC liver failure [6]. Previous study also reported that hepatic encephalopathy was always the factor reducing the survival in patients with liver cirrhosis [25]. However, the acute liver failure is characterized by absence of any preexisting liver disease, but the occurrence of AOC liver failure is dependent on the presence of cirrhosis. These essential differences between acute liver failure and AOC liver failure may lead to different occurrence mechanism of hepatic encephalopathy. It speculated that the occurrence mechanism of hepatic encephalopathy in patients with AOC liver failure may be a mechanism associated with survival. In addition, Ciocca M found the encephalopathy III/IV was associated with high rate of death in patients with acute liver failure [16], Thus we inferred that the grade of encephalopathy may be a factor influencing the prognostic value of hepatic encephalopathy in patients with acute liver failure. More studies should be performed to confirm the above speculations.
Besides, we also found the follow up duration also a factor influencing the association between hepatic encephalopathy and survival of patients with liver failure. Among these 3 studies with 6 months follow up [12,22,23], only one study was performed with participants of AOC liver failure patients and showed significant association between hepatic encephalopathy and survival, but no significant association was found in patients with autoimmune-hepatitis-induced liver failure or severe hepatitis B-induced liver failure. The negative results in the analysis based on studies with 6 months follow up may be caused by the negative results in that two studies. Thus, the influence of follow up duration on that association should be further investigated in further studies.
This is the first meta-analysis to investigate the prognostic value of hepatic encephalopathy in patients with liver failure. However, some disadvantages should be noted. Firstly, some retrospective studies were also included because prospective studies were two fewer to do a meta-analysis. Secondly, significant heterogeneity among studies still existed in subgroup analysis and many other corresponding (such as age, sex and culture) may be also sources of heterogeneity. Furthermore, only short term follow up was performed in these included studies. More prospective studies with long term follow up should be performed in future.
In conclusion, hepatic encephalopathy is one of the prognostic factors of survival in liver failure patients. This prognostic value of hepatic encephalopathy may be affected by the type of liver failure.AbbreviationsOR
odds ratio
CIconfidence intervals
AOCacute-on-chronic
NOSNewcastle-Ottawa Scale
Author roles in manuscript creationHY and YC contributed to the study design, conducting the study, data collection, data analysis and writing of the manuscript. PJ contributed to data interpretation and discussion. All authors read and approved the final manuscript.
Financial supportNone.
Conflict of interestThe authors have not declared any conflicts of interest.
Informed patient consentNot applicable.