Background. Despite the introduction of direct antiviral agents, pegylated interferon remains the mainstay of treatment for chronic hepatitis C. However, pegylated interferon is associated with a high rate of severe adverse events and decreased quality of life. Specific interventions can improve adherence and effectiveness. We aimed to determine whether implementing a multidisciplinary approach improved outcomes in the treatment of chronic hepatitis C.
Material and methods. We analyzed consecutive patients treated with pegylated interferon plus ribavirin between August 2001 and December 2011. We compared patients treated before and after the implementation of a multidisciplinary approach in 2007. We compared the baseline demographic and clinical characteristics and laboratory findings between groups, and used bivariate logistic regression models to detect factors involved in attaining a sustained virological response, calculating the odds ratios with their respective 95% confidence intervals. To evaluate the effect of the multidisciplinary team, we fitted a multivariate logistic regression model to compare the sustained virological response after adjusting for unbalanced variables and predictive factors.
Results. We included 514 patients [228 (44.4%) in the pre-intervention cohort]. Age, viral genotype, previous treatment, aspartate transaminase, ferritin, and triglyceride were prognostic factors of sustained virological response. After adjusting for prognostic factors, sustained virological response was higher in the multidisciplinary cohort (58 vs. 48%, p = 0.038). Despite higher psychiatric comorbidity and age in the multidisciplinary cohort, we observed a trend toward a lower rate of treatment abandonment in this group (2.2 vs. 4.9%, p = 0.107).
Conclusion. Multidisciplinary management of chronic hepatitis C improves outcomes.
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease; HCV infects 130 to 210 million individuals worldwide.1,2 In Spain, where about 800,000 people are infected, the prevalence ranges from 1.6 to 2.6%, depending on the area.3,4 HCV infection causes chronic hepatitis (CHC), ultimately leading to cirrhosis and hepatocellular carcinoma in many patients. CHC is the most frequent cause of liver transplantation in Spain and in other developed countries.3
A sustained virologic response (SVR) is defined as undetectable HCV RNA 12 weeks or 24 weeks after the end of therapy, as assessed by a sensitive molecular method with a lower limit of detection ≤ 15 IU/mL.2 An SVR may stop the progression of CHC to cirrhosis. Until recently, the standard treatment for CHC was pegylated interferon plus ribavirin.2 This combination achieved a SVR in 60% of patients;3,5,6 however, in the most prevalent (80-85%) genotype in our area (genotype 1), the standard therapy achieved an SVR of only 50%.3,5,7 Moreover, this treatment has drawbacks that reduce patients’ quality of life: its subcutaneous administration requires frequent trips to the hospital, and its high rate of severe adverse events requires close monitoring.3,8
The adverse effects of this treatment may require further treatments such as epoetin for severe anemia, omeprazole for dyspepsia, selective serotonin reuptake inhibitors for depression, or benzodiazepine for insomnia. Adverse effects sometimes make it necessary to reduce the dose or even to discontinue treatment. All these factors decrease the SVR rate,9,10 so improving adherence by treating adverse effects promptly might help more patients achieve SVR.
Until 2011, the combination of pegylated interferon and ribavirin was the approved treatment for chronic hepatitis C.1 In 2011, the direct-acting antiviral agents telaprevir and boceprevir were licensed for use in HCV genotype 1 infection. Both must be administered in combination with pegylated interferon and ribavirin. However, the side-effect profiles of these triple combination therapies and the costs per SVR are high. These treatments should ideally no longer be used in patients infected with HCV, as more efficacious and better tolerated options are available. Since 2014, new, highly effective direct-acting antiviral agents with few adverse events have been licensed in Europe for use as part of interferon-free combination therapies for HCV infection (sofosbuvir, daclatasvir, simeprevir, paritaprevir/ombitasvir/ritonavir and dasabuvir and sofosbuvir/ledipasvir).11,12 These new drugs are easily tolerated, but pose new challenges for hematologists, including comorbidities (e.g., heart disease, psychiatric disorders, or decompensated liver disease) and drug interactions. The complexity of these antiviral treatments demands specific interventions in the setting of multidisciplinary approach. Some studies have shown increased adherence and effectiveness with the implementation of these interventions in the setting of multidisciplinary teams (psychiatrist, pharmacist, or dermatologist) to improve the management of adverse effects.9,13,14 However, there are few data about the impact of a multidisciplinary approach on patients with CHC.
In 2007 we initiated a program for the treatment of patients with HCV by a multidisciplinary team comprising hepatologists, nurses, pharmacologists, psychiatrists, and dermatologists.
The aim of this study was to evaluate the effects of this multidisciplinary approach on the effectiveness of treatment.
Material and MethodsPatientsFrom August 2001 to December 2011, we included all consecutive patients with CHC treated with pegylated interferon plus ribavirin.
We excluded patients co-infected with human immuno-deficiency virus or hepatitis B virus.
Our hospital’s ethics committee approved the study (CEIC 2010612), and all patients provided written informed consent.
All patients were referred to our hospital by general practitioners to evaluate chronic hepatitis stage and treatment. All patients were from the same area with a population of approximately 400,000 inhabitants.
Multidisciplinary approachBefore the multidisciplinary approach was implemented, patients were managed only by their hepatologist and a nurse. They had the option of calling or coming to the day hospital if they had a problem, but the nurse was not specifically trained for this. If adverse effects appeared during treatment, referral to specialists (e.g., psychiatrists) was not protocolized. In 2007, we implemented a multidisciplinary program in our hepatology unit to standardize the management of patients with CHC treated with pegylated interferon plus ribavirin. We constituted a multidisciplinary team in which the following professionals played the following roles.
• Hepatologists attended patients on an outpatient basis. All patients had appointments before starting treatment (baseline) and then in the 4th, 12th, and 24th weeks after starting treatment, at the end of treatment, and 24 weeks after the end of treatment. Throughout this period, patients were encouraged to telephone if they had any concerns related with their treatment.
• A nurse was responsible for the treatment education program. This program included an appointment for patients to learn how to
- a)
Administer subcutaneous pegylated interferon themselves.
- b)
Manage adverse events.
- c)
Treat headache, fever or asthenia, and
- d)
Recognize the signs of some complications of treatment.
The nurse also managed phone calls from patients from 7:00 am to 5:00 pm.
- •
A pharmacist had monthly appointments with patients to provide the medication, to help manage adverse events, and to try to improve adherence.
- •
A psychiatrist evaluated and, when necessary, treated psychiatric complications that appeared during treatment. Antiviral treatment was administered only after a positive psychiatric evaluation. Patientsz psychological state was assessed at baseline, in the 4th, 12th, and 24th weeks after starting treatment, at the end of treatment, and 24 weeks after completing treatment. Assessment consisted of two self-administered questionnaires. The Hospital Anxiety and Depression Scale (HADS)15–17 and the General Health Questionnaire (GHQ-28) Spanish version.18,19 The HADS comprises two 7-item scales designed to rate depression (HADS-D) and anxiety (HADS-A) in medical patients10,15,16 and is considered a valid measure of the severity of mood disorders.15,16 Patients scoring 7 or more points in either the HADS-D or HADS-A were evaluated by the psychiatrist. The GHQ-28 aims to detect patients with a diagnosable psychiatric disorder.19 Patients scoring over 7 points were evaluated by the psychiatrist.18 Patients received psychiatric attention when the questionnaires showed any risk of psychiatric disease or when referred by their hepatologist. The psychiatrist was also telephoned in psychiatric emergencies. When a suicide risk or psychotic episode was detected, treatment was stopped. These patients were followed and treated according to the psychiatrist’s orders. When active psychiatric disease was detected at baseline, treatment was postponed until the patient’s disease had stabilized. Patients with a history of psychiatric disease were not excluded, but they were administered antiviral treatment only if the disease was stable.
- •
A dermatologist treated skin problems that developed due to antiviral treatment. Patients were referred by the hepatologist; the dermatologist was responsible for treatment and follow-up of the skin lesions until resolution.
All the team was from the same hospital. The multidisciplinary team had regular meetings to discuss management issues and results, followed uniform criteria, and discussed doubts in multidisciplinary team meetings.
Groups of analysisWe defined two cohorts:
- •
Pre-intervention cohort. Patients with CHC treated between August 2001 and December 2006, before the implementation of the multidisciplinary approach, and
- •
Multidisciplinary treatment cohort. Patients with CHC treated between January 2008 and December 2011, after the implementation of the multidisciplinary approach.
Patients, who initiated treatment in 2007, the year the multidisciplinary team was established, were excluded. There were 286 patients in the pre-intervention cohort and 228 in the multidisciplinary treatment cohort.
Variables analyzedWe recorded demographic data, medical history, HCV characteristics, and variables used to monitor treatment such as hemoglobin, aspartate transaminase (AST), alanine transaminase (ALT), platelet count, and viral load (4, 12, 24, and 48 weeks after starting treatment and 24 weeks after finishing treatment). We also recorded adverse events and whether they led to changes in dosage, whether they required additional medications, and whether they required termination of treatment.
The main outcome variable was SVR, defined as undetectable HCV-RNA 24 weeks after the end of treatment. As patients with genotypes 1 or 4 differ from those with genotypes 2 or 3 in their response to antiviral treatment,3,9 we analyzed SVR in these two groups separately.
Liver biopsyPretreatment liver biopsies were obtained before viral treatment when considered necessary. The Knodell Histology Activity Index (HAI) was used to determine the grade and stage of fibrosis.20,21 Patients who underwent biopsy were divided in two groups according to fibrosis in the HAI score: patients without significant fibrosis (HAI fibrosis score = 0 or 1) and those with significant fibrosis (HAI fibrosis score = 3 or 4).21
Antiviral treatment and dosagePatients with genotype 1 or 4 received treatment with either pegylated interferon alpha 2a (180 µg/week) plus ribavirin (1,000 mg/day if body weight was < 75 kg or 1,200 mg/day if body weight was ≥ 75 kg) or interferon alpha 2b (1.5 µg/kg/week) plus ribavirin (10.6 mg/kg/week) for 48 weeks.1,3
Patients with genotype 2 or 3 were treated for 24 weeks with the same regimen of pegylated interferon alpha 2a or alpha 2b plus 800 mg of ribavirin divided in two daily doses.1,3
The stop rules for treatment differed according to HCV genotype and study period. Prior to 2004, we stopped treatment in patients with genotype 1 or 4 when HCV RNA was detected in week 24 of treatment, but there was no stop rule for those with genotype 2 or 3. After 2004, the treatment of patients with genotype 1 or 4 was guided by the viral load: we stopped treatment when HCV RNA at week 12 had decreased < 2 log or were still detectable at week 24. All patients who stopped treatment because of stop rules, abandoned treatment prematurely due to adverse events, or relapsed after treatment were considered non-responders.
Two methods were used in for assaying HCV RNA. Until 2006, RNA was measured using the Cobas Amplicor technique, with manual RNA extraction followed by amplification and detection in a Cobas Monitor analyzer (Roche Diagnostics) with a detection limit of < 600 IU/mL. After 2006, the Cobas Ampliprep/Cobas Taqman HCV (Roche Diagnostics) test was used. This is a real-time, in vitro nucleic acid amplification test for quantitative measurement of HCV RNA in plasma or serum (the Cobas Ampliprep system was used for automated sample processing and the Cobas Taqman analyzer was used for subsequent automated RNA amplification and detection). The Cobas Ampliprep/Cobas Taqman assay allows for automated sample preparation (RNA isolation), followed by automated reverse transcription, PCR amplification, and RNA detection. The detection limit of this technique is 15 IU/mL.
Statistical analysisTo compare the baseline demographical and clinical characteristics and laboratory findings between groups, we used χ2 tests for categorical variables and t-tests for quantitative variables (or the Mann-Whitney test for variables with non-normal distributions).
To detect factors predictive of SVR, we used bivariate logistic regression models, calculating the odds ratios with their respective 95% confidence intervals.
To evaluate the effect of the multidisciplinary team, we fitted a multivariate logistic regression model to compare the SVR after adjusting for unbalanced variables and risk factors.
Statistical significance was set at 0.05 for all tests. We used SAS v9.2 (SAS Institute Inc., Cary, NC, USA) for all analyses.
ResultsBaseline findingsDemographic and clinical characteristicsBetween 2001 and 2011, a total of 563 patients received pegylated interferon plus ribavirin at our hospital. We excluded the 49 patients who started treatment during 2007; thus, 514 patients were included in the study. Of these, 286 (55.6%) (pre-intervention cohort) initiated treatment prior to the implementation of the multidisciplinary approach and 228 (44.4%) (multidisciplinary treatment cohort) initiated treatment after the implementation of the multidisciplinary approach.
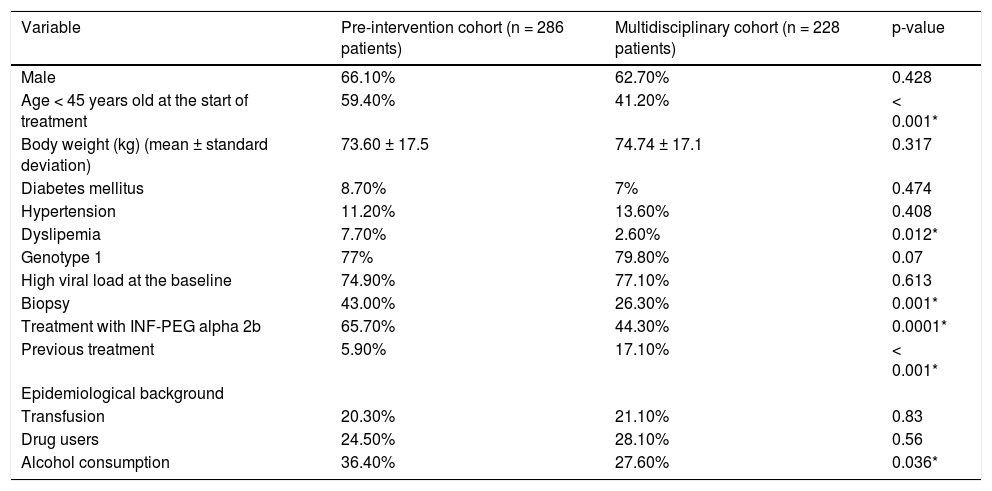
Table 1 reports the demographic and clinical characteristics of patients in the two groups. Both groups had more men than women. Patients from the pre-intervention cohort were younger than those from the multidisciplinary cohort (43.2 ± 10.8 vs. 48.54 ± 11 years, p < 0.0001). The prevalence of dyslipemia was higher in the pre-intervention cohort (7.7 vs. 2.6%, p = 0.0120). Genotypes 1 and 4 were the most frequent in both groups (77% in the pre-intervention cohort vs. 80% in the multidisciplinary cohort, p = 0.0696). Liver biopsy was performed more frequently in the pre-intervention cohort (43 vs. 26%, p < 0.0001). In the pre-intervention cohort 51.6% of patients who where biopsied had no significant fibrosis; by contrast, in the multidisciplinary cohort, only 26.7% of patients biopsied had no significant fibrosis. The percentage of patients who had undergone a previous treatment was higher in the multidisciplinary cohort (17 vs. 6%, p < 0.0001). The percentage of patients with a history of alcohol consumption (defined as ≥ 60 g/day for men and ≥ 40g/day for women) was higher in the pre-intervention cohort (36.4 vs. 27.6%, p = 0.036). The percentage of patients who received pegylated interferon alpha 2b was higher in the pre-intervention cohort (65.7 vs. 44.3%, p < 0.0001). There were no differences regarding SVR among patients treated with pegylated interferon alpha 2a versus alpha 2b (49.8 vs. 48.7%, respectively, p = 0.8042).
Demographical and clinical and virological characteristics of patients in each cohort.
| Variable | Pre-intervention cohort (n = 286 patients) | Multidisciplinary cohort (n = 228 patients) | p-value |
|---|---|---|---|
| Male | 66.10% | 62.70% | 0.428 |
| Age < 45 years old at the start of treatment | 59.40% | 41.20% | < 0.001* |
| Body weight (kg) (mean ± standard deviation) | 73.60 ± 17.5 | 74.74 ± 17.1 | 0.317 |
| Diabetes mellitus | 8.70% | 7% | 0.474 |
| Hypertension | 11.20% | 13.60% | 0.408 |
| Dyslipemia | 7.70% | 2.60% | 0.012* |
| Genotype 1 | 77% | 79.80% | 0.07 |
| High viral load at the baseline | 74.90% | 77.10% | 0.613 |
| Biopsy | 43.00% | 26.30% | 0.001* |
| Treatment with INF-PEG alpha 2b | 65.70% | 44.30% | 0.0001* |
| Previous treatment | 5.90% | 17.10% | < 0.001* |
| Epidemiological background | |||
| Transfusion | 20.30% | 21.10% | 0.83 |
| Drug users | 24.50% | 28.10% | 0.56 |
| Alcohol consumption | 36.40% | 27.60% | 0.036* |
Mean plus-minus standard deviation and percentages (%). INF-PEG: pegylated interferon.
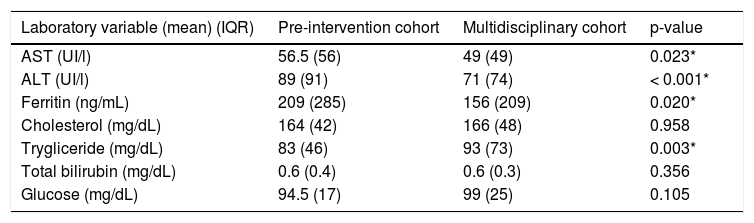
At baseline, ALT, AST, and ferritin were higher in the pre-intervention cohort, and triglycerides were higher in the multidisciplinary cohort; cholesterol, bilirubin, and glucose did not differ between groups (Table 2).
Laboratory findings of patients in each cohort.
| Laboratory variable (mean) (IQR) | Pre-intervention cohort | Multidisciplinary cohort | p-value |
|---|---|---|---|
| AST (UI/l) | 56.5 (56) | 49 (49) | 0.023* |
| ALT (UI/l) | 89 (91) | 71 (74) | < 0.001* |
| Ferritin (ng/mL) | 209 (285) | 156 (209) | 0.020* |
| Cholesterol (mg/dL) | 164 (42) | 166 (48) | 0.958 |
| Trygliceride (mg/dL) | 83 (46) | 93 (73) | 0.003* |
| Total bilirubin (mg/dL) | 0.6 (0.4) | 0.6 (0.3) | 0.356 |
| Glucose (mg/dL) | 94.5 (17) | 99 (25) | 0.105 |
Median (interquartile range). AST: aspartate, transaminase. ALT: alanine transaminase. IQR: interquartile range.
Adverse effects requiring a modification of pegylated interferon dose appeared in 7.7% of patients in the preintervention cohort and in 3.5% of those in the multidisciplinary cohort. Ribavirin dosage was changed in 9.1% in the pre-intervention cohort and in 12.3% in the multidisciplinary cohort (p = 0.24). The most frequent adverse effects requiring discontinuation of treatment were hyperthyroidism (8.6% in the pre-intervention cohort vs. 5.9% in the multidisciplinary cohort), general discomfort (11.4% in the pre-intervention cohort vs. 0% in the multidisciplinary cohort), anemia (2.9% in the preintervention cohort vs. 5.9% in the multidisciplinary cohort), depression (2.9% in the pre-intervention cohort vs. 5.9% in the multidisciplinary cohort), anxiety crisis (8.6% in the pre-intervention cohort vs. 0% in the multidisciplinary cohort), hypothyroidism (2.9% in the pre-intervention cohort vs. 0% in the multidisciplinary cohort), psychotic break (2.9% in pre-intervention cohort vs. 0% in the multidisciplinary cohort), severe asthenia (14.3% in the pre-intervention cohort vs. 0% in the multidisciplinary cohort), severe thrombocytopenia (5.7% in the pre-intervention cohort vs. 5.9% in the multidisciplinary cohort), dermatological disorders (8.6% in the pre-intervention cohort vs. 0% in the multidisciplinary cohort).
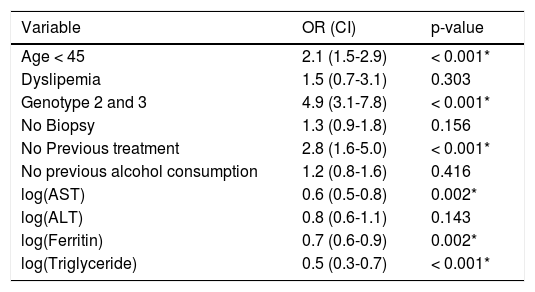
Evaluation of SVRAge, genotype, previous treatment, AST, ferritin, and triglycerides were independent prognostic factors for SVR (Table 3). The mean age of patients who achieved an SVR was 45 years, and the mean age of those who did not was 49 years.
Predictive factors of sustained virological response.
| Variable | OR (CI) | p-value |
|---|---|---|
| Age < 45 | 2.1 (1.5-2.9) | < 0.001* |
| Dyslipemia | 1.5 (0.7-3.1) | 0.303 |
| Genotype 2 and 3 | 4.9 (3.1-7.8) | < 0.001* |
| No Biopsy | 1.3 (0.9-1.8) | 0.156 |
| No Previous treatment | 2.8 (1.6-5.0) | < 0.001* |
| No previous alcohol consumption | 1.2 (0.8-1.6) | 0.416 |
| log(AST) | 0.6 (0.5-0.8) | 0.002* |
| log(ALT) | 0.8 (0.6-1.1) | 0.143 |
| log(Ferritin) | 0.7 (0.6-0.9) | 0.002* |
| log(Triglyceride) | 0.5 (0.3-0.7) | < 0.001* |
AST: aspartate transaminase. ALT: alanine transaminase. log: logarithm. OR: odds ratio. CI: confidence interval.
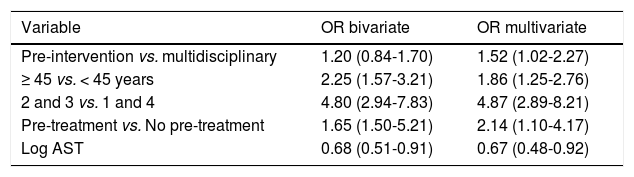
When comparing the SVR without adjusting for covariates, we found similar results in the two groups (46.9% in the pre-intervention cohort vs. 50.4% in the multidisciplinary cohort, p = 0.42). After adjusting for prognostic factors, the SVR was higher in the multidisciplinary cohort (58 vs. 48%, p = 0.038) (Table 4).
Bivariate and multivariate model results (OR with 95% confidence intervals for SVR).
| Variable | OR bivariate | OR multivariate |
|---|---|---|
| Pre-intervention vs. multidisciplinary | 1.20 (0.84-1.70) | 1.52 (1.02-2.27) |
| ≥ 45 vs. < 45 years | 2.25 (1.57-3.21) | 1.86 (1.25-2.76) |
| 2 and 3 vs. 1 and 4 | 4.80 (2.94-7.83) | 4.87 (2.89-8.21) |
| Pre-treatment vs. No pre-treatment | 1.65 (1.50-5.21) | 2.14 (1.10-4.17) |
| Log AST | 0.68 (0.51-0.91) | 0.67 (0.48-0.92) |
SVR: sustained virologic response. vs: versus. OR: odds radio. log: logarithm. AST: aspartate, transaminase.
When we analyzed patients with genotypes 1 and 4 separately from those with genotypes 2 and 3, the SVR in patients with genotypes 1 and 4 was higher in the multidisciplinary cohort (41 vs. 30.5%, p = 0.039), but did not differ between groups in those with genotypes 2 and 3 (p = 0.889).
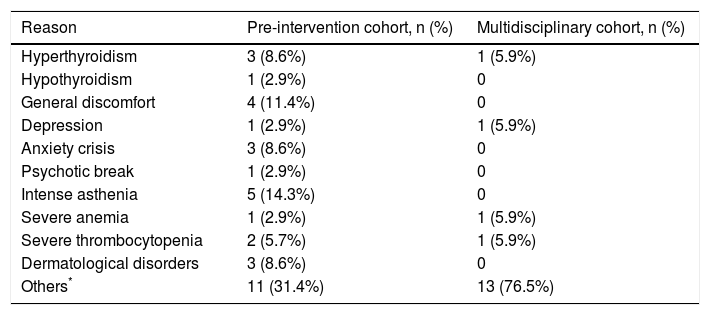
Abandonment rateA smaller percentage of patients in the multidisciplinary cohort abandoned treatment, but this trend did not reach statistical significance (2.2 vs. 4.9%, p = 0.11). Hyperthyroidism was the most common reason for discontinuing treatment (3 patients in the pre-intervention cohort and 1 in the multidisciplinary cohort). Sometimes the reasons for abandoning treatment were directly related to the treatment (e.g., depression, severe anemia, or severe thrombocytopenia) and sometimes they were not (e.g., cerebral hemorrhage, epileptic seizure, heart attack, etc.) (Table 5).
Reasons to abandon antiviral treatment.
| Reason | Pre-intervention cohort, n (%) | Multidisciplinary cohort, n (%) |
|---|---|---|
| Hyperthyroidism | 3 (8.6%) | 1 (5.9%) |
| Hypothyroidism | 1 (2.9%) | 0 |
| General discomfort | 4 (11.4%) | 0 |
| Depression | 1 (2.9%) | 1 (5.9%) |
| Anxiety crisis | 3 (8.6%) | 0 |
| Psychotic break | 1 (2.9%) | 0 |
| Intense asthenia | 5 (14.3%) | 0 |
| Severe anemia | 1 (2.9%) | 1 (5.9%) |
| Severe thrombocytopenia | 2 (5.7%) | 1 (5.9%) |
| Dermatological disorders | 3 (8.6%) | 0 |
| Others* | 11 (31.4%) | 13 (76.5%) |
Antiviral treatment for CHC is complex and requires careful monitoring and specialized care for the multiple side effects; thus, a multidisciplinary approach to management may improve outcomes. Our results suggest that multidisciplinary management improves the rate of SVR in patients with CHC undergoing antiviral therapy. We found that the SVR rate improved from 46.9% before the implementation of the multidisciplinary approach to 50.4% afterward; however, this difference was not statistically significant until after correcting for confounding factors.
The complications that are most likely to affect adherence are psychiatric disorders; therefore, strict control of patients’ psychiatric status is necessary.22 Depressive disorders impair the quality of life and have a profound effect on adherence to treatment.23,24 The multidisciplinary approach helps ensure these psychiatric complications are adequately treated. Before the implementation of multidisciplinary management, our hepatologists treated mild psychiatric disorders themselves with antidepressants or benzodiazepines, sometimes after delays. Antiviral treatment was discontinued when patients developed severe psychiatric complications. Including a psychiatrist in the multidisciplinary team enabled us to detect and treat psychiatric complications promptly, preventing the development of more severe symptoms that would have required us to stop antiviral treatment. Other studies have shown that HCV-infected patients with stable psychiatric disease can be safely treated with pegylated interferon plus ribavirin.22,24,25 Cabré, et al.22 showed that detecting psychiatric adverse effects early and managing them with a multidisciplinary team optimizes treatment adherence and efficiency.
Various prognostic factors of SVR have been reported.1,3,9,26 Adherence to treatment is one of the most important factors for achieving an SVR.8 Severe adverse effects can reduce adherence, leading to dose modifications and lower virologic response.8 Adherence improves when adverse effects are well controlled.3,9 Moreover, advice and control from a trained pharmacologist seems to improve treatment adherence.25,27,28 One limitation of this study is that measurement of adherence was initiated with the multidisciplinary team. Therefore, since we did not measure adherence in the pre-treatment group, we cannot compare the two groups or analyze adherence in subsets of patients such as those referred to psychiatrists or the effect of adherence in achieving an SVR.
To our knowledge, only one published study examined the effects of implementing a multidisciplinary team to manage antiviral therapy. Our results corroborate those reported by Carrion, et al.,9 who showed that a multidisciplinary team is cost-effective.
Our study aimed to evaluate the effectiveness of the multidisciplinary approach, not the costs. Our results showed that managing these patients in the setting of multidisciplinary improves the SVR rate.
The study comprises 10 years (from 2001 to 2011) and over this time methods of RNA-HCV detection changed. Nevertheless, these changes are unlikely to affect our measures of the effectiveness of treatment because SVR is measured in the 24th week, and the recurrence of chronic hepatitis C after the 24th week is extremely low, regardless of the technique used.3 The long duration of the study also makes it difficult to analyze other factors that could influence SVR that were discovered after the initiation of the study, such as the ILB28 genotype.29,30 Since we did not store DNA, we are unable to analyze this factor.
In our study, multidisciplinary management reduced the number of patients who abandoned antiviral treatment, although the reduction did not reach statistical significance. Several factors might contribute to this failure to reach significance. First, the number of patients who abandoned treatment because of adverse effects were remarkably low in both groups compared to previously published studies,31 probably because prior to the implementation of the multidisciplinary team, hepatologists and nurses provided specific support to patients receiving antiviral treatment, and patients had access to nurses, to a hotline, and to dedicated emergency care on weekdays. Thus, the multidisciplinary approach was implemented with the aim of extending and improving the structures already in place. Second, before the initiation of the multidisciplinary team, patients with depression or anxiety disorders were not accepted for antiviral treatment; unfortunately, we did not record the number of patients refused care for depression or anxiety, so we cannot quantify the effect of this measure on treatment abandonment. By contrast, after the initiation of multidisciplinary team, patients with depression or anxiety detected on the questionnaires were evaluated by a psychiatrist and could start treatment when their condition stabilized. Thus, the multidisciplinary treatment cohort included more patients with stable psychiatric disease, and having a similar rate of patients who abandoned treatment due to psychiatric complications in a group with more psychiatric comorbidities could actually be considered an improvement.
Our study has some limitations. The pre-intervention cohort and the multidisciplinary cohort were not parallel and did not coincide in time; thus, the better response to antiviral therapy in the multidisciplinary cohort may be at least partly due to improvements in care apart from the multidisciplinary team. However, the greater complexity of the multidisciplinary treatment cohort may partly mitigate this effect: patients in this group were older and were more likely to have received previous antiviral treatments. Another limitation of our study that could influence the rate of SVR is the fact that fibrosis could not be evaluated. The number of patients biopsied differed between groups (Table 1). The criteria for liver biopsy varied over time, and the decision of whether to obtain biopsies was left to the discretion of the attending physicians On the other hand, currently, the most important method to measure fibrosis is elastography, but this technique was unavailable at our center during the study. The large number of patients who did not undergo liver biopsy precludes an analysis of differences in the fibrosis stage between the two groups. However, it seems that the number of patients with significant fibrosis at liver biopsy was higher in the multidisciplinary group. Significant fibrosis makes it more difficult to achieve an SVR and could also mitigate the effect of the multidisciplinary team.
For ethical and logistical reasons, we did not randomize patients to receive treatment with the support of the multidisciplinary team: psychiatric and dermatological adverse events would almost certainly be better managed by specialists other than hepatologists. Another limitation is that we measured quality of life only after implementing the multidisciplinary approach in 2007, so we are unable to compare this important outcome variable between the two groups.
In conclusion, the multidisciplinary management of antiviral treatment in CHC improved the rates of SVR, probably by decreasing the percentage of patients who abandon treatment and improving adherence by closer monitoring and more effective management of adverse events.
Abbreviations- •
ALT: alanine transaminase.
- •
AST: aspartate transaminase.
- •
CHC: chronic hepatitis C.
- •
GHQ-28: general health questionnaire.
- •
HADS: hospital anxiety and depression scale.
- •
HADS-A: hospital anxiety and depression, rate anxiety.
- •
HADS-D: hospital anxiety and depression, rate depression.
- •
HAI: Knodell histology activity index.
- •
HCV: hepatitis C virus.
- •
SVR: sustained virologic response.
There are no conflicts of interest, no financial support.
AcknowledgementsAriadna Figuerola, Sílvia Rodríguez Cerrillo, Marcos Catalan, and John Giba helped redact the manuscript.