Fibrosing cholestatic hepatitis (FCH) is a less common but well-recognized severe complication of recurrent hepatitis C virus (HCV) infection post-liver transplant. This condition is fatal without successful treatment and to date; post-transplant antiviral interferon-based antiviral therapy has been associated with guarded success. The new era of protease inhibitors in the treatment of chronic HCV infection may alter the dismal outcome of this condition. To date, however, the experience with protease inhibitors in this condition is unreported. We report a post-liver transplant recipient with HCV associated FCH treated successfully with boceprevir, peginteferon and ribavirin for severe FCH. The patient was young woman who was a null responder pre-transplant to peginterferon and ribavirin. The peak serum bilirubin 391 µmol/L normalized to 15 µmol/L by week 8 of therapy. The pre-treatment HCV viral load of > 78 million IU/mL, decreased to 78 IU/mL at week 8 of therapy and was undetectable by week 12 and at the end of 48 week of treatment. 12 weeks post treatment, the HCV viral load remains undetectable. Significant anemia and neutropenia were encountered. Tacrolimus dosage titrated to trough levels, required marked reduction to 0.5 mg three times weekly. Despite the suboptimal peginterferon and ribavirin dosing, limited by adverse effects, full boceprevir dosing was maintained, with resolution of liver dysfunction. Boceprevir was obtained on compassionate grounds from the manufacturer before its licensure in Canada and this was the first use of boceprevir in the world for post-transplant FCH.
FCH is a severe and rapidly progressive disease associated with marked liver dysfunction. FCH is a recognized complication of recurrent hepatitis C or B infection after liver transplantation, occurring in approximately 5–10% of cases.1–3 FCH has also been reported outside of the transplant setting in patients infected with Hepatitis B or C virus, receiving immunosuppression therapy or chemotherapy and those with acquired immunodeficiency syndrome.4–6 FCH histology is characterized by portal fibrosis with immature fibrous bands extending into sinusoidal spaces, ductular proliferation, marked canalicular, hepatocellular cholestasis, ballooning hepatocytes and mild to moderate inflammation.7–8 Post-transplant FCH is associated with the use of high doses of immunosuppression ie. treatment of acute graft rejection (AGR), donors of old age, and enhanced divergence of quasispecies.9–12 Successful treatment is limited to isolated case reports, and the majority of cases are fatal despite attempts at treatment and adjustment of immunosuppression.13–14
Until recently, treatment of chronic hepatitis C virus infection, consisting of peginterferon and ribavirin, has been challenging, especially for genotype 1. Directly acting antiviral agents (DAA) belong to a new class of drug therapy that has shown significant improvement in treatment outcomes. Such agents that target HCV NS3/4A protease include boceprevir and telaprevir. These drugs are approved by the US Food and Drug Administration (FDA) for use with a peginterferon alfa-2a or 2b and ribavirin for the management of chronic HCV infection in patients with genotype 1.15–16 As of this writing, these agents, are not licensed in the post-liver transplant setting and phase 2 post-liver transplant clinical trials have only started enrollment and specifically exclude patients with FCH. We report the first patient with post-transplant FCH successfully treated with boceprevir in combination with peginterferon and ribavirin.
Case ReportA 36 year old woman acquired HCV-genotype 1 infection from a blood transfusion during bone marrow transplantation for acute myeloid leukemia 20 years previously. Pre-transplant she received a12 week course of peginterferon alfa-2a and ribavirin for HCV infection treatment, but the reduction in HCV RNA level was only 1.4 log10, subsequently, she received a further 12 weeks course of peginterferon alfa-2b and ribavirin and this again was discontinued for lack of significant viral response (HCV RNA level reduced by 0.8 log10). This categorized her as a null responder (i.e. failure to achieve at least a two log decrease in viral load at week 12 of antiviral therapy). She developed decompensated liver cirrhosis and portal hypertension requiring cadaveric liver transplant. Her immunosuppressant therapy regimen following transplant included mycophenolate mofetil and tacrolimus, with tapering course corticosteroids. Recurrent HCV infection with elevated liver enzymes and detectable HCV RNA level, was documented by biopsy at 4 months post-transplant (i.e. the biopsy had graft hepatitis with no features of acute graft rejection). She also suffered recurrent transudative ascites without a clear etiology found, including lack of evidence of portal hypertension or peritoneal disease. The ascites was refractory and was treated with a surgical peritoneovenous (LeVeen) shunt placement.
Fifteen months post-transplant, there was a marked deterioration in liver biochemistry:
- •
ALT 61 U/L (20–65).
- •
AST 129 U/L (10–38).
- •
ALP 195 U/L (50–160).
- •
GGT 196 U/L (10–55).
- •
Total bilirubin 85 µmol/L (0–18).
- •
Direct bilirubin 75 µmol/L
- •
Partial thromboplastin time (PTT) 52 sec.
- •
INR 2.2 (warfarin was started to decrease the likelihood of LeVeen shunt-related thrombosis).
- •
Serum creatinine 117 µmol/L (40–95).
- •
Hemoglobin (Hb) level 111 g/L.
- •
White cell count (WBC) 1.5 × 109/L.
- •
Neutrophils 1.1 × 109/L.
Abdominal imaging including Doppler ultrasound scan (USS) and contrast enhanced computerized tomography (CT) scan were normal. Cholangiogram (via ERCP) did not show biliary abnormality. The HCV RNA level was 78,371,051 IU/mL (7.9 log10). A liver biopsy revealed interface hepatitis with predominantly lymphocytes, scattered neutrophils and very rare esinophil and plasma cells. Within the lobules there were scattered ballooned hepatocytes as well as dead hepatocytes. Early fibrosis was noted around the portal triads with tendrils extending into the lobules but without significant architectural distortion. There was centrilobular cholestasis but no endothelitis to indicate rejection. The biopsy was consistent with fibrosing cholestatic hepatitis.
Attempts at reducing immunosuppression were not beneficial as the liver biochemistry continued to deteriorate with worsening jaundice. As published clinical studies of protease inhibitor in previously treated non-transplant patients, but exclusive of null responders, reported an approximate 40–52% sustained virologic response in non-responders 17, we approached the manufacturer of boceprevir (Merck Canada Inc, Kirkland QC) for provision on compassionate basis. At the time, protease inhibitors were not licensed in Canada, and had only recently been licensed in the United States. There was also no experience in the liver transplant setting with these agents at the time. After discussion with their global head office, Merck Canada agreed to provide boceprevir. Merck Global had not previously provided or authorized boceprevir in the posttransplant setting anywhere in the world. Due to provision of a non-licensed medication, informed consent was obtained from the patient.
The lead-in phase of therapy was started with adjusted doses of peginterferon alfa-2b (48 mcg) and ribavirin (400 mg bid). Immunosuppression was altered with discontinuation of mycophenolate mofetil and continuation of tacrolimus monotherapy. Boceprevir 800 mg tid was introduced at the beginning of week 4 as patient condition was rapidly deteriorating. The dose of tacrolimus was reduced by 50% (0.5 mg twice a day). Initially, the patient’s liver and kidney function deteriorated (total bilirubin 391 µmol/L, creatinine 486 µmol/L) and the trough tacrolimus trough level acutely increased to 24.3 µmol/L. There was an acute decrease in hemoglobin level (55 g/L) with no evidence of bleeding or hemolysis, with significant neutropenia (neutrophils 0.1). In addition, she developed tense ascites, despite a functioning peritoneovenous (LeVeen) shunt. The patient was hospitalized and managed conservatively with fluid support, packed red blood cell (PRBC) transfusion and therapeutic paracenteses. Antiviral and immunosuppressive treatment was held for one week including peginterferon, ribavirin, boceprevir and tacrolimus. Therapy was resumed within a week with adjusted tacrolimus dose (0.5 mg three times a week). Weekly granulocyte colony-stimulating factor (G-CSF) 300-mcg subcutaneous injection, and weekly epoetin alpha (Eprex) 10,000-unit subcutaneous injection was started. She continued on reduced peginterferon alfa-2b (48 mcg once weekly) and ribavirin (200 mg bid.) but full dose boceprevir (800 mg tid). With this regimen, the white cell count, neutrophils and hemoglobin remained stable (ranging from 63 g/L to 103 g/L), with less requirement of blood transfusion (2 units of PRBCs every 12 weeks) and the frequency of G-CSF was reduced to once every 2 weeks. The ascites resolved completely with no need for oral diuretics and the liver biochemistry including bilirubin normalized by week 8 (Figure 1).
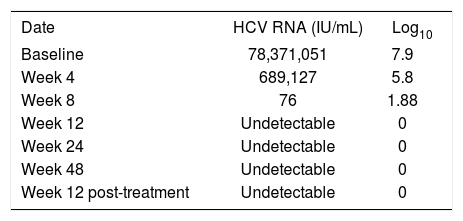
The virologic response was satisfactory despite adjusted low doses of peginterferon and ribavirin. The hepatitis C virus viral load decreased > 1 log at week 4 (5.8 log10), and at week 8, HCV viral load being 76 IU/mL (1.88 log10). At week 12, HCV viral load was undetectable and remained so up to week 48 of treatment and 12 weeks post-treatment (Table 1). The planned duration of treatment was 48 weeks, as HCV RNA level was detectable at week 8. However, because therapy was interrupted early in the course (at week 6), the patient received an empiric extra 6 weeks of triple therapy after the 48 weeks mark (i.e. total duration of treatment was 54 weeks).
DiscussionIn our patient, who had previously been a null responder to peginterferon and ribavirin pre-trans-plant, boceprevir use, in combination with reduced peginterferon and ribavirin was life saving. The effect of boceprevir at the time, was unknown in a previously treated null-responder group of patients, as they were excluded from the published study.17 Subsequently, a study that is unpublished, was presented at the American Association for the Study of Liver Conference reporting an approximately 40% SVR with boceprevir in non-transplant null responders18 The effect of boceprevir in post-transplant recipients, especially those with fibrosing cholestatic hepatitis (FCH), however, remains unknown with no published reports. Despite, the patient having a null-response to previous treatment (peginterferon and ribavirin), the decision was to proceed with a trial of triple therapy as the outcome of FCH is otherwise poor.
Our center is experienced using protease inhibitors in patients with chronic HCV infection in the non-transplant setting. Despite this, the lack of data about safety using protease inhibitors in transplant recipients made treating this patient very challenging. With boceprevir, it is known that a significant reduction (≥ 1 log10) in HCV RNA levels at week 4 of the peginterferon and ribavirin lead-in phase is associated with a significantly higher chance for SVR. This indicates virus sensitivity to peginterferon and ribavirin. Lack of this response is associated with reduced sustain virology response (SVR) rates, increased rates of virological failure, and resistance mutations.19 In our case, the lead-in phase was only 3 weeks as the patient’s liver function was progressively worsening. Boceprevir was started early because of a concern that if we waited, she might not tolerate the protease inhibitor triple combination therapy later. Despite the abbreviated lead-in, she had good response at week 4 (> 1 log10 reduction).
Protease inhibitors are potent CYP3A4 and p-glycoprotein inhibitors. They have significant drug interaction with calcineurin inhibitors and they increase the drug bioavailability and reduce drug metabolism, thereby significantly increasing blood concentrations. While no pharmacokinetic studies have been performed with boceprevir and calcineurin inhibitors, the effect of telaprevir on the pharmacokinetic parameters of tacrolimus and cyclosporine have been studied in healthy volunteers, showing increases with both (tacrolimus increase AUC 70-fold, Cmax 9.3 fold; cyclosporine increase AUC 4.6-fold, Cmax 1,4 fold).20 Concomitant administration of boceprevir with tacrolimus requires significant a dose reduction and prolongation of the dosing interval for tacrolimus, with close monitoring of tacrolimus blood concentrations and frequent assessments of renal function and tacrolimus-related side effects. Despite a reduced tacrolimus dosage pre-treatment, our patient still developed very high tacrolimus trough levels (i.e. > 20 ng/mL) requiring very attenuated tacrolimus dosing (i.e. a few times a week). It may be suggested that calcineurin inhibitors be held at the initiation of protease inhibitors and dosed based on close monitoring of blood levels in each individual patient. Anemia was another major issue during the treatment course. The etiology of anemia in this case was multifactorial, related to ribavirin effect on red blood cells, boceprevir and anti-rejection immunosuppressive effect s on bone marrow. Adjuvant therapies with erythropoietin stimulating agents and blood transfusions were required. In the published reports, the mean hemoglobin concentration reached a nadir approximately four to eight weeks after starting boceprevir.21 This was also observed in our patient, as the majority of her blood product transfusion requirements were in first ten weeks of treatment. Thereafter, she required less PRBCs transfusion (2 units every 12 weeks) although epoetin alpha was still required for hemoglobin stabilization.
Overall, the patient has had an excellent viral response to therapy, as her HCV RNA level was 76 copies/mL at week 8, and was undetectable at week 12, 24, 48 and 12 weeks post-treatment (the 24 week post-treatment HCV determination is pending as of this writing but it is expected that the patient will achieve a sustained virologic response). This was achieved despite suboptimal peginterferon and ribavirin dosing, with optimal boceprevir dosing, as dosing was limited by hematologic side effects. Most importantly, the liver dysfunction, both clinical and biochemical, associated with FCH have resolved with resolution appearing at week 8 of antiviral treatment. These results are promising for the management of both FCH and the post-transplant management of HCV genotype 1 that were previously treated pre-transplant and were null-responders.
ConclusionThe use of protease inhibitors in liver transplant recipients is challenging and requires careful adjustment of tacrolimus doses with frequent and close monitoring of the patient. Despite these challenges, our experience demonstrates that protease inhibitors can be life-saving in liver transplant recipients with FCH.