Evidence has been accumulated during the last decade showing that HCV infection plays an important activity at hepatic and extra-hepatic level. Chronic HCV is associated with a large spectrum of extra-hepatic manifestations including lympho-pro-liferative diseases and metabolic abnormalities (such as insulin resistance and fatty liver disease).
Material and methodsWe have performed an extensive review of the medical literature regarding the increased risk of cardiovascular and kidney disease that has been observed in various groups of HCV-infected patients. The potential link between such increased risk and the metabolic consequences of chronic HCV infection has been explored.
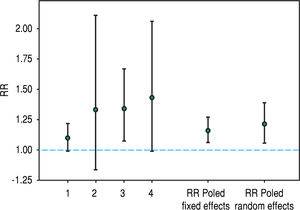
ResultsAccording to a systematic review with a meta-analysis of longitudinal studies (n = 9 clinical observational studies; n = 1,947,034 unique patients), we found a strong relationship between positive anti-HCV serologic status and increased incidence of chronic kidney disease in the adult general population, the summary estimate for adjusted hazard ratio was 1.43 (95% confidence intervals, 1.23; 1.63, P = 0.0001) (random-effects model) in anti-HCV positive patients. In another meta-analysis of clinical observational studies (n = 145,608 unique patients on long term dialysis; n = 14 observational studies), anti-HCV sero-positive status was an independent and significant risk factor for death in patients on maintenance dialysis. The summary estimate for adjusted relative risk (all-cause mortality) was 1.35 with a 95% confidence interval (CI) of 1.25; 1.47 (P < 0.01) in anti-HCV positive patients on maintenance dialysis. An updated and stratified analysis (n = 4 studies, n = 91,916 patients on maintenance dialysis) resulted in an adjusted HR for cardiovascular mortality among anti-HCV positive patients of 1.21 (95% CI, 1.06; 1.39) (P < 0.01); the homogeneity assumption was not rejected. The mechanisms underlying such relationships remain unclear; it has been suggested that HCV promotes atherogenesis through direct and indirect mechanisms.
ConclusionsClinical trials are under way to assess whether the clearance of HCV RNA from serum by direct-acting antiviral drugs reduces all-cause or disease-specific (cardiovascular) mortality among patients on maintenance dialysis.
Hepatitis C virus infection is still frequent among patients undergoing long-term dialysis all over the world. Hepatitis C virus was identified in 1989; since then, an enormous body of evidence has been accumulated showing a large frequency of anti-HCV positive patients on maintenance dialysis patients in developed countries. The epidemiology of HCV infection in patients on long-term dialysis in developed countries has been studied by large multicenter surveys; prevalence and incidence rates are decreasing due to the implementation of infection control procedures, as recommended in several clinical guide-lines.1,2
According to the DOPPS (the Dialysis Outcomes and Practice Patterns Study), a large observational survey published more than 10 years ago, the prevalence of anti-HCV antibody positive patients was 13.5% in a large cohort (n = 8,615) of patients on maintenance dialysis in 308 Dialysis Centers in developed world (France, Germany, Italy, Japan, Spain, United Kingdom, and USA).3 An update of the DOPPS was recently made- the rate of anti-HCV positive patients undergoing maintenance dialysis (n = 49,762) in various developed countries (n = 12) was 9.5%.4
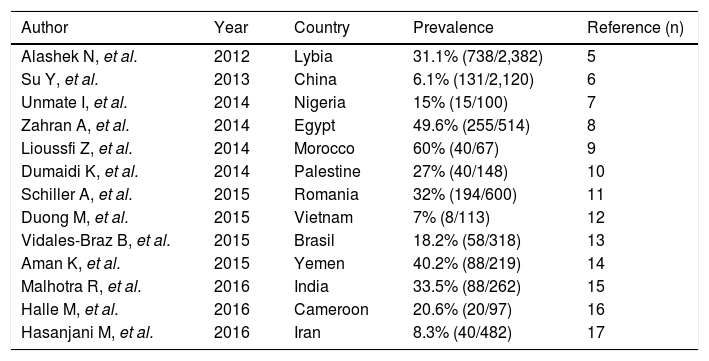
The epidemiology of HCV infection among patients undergoing maintenance dialysis in developing countries is less known, the information is mostly based on small, single-center surveys. As listed in table 1, the evidence accumulated to date shows important heterogeneity and very high (> 50%) prevalence rates.5-17
Prevalence rates of anti-HCV positive patients on maintenance dialysis in developing countries.
| Author | Year | Country | Prevalence | Reference (n) |
|---|---|---|---|---|
| Alashek N, et al. | 2012 | Lybia | 31.1% (738/2,382) | 5 |
| Su Y, et al. | 2013 | China | 6.1% (131/2,120) | 6 |
| Unmate I, et al. | 2014 | Nigeria | 15% (15/100) | 7 |
| Zahran A, et al. | 2014 | Egypt | 49.6% (255/514) | 8 |
| Lioussfi Z, et al. | 2014 | Morocco | 60% (40/67) | 9 |
| Dumaidi K, et al. | 2014 | Palestine | 27% (40/148) | 10 |
| Schiller A, et al. | 2015 | Romania | 32% (194/600) | 11 |
| Duong M, et al. | 2015 | Vietnam | 7% (8/113) | 12 |
| Vidales-Braz B, et al. | 2015 | Brasil | 18.2% (58/318) | 13 |
| Aman K, et al. | 2015 | Yemen | 40.2% (88/219) | 14 |
| Malhotra R, et al. | 2016 | India | 33.5% (88/262) | 15 |
| Halle M, et al. | 2016 | Cameroon | 20.6% (20/97) | 16 |
| Hasanjani M, et al. | 2016 | Iran | 8.3% (40/482) | 17 |
The aim of this paper is to make a review of the medical literature regarding the increased risk of cardiovascular and kidney disease that has been observed in various groups of HCV-infected patients. The link between such increased risk and the metabolic consequences of chronic HCV infection has been explored.
Material and MethodsData Sources and SearchesWe reviewed English-language citations from the National Library of Medicine's Medline database from 1989 through July 1, 2016. Data on HCV status were not available before 1989, when the first –generation assay for anti-HCV antibody was manufactured. Four Medline database engines (Ovid, PubMed, Embase, and Grateful Med) were used. Medline searches were limited to human studies. An additional search was performed with electronic searches of the Current Contents Cochrane Library; manual searches of selected specialty journals were performed to identify all pertinent literature. Unpublished studies and abstracts were not considered for inclusion in this review. The relationships between CKD, HCV and anti-HCV therapy in the general population were explored with the following search terms: ‘chronic kidney disease’ OR ‘CKD’ OR ‘kidney failure’ OR ‘kidney injury’ OR ‘renal disease’ OR ‘renal failure’ OR ‘renal insufficiency’ AND ‘hepatitis C’ OR ‘hepatitis C virus infection’ OR ‘HCV’ OR ‘chronic HCV’ AND ‘anti-HCV therapy’ OR ‘anti-HCV treatment’ OR ‘interferon’. The impact of HCV upon survival in the dialysis population was evaluated with the following search terms: ‘hepatitis C’ OR ‘hepatitis C virus infection’ OR ‘HCV’ OR ‘chronic HCV’ AND ‘death risk’ OR ‘mortality’ OR ‘survival’ AND ‘chronic kidney disease’ OR ‘dialysis’ OR ‘end-stage renal disease’ OR ‘haemodialysis’ OR ‘peritoneal dialysis’.
ResultsHCV and survival among patients on dialysisIt is well known that cardiovascular diseases are the major causes of morbidity and mortality in patients with CKD Stage 5 including those on regular haemodialysis. In addition, the frequency of cardiovascular diseases among patients with chronic kidney disease is greater than that observed among patients with intact kidney function.18It has been observed a great prevalence of traditional factors (age, male gender, arterial hypertension, diabetes mellitus, dyslipidemia, and physical inactivity) for cardiovascular disease among patients with CKD stage 5. In addition, patients with CKD Stage 5 have disease-related risk factors for cardiovascular disease that include anaemia, hyper-ho-mocysteinemia, hyperparathyroidism, oxidative stress, hy-poalbuminemia, and chronic inflammation, among others.
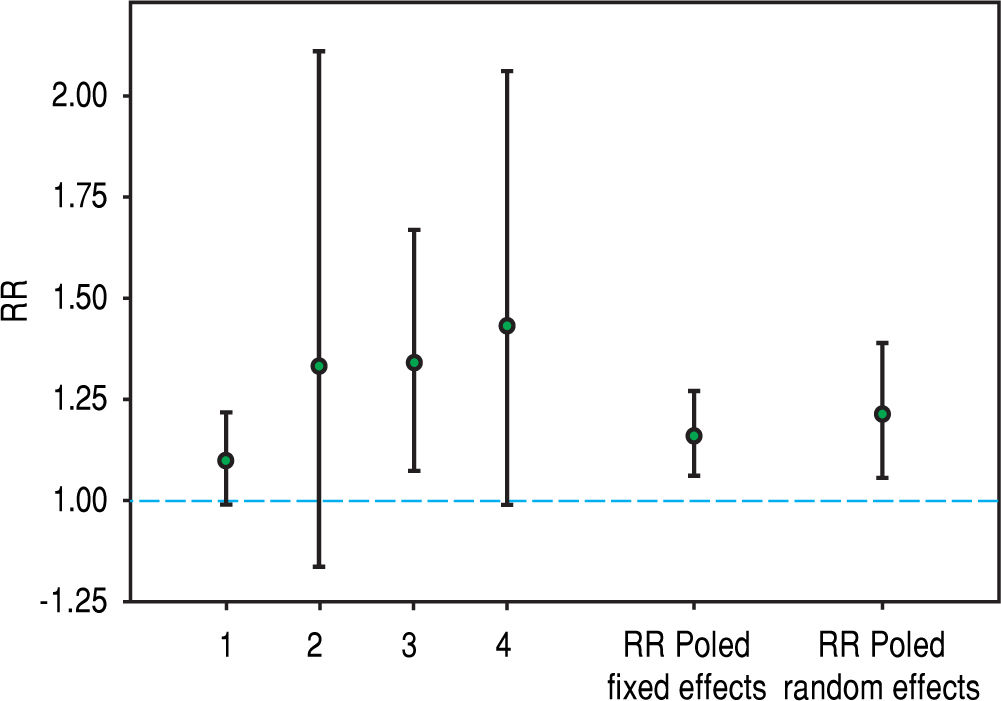
Recently, hepatitis C virus has been included in the group of non-traditional (and modifiable) risk factors for death in patients undergoing maintenance dialysis. In fact, numerous observational (population-based and hospital-based) studies have been published during the last twenty years suggesting a detrimental role of HCV upon survival among patients on regular dialysis. We have performed a systematic review and meta-analysis of clinical studies, and 145,608 unique patients on long term dialysis (n = 14 observational studies) were retrieved; pooling of study results demonstrated that anti-HCV sero-positive status was an independent and significant risk factor for death in patients on maintenance dialysis.19 The summary estimate for adjusted relative risk (all-cause mortality) was 1.35 with a 95% confidence interval (CI) of 1.25; 1.47 (P < 0.01) in anti-HCV positive patients. It remains unclear if the elevated mortality risk due to HCV infection is attributable only to an increase in liver disease-related deaths. A stratified analysis showed that the adjusted RR for liver disease-related death was 3.82 (95% CI, 1.92; 7.61); heterogeneity statistics, R(i) = 0.58 (P-value by Q-test = 0.087). We updated our stratified analysis regarding the impact of anti-HCV seropositive status on CV risk in the dialysis population. This information was given in four reports (n = 91,916 patients).20-23 The adjusted HR for cardiovascular mortality that is associated with anti-HCV seropositive status was 1.21 (95%CI, 1.06; 1.39) (Figure 1); according to asymptotic and bootstrap heterogeneity tests, the homogeneity assumption was not rejected.
These data confirm the extra-hepatic activity of chronic hepatitis C virus infection among patients with intact or impaired kidney function. The increased cardiovascular risk in anti-HCV positive patients undergoing maintenance dialysis may stem from insulin resistance and fatty liver disease, contributing to endothelial dysfunction and increase in systemic inflammatory markers.24-28 Insulin resistance and hepatic steatosis may contribute to the occurrence of other components of the metabolic syndrome such as arterial hypertension and dyslipidemia.
HCV and survival among patients with intact kidney functionHistorically, HCV infection has been considered to affect only the liver, via the development of cirrhosis and its complications. HCV infection has also been shown to increase the risk of overall mortality and the occurrence of extra-hepatic complications, such as lympho-proliferative disorders and metabolic alterations (insulin resistance and diabetes mellitus). HCV-driven B cell lympho-prolifera-tive disorders likely comprise a spectrum of disease, varying from asymptomatic clonal B cell expansions to pathogenic cryoglobulinemia and lymphoma.
Since 2001, numerous observational studies have investigated the relationship between HCV infection and cardiovascular risk in the adult general population. A novel meta-analysis of observational studies (n = 22; n = 730,645 unique patients) found a negative impact of HCV infection upon cardiovascular mortality, aOR, 1.65 (95% CI, 1.07; 2.56, P = 0.02), and development of carotid plaques aOR, 2.27 (95% CI, 1.76; 2.94, P < 0.01).29 In addition, a detrimental role of HCV on cerebro-cardiovascular events, aOR, 1.30 (95% 1.10; 1.55, P = 0.002) was observed. The authors also observed significant heterogeneity in the first and third subset of studies (I2 = 76, P = 0.02 and I2 = 91%, P < 0.001, respectively), and this precluded definitive conclusions. In other words, aggregate data from 22 studies showed that compared to uninfected controls, HCV-infected individuals have increased risks of cardiovascular mortality and subclinical atherosclerosis in the adult general population.
The meta-analyses reported above have limitations, which are intrinsic to the nature of included studies and provide the basis for future research. These are based on observational studies and, consequently, could be biased by residual confounding (confounding remaining after adjustment). As an example, information on some covariates (such as injection drug use or exposure to nephrotoxic drugs) was not given in all the included studies. It was believed that meta-analyses should be conducted only on RCTs in order to reduce the risk of misleading conclusions. However, many diseases can only be studied obser-vationally. If these studies have good quality, it is appropriate to include them in a meta-analysis. Our conclusion is that awaiting further studies, patients with HCV infection, either undergoing dialysis or having intact kidney function, should be regarded as being at increased risk of cardiovascular risk, regardless of the presence of conventional risk factors for kidney disease.
Impact of HCV upon CKD: data from the general populationKnowledge has been recently gathered regarding a link between hepatitis C virus and chronic kidney disease. Hepatitis C virus and chronic kidney disease are prevalent in the general population and associated in various ways-patients with end-stage renal disease are at increased risk of acquiring HCV and some types of renal disease are precipitated by HCV infection. Conventional risk factors for chronic kidney disease such as ageing, diabetes, hypertension and metabolic syndrome do not fully explain the current frequency of chronic kidney disease in the adult general population of developed countries. In addition to these conventional risk factors, various pieces of evidence in the last decade have implicated hepatitis C virus infection as a cause of kidney disease. Numerous surveys have been performed in the last decade to address this point. A meta-analysis of observational studies with cross-sectional design (n = 8 studies, n = 788,027 unique patients) did not find an increased prevalence of CKD among patients with HCV, adjusted OR with HCV across the studies, 1.16 (95% CI, 0.98; 1.33, P = NS).30 Pooling results from longitudinal studies (n = 9; n = 1,947,034 unique patients) demonstrated a relationship between anti-HCV positive serologic status and an increased incidence of chronic kidney disease in the adult general population- the summary estimate for adjusted hazard ratio with HCV was 1.43 (95% CI, 1.23; 1.63, P = 0.0001), according to a random-effect model. Significant heterogeneity (test for homogeneity of the aHR across the nine studies = Q-value [by χ2 test] of 123.6, P = 0.001) was noted and this precluded more definitive conclusions.30 According to an updated systematic review with meta-analysis of cross-sectional studies (n = 7), HCV positive serology was an independent risk factor for proteinuria in the adult general population; adjusted odds ratio with HCV, 1.50 (95% confidence intervals, 1.23; 1.82, P = 0.0001) (n = 7 studies, n = 141,085 unique pa-tients).31-37
Epidemiologic data regarding the prevalence of chronic kidney disease in HCV-infected patients were until recently limited and used variable criteria for the definition of CKD; also, the demographic/clinical characteristics of the representative patient population were variable. Three studies performed in the US a few years ago38-40 reported that the unadjusted prevalence of low GFR (GFR < 60 mL/min/1.73 m2) ranged at baseline between 5.1% and 8.0% among anti-HCV seropositive individuals. The unadjusted prevalence of renal insufficiency (serum creatinine> 1.5 mg/dL) in a large study of anti-HCV seropositive veterans from the US was 4.8%.41 In a large cohort of HCV positive/HIV positive patients from North America, the unadjusted frequency of low GFR (GFR < 60 mL/min/1.73 m2) at baseline ranged between 3.7% and 4%.42 Based on modified K/DOQI guidelines, serial measurements of eGFR and proteinuria were obtained in a large cohort of metropolitan residents in the US- the prevalence of CKD was greater among anti-HCV positive/HCV RNA positive patients compared to (age-, race-, and gender-matched) anti-HCV negative controls, 9.1% vs. 5.1%, P < 0.04.43
There is emerging information concerning the role of HCV in extra-hepatic manifestations, including metabolic derangements and, more recently, accelerated atherosclerosis. An accelerated atherosclerosis at kidney level induced by HCV has been mentioned. Atherosclerosis, either sublinical or manifest, is a chronic inflammatory disease; in addition to the ‘traditional’ factors of atherosclerosis other ‘novel’ factors (such as chronic HCV infection) have been advocated. The possible role of an infectious agent in the development of experimental atherosclerosis in rodents was reported more than a century ago44 and this hypothesis had gained renewed interest recently.45It is likely that HCV promotes atherogenesis through several direct and indirect biological mechanisms. HCV RNA sequences have been isolated from carotid plaques:46 HCV could play a direct atherogenic role by inducing arterial inflammation. Chronic HCV causes hepatic, systemic inflammation and hepatic steatosis which is currently considered an early mediator of atherosclerosis. Steatosis promotes the development of atherosclerosis through multiple factors including insulin resistance, hypo-adiponectinemia, metabolic syndrome, oxidative stress, hyper-homocysteinaemia, and increased synthesis of tumor necrosis factor-alpha.24-28 These activities occur independently of traditional known risk factors such as smoking, hypercholesterolemia, and hypertension. In addition to local hepatic inflammation, a concomitant low-grade systemic inflammation has been mentioned in several reports, as suggested by activation of blood mono-cytes, higher levels of systemic markers of inflammation, particularly fibrinogen, C reactive protein, erythrocyte sedimentation rate, and N-terminal pro-brain natriuretic peptide.
Impact of HCV upon CKD: additional dataChronic kidney disease affects 4%-13% of the Western adult population and over 25% of individuals older than 65 years. Recent data emphasized the rising prevalence of CKD in the general population, this is related to the rising epidemic of its risk factors such as overweight, diabetes mellitus, arterial hypertension, smoking, and ageing. The high morbidity, mortality and health care costs associated with CKD have led investigators to search for non-traditional risk factors such as chronic hepatitis C virus infection. Hepatitis C virus infection is endemic worldwide, with an estimated global prevalence of 3% (approximately 170 million infected people). Hepatitis C virus has hepatic and extra-hepatic manifestations and has been implicated in perturbation of multiple organ systems including the ocular, skeletal, nervous, cardiovascular systems and skin. In addition, HCV has a deleterious impact on psyco-social status.
Renal involvement by hepatitis C virus was first reported more than two decades ago; the knowledge of the association between HCV and chronic kidney disease (low eGFR or abnormal proteinuria) in the adult general population was limited and controversial until a few years ago. A growing body of evidence has recently highlighted the detrimental role of HCV infection on the risk of chronic kidney disease. Cohort studies performed in various patient groups38-43,47,48 demonstrated a significant relationship between anti-HCV positive serologic status and accelerated progression of chronic kidney disease. Soma, et al. evaluated 123 patients (24 anti-HCV seropositive) with type 2 diabetic-related nephropathy over a 27-month follow-up.47 In their multiple linear regression analysis adjusted for the effect of several covariates (age, gender, blood pressure, gli-catedhaemoglobin, urinary protein excretion, and histologic parameters) urinary protein excretion (P = 0.011), severe arteriolar hyalinosis (P = 0.006), and anti-HCV positive (P < 0.001) serologic status were significantly associated with a decline of renal function as reflected by the slope of reciprocal serum creatinine (1/Scr).
Noureddine and coworkers49 at the Indiana University School of Medicine retrospectively identified 111 patients (21% showing anti-HCV antibody in serum) with primary glomerulonephritis on kidney biopsy over 4 years, and evaluated the progression of CKD over time. The hepatitis C-positive subjects were more likely to be African American (P = 0.031), followed for fewer days (P = 0.007) and have diabetes and focal segmental glomerulosclerosis on biopsy (P < 0.001). Longitudinal follow-up of CKD progression using multiple creatinine measures analyzed by repeated measures ANCOVA demonstrated an increase in serum creatinine of 1.3 mg/dL/year for hepatitis C subjects compared to either hepatitis C-negative or those not tested (P < 0.001).
Ble, et al.50 discovered that a sustained viral response was associated with improved kidney function among liver transplant recipients treated for HCV infection. All treated LT recipients with a minimum observation period of at least 1 year since the end of antiviral treatment (n = 175 LT patients) were identified. In the subset of patients (n = 99) with stage 2 CKD before antiviral treatment, the median changes in the MDRD-4 values before treatment to 1, 3, and 5 years after treatment were -0.5, 4.5, and 9.4 mL/min for the SVR patients and -1, -0.3, and -1.5 mL/min for the non-responder recipients (NRs) (P = 0.6, P = 0.06, and P = 0.004, respectively). In the multivariate analysis, there was a relationship between kidney dysfunction at the last follow-up and NRs [relative risk (RR) 3.8; 95% confidence intervals (CIs) = 1.3; 11.23, P = 0.01], MDRD-4 values at the end of treatment (EOT) (RR = 1.022, 95% CI = 1.001; 1.04, P = 0.04), and female gender (RR = 5.6, 95% CI = 1.84; 17.5, P = 0.002).
Another piece of evidence regarding the link between chronic HCV and CKD comes from additional epidemio-logical studies. Various authors found that the prevalence of anti-HCV antibody was significantly higher in patients with chronic kidney disease before reaching end-stage renal disease than in a healthy population, independent of blood transfusions.51,52 Iwasa, et al.52 enrolled 400 patients who began regular haemodialysis between February 2003 and June 2007 at Tokyo University School of Medicine. As healthy controls they used healthy blood donors (n = 70,717) with data were obtained from Tokyo Metropolitan Red Cross Blood Center. The anti-HCV antibody prevalence rate among the patients who were new to haemodialysis was 7.3%, as opposed to 0.15% in the healthy volunteers. The prevalence of anti-HCV seropositive status in the 31-45-, 46-60-, and 61-year-old groups was significantly higher among the haemodialysis patients than among the healthy volunteers (P = 0.0209, P < 0.0001, and P < 0.0001, respectively). Among the anti-HCV-antibody-positive patients, 55.2% had received a blood transfusion. The rate was significantly higher than among the anti-HCV-antibody-negative patients (19.4%, P < 0.0001). The authors concluded that the frequency of anti-HCV antibody positivity was greater in patients new to haemodialysis than in healthy volunteers. Older age, blood transfusion, male gender, and diabetic ne-phropathy seemed to be risk factors for anti-HCV antibody positivity in Japan.
The relationship between anti-HCV antibody positive status and chronic kidney disease has been also addressed among HIV infected populations. In the Women's Intera-gency HIV study, anti-HCV positive serologic status was independently associated with a fully adjusted net decrease in eGFR of approximately 5% per year (95% confidence interval, 3.2 to 7.2) relative to women who were seronega-tive.53 One systematic review had been already published on the impact of HIV/HCV co-infection on the incidence of CKD;54 we performed a meta-analysis and retrieved 19 studies (n = 146,1151 unique patients with HIV infec-tion).55 Aggregation of longitudinal studies (n = 8; n = 105,462 unique patients) showed a link between anti-HCV positive serologic status and increased risk of reduced glomerular filtration rate among HIV-infected patients, the summary estimate for adjusted hazard ratio (aHR) was 1.64 (95% CI, 1.28; 2.0, P < 0.001) of incidence of CKD in HIV-HCV co-infected individuals compared with mono-infected HIV positive individuals. No between-studies heterogeneity was found (P-value by Q test = 0.08). Anti-HCV antibody positive serologic status was an independent risk factor for proteinuria in HIV-infected population; adjusted effect estimate, 1.23 (95% CI, 1.18; 1.28, P = 0.001) (n = 6 studies, n = 26,835 unique patients). In meta-regression, we noted the impact of ageing (P = 0.0001) upon the adjusted hazard ratio of incidence of reduced glomerular filtration rate among HCV-HIV co-infected populations.
HCV co-infection appears as a risk factor for increased healthcare resource utilization in HIV-infected individuals in the USA; multivariate Poisson model showed that HCV co-infection was associated with higher frequency of emergency department visits, aRR 2.07 (95% CI 1.49; 2.89), P < 0.001. In addition to liver injury, renal disease (37% vs. 12%) played a larger role.56
Classically, HCV infection predisposes to cryoglob-ulinemicmembrano-proliferative glomerulonephritis or non-cryoglobulinemic glomerular disease; however, HCV-positive individuals may also be at risk for kidney injury secondary to end-stage liver disease, injecting drug use, concomitant HIV or HBV co-infection. Also, an accelerated atherosclerosis may increase the risk of developing kidney disease among HCV-infected individuals. The data reported above promote the screening for CKD among HCV-infected individuals even in the absence of traditional risk factors for kidney disease.
HCV, metabolic changes and chronic kidney diseaseData from the 1988 to 1994 NHANES (National Health and Nutrition Examination Survey) have shown a significant and independent relationship between insulin resistance, diabetes mellitus and HCV infection. The rapid increase in the prevalence of obesity has been cited to explain the absence of relationships between insulin resistance, HCV, and diabetes in later NHANES surveys (1998-2008). Multiple additional studies have suggested a link between chronic HCV, insulin resistance, type 2 diabetes mellitus, and subsequent steatosis. In their longitudinal study on 4,958 individuals from southern Taiwan, Wang, et al. found that anti-HCV positive serologic status (hazard ratio, 1.7, 95% CI, 1.3-2.1), HBV/HCV co-infection (hazard ratio = 1.7), overweight, obesity, and ageing were significantly associated with diabetes (P < 0.05).57 The authors concluded that HCV infection is an independent risk factor for diabetes, especially for HCV positive patients who are younger or have a greater body mass index. Clearance of HCV RNA from serum by combined antiviral therapy (pegylated interferon and ribavirin) reduced insulin resistance at 12 and 24 weeks and at the end of antiviral therapy (24 or 48 weeks).58
Another metabolic consequence of chronic HCV is hepatic steatosis. Hepatic steatosis is a distinguishable feature of HCV infection. It remain uncertain whether steatosis is directly related to the presence of HCV or results from host-related factors. Growing experimental and epidemiological data suggests that HCV infection, non-alcoholic fatty liver disease and CKD share common pathogenic mechanisms and interactions. NAFLD encompasses a histologic spectrum ranging from simple steatosis to non-alcoholic steato-hepatitis NASH, the latter with or without advanced fibrosis. In a recent systematic review with meta-analysis of clinical studies (n = 32 studies, n = 63,902 participants), Musso, et al. found that NAFLD was associated with a greater risk of prevalent (Odds Ratio, 2.12, 95% CI, 1.69; 2.66) and incident (HR, 1.79, 95% CI, 1.65; 1.95) chronic kidney disease. These associations remained statistically significant in diabetic and non-diabetic individuals, as well as in studies adjusting for conventional risk factors for CKD and were independent from insulin resistance and obesity.59
Anti-HCV therapy and extra-hepatic outcomesOf note, antiviral therapy towards HCV significantly improves hepatic and extra-hepatic outcomes in the general population60 and among patients co-infected with HIV and HCV.61 A population-based study in Taiwan has retrospectively shown that interferon-based therapy (IBT) reduces the risk of stroke in patients with chronic hepatitis C. 208 patients who received IBT for more than 3 months were included in the IBT cohort, and 2,875 subjects who did not receive IBT between 2004 and 2008 were included in the non-IBT cohort. The median duration of follow-up was 4.78 years. Use of IBT (defined as treatment with in-terferon alpha, pegylated interferon alpha-2a or pegylated interferon alpha-2b for at least 3 months) significantly reduced the risk of stroke in HCV patients (adjusted HR = 0.39, 95% CI = 0.16; 0.95, P = 0.039) after adjusting for known prognostic factors (age, gender, co-morbidities, health behaviour, and drugs).60
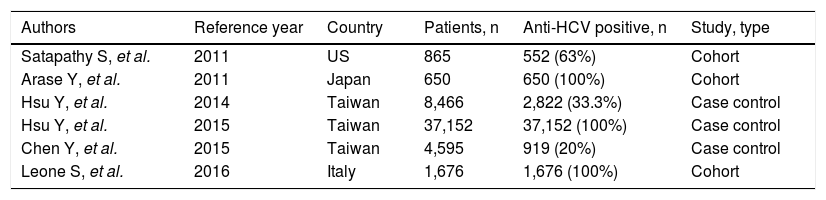
According to an updated review, a total of six studies have addressed the impact of IFN-based regimens on the progression of chronic kidney disease.43,62-66 In five43,62-65 multivariate analysis suggested that treatment of HCV infection may improve per se renal survival (Table 2). In the nationwide cohort study based on the Taiwan National Health Insurance Research Database (NHIRD), a total of 12,384 eligible patients who had received antiviral treatment (pegylated interferon plus ribavirin) towards HCV were matched 1: 2 with 24,768 untreated controls.62 The treated and untreated cohorts were followed for a mean duration of 3.3 (+2.5) and 3.2 (+2.4) years, respectively. The calculated 8-year cumulative incidences of end-stage renal disease between treated and untreated patients were 0.15% vs. 1.32% (P < 0.001). Multivariate-adjusted Cox regression revealed that antiviral treatment was associated with lower risk of end-stage renal disease (HR, 0.15; 95% CI, 0.07; 0.31, P < 0.001). Antiviral treatment was also associated with an adjusted HR of 0.77 (95% CI, 0.62; 0.97, P = 0.026) for acute coronary syndrome, and 0.62 (95% CI, 0.46; 0.83, P = 0.001) for ischemic stroke. These associations were invalid in incompletely treated patients (duration of antiviral treatment, < 16 weeks). Various confounders entered the multivariate analysis, including death as a competing cause of risk. An important limitation of the current study is that the NHIRD does not contain information regarding virology such as the viral genotype, the sustained viral response to antiviral therapy, and the HCV viral load.
Clinical studies addressing the association between interferon-based regimens for HCV and the risk of chronic kidney disease in the general population.
| Authors | Reference year | Country | Patients, n | Anti-HCV positive, n | Study, type |
|---|---|---|---|---|---|
| Satapathy S, et al. | 2011 | US | 865 | 552 (63%) | Cohort |
| Arase Y, et al. | 2011 | Japan | 650 | 650 (100%) | Cohort |
| Hsu Y, et al. | 2014 | Taiwan | 8,466 | 2,822 (33.3%) | Case control |
| Hsu Y, et al. | 2015 | Taiwan | 37,152 | 37,152 (100%) | Case control |
| Chen Y, et al. | 2015 | Taiwan | 4,595 | 919 (20%) | Case control |
| Leone S, et al. | 2016 | Italy | 1,676 | 1,676 (100%) | Cohort |
Another survey from the NHIRD database, a single payer system that covers nearly the entire population of Taiwan, compared the risk of chronic kidney disease in patients (n = 919) who received three months or more of interferon-based therapy with controls (n = 3,676 patients; untreated cohort) matched by propensity score. The adjusted HR for CKD in patients treated with inter-feron-based therapy was 0.42 (95% CI, 0.20; 0.92, P = 0.03). Multivariable stratified analysis revealed that the greater risk reduction of CKD occurred among HCV-in-fected patients with hyperlipidemia, diabetes mellitus, arterial hypertension, and those without coronary artery disease.63
Improved renal (and cardiovascular) outcomes after antiviral treatment for hepatitis C virus infection have been observed in diabetic patients residents in Taiwan, according to the NHIRD. A total of 1,411 eligible patients who received pegylated interferon plus ribavirin (treated cohort), and matched 1:1 with 1,411 untreated controls by propensity scores were enrolled. The authors included an additional group of 5,644 diabetic patients without HCV infection (uninfected cohort) matched 4:1 with the treated cohort. From 2003 to 2011, the 8-year cumulative incidences of end-stage renal disease in the treated, untreated, and uninfected cohorts were 1.1% (95% CI, 0.3; 2.0), 9.3% (95% CI, 5.94; 12.7), and 3.3% (95% CI, 2.3; 4.3) (P < 0.001). As compared with the untreated cohort, antiviral treatment was associated with multivariate-adjusted hazard ratio of 0.16 (95% CI, 0.07; 0.33) for ESRD and 0.53 (95% CI, 0.3; 0.93) for ischemic stroke.64
Similar findings have been obtained from studies performed in other countries. Arase, et al. retrospectively followed 650 HCV-infected, liver cirrhotic patients who were treated with interferon and had an estimated glomer-ular filtration rate (eGFR) of > 60 mL/min per 1.73m2 after termination of interferon therapy.65 The cumulative development rate of end-stage renal disease was determined to be 0.4% at the 5th year, 1.6% at the 10th year and 2.8% at the 15th year by Kaplan-Meier method. Multivari-ate Cox proportional hazard analysis showed that ageing, diabetes, hypertension, and non-clearance of HCV (HR, 2.67; 95% CI, 1.34; 5.32; P = 0.005) were risk factors for CKD. In a hospital-based study from the US, 552 HCV-infected American patients were retrospectively evaluated and 159 received IFN therapy during a 7-year follow-up. The prevalence of CKD in the anti-HCV positive group was greater compared to controls (313 patients without HCV infection matched for age, race, gender), 9.6% vs. 5.1%, P = 0.02. Multivariate logistic regression analysis, performed in patients with serum positive for antibody for HCV, showed that history of IFN treatment was an independent negative predictor for CKD (OR, 0.18; 95% CI, 0.06; 0.56, P < 0.003).65
The Italian multicenter survey demonstrated conflicting findings. The impact of sustained viral response to hepatitis C virus therapy on the outcome of extra-hepatic complications (occurrence of CKD, diabetes, and cardiovascular disease) was addressed in a cohort of human immunodeficiency virus –infected patients. A total of 1,676 patients from the Italian Management of Standardized Evaluation of Retroviral HIV Infection (MASTER) cohort were included, 340 (20.3%) patients initiated an interferon-based regimen and 102 (30%) achieved SVR during the observation period. In the Cox regression model for treated patients, there was no association between SVR and lower risk of CKD, 1.05 (0.29; 3.9) (NS).66
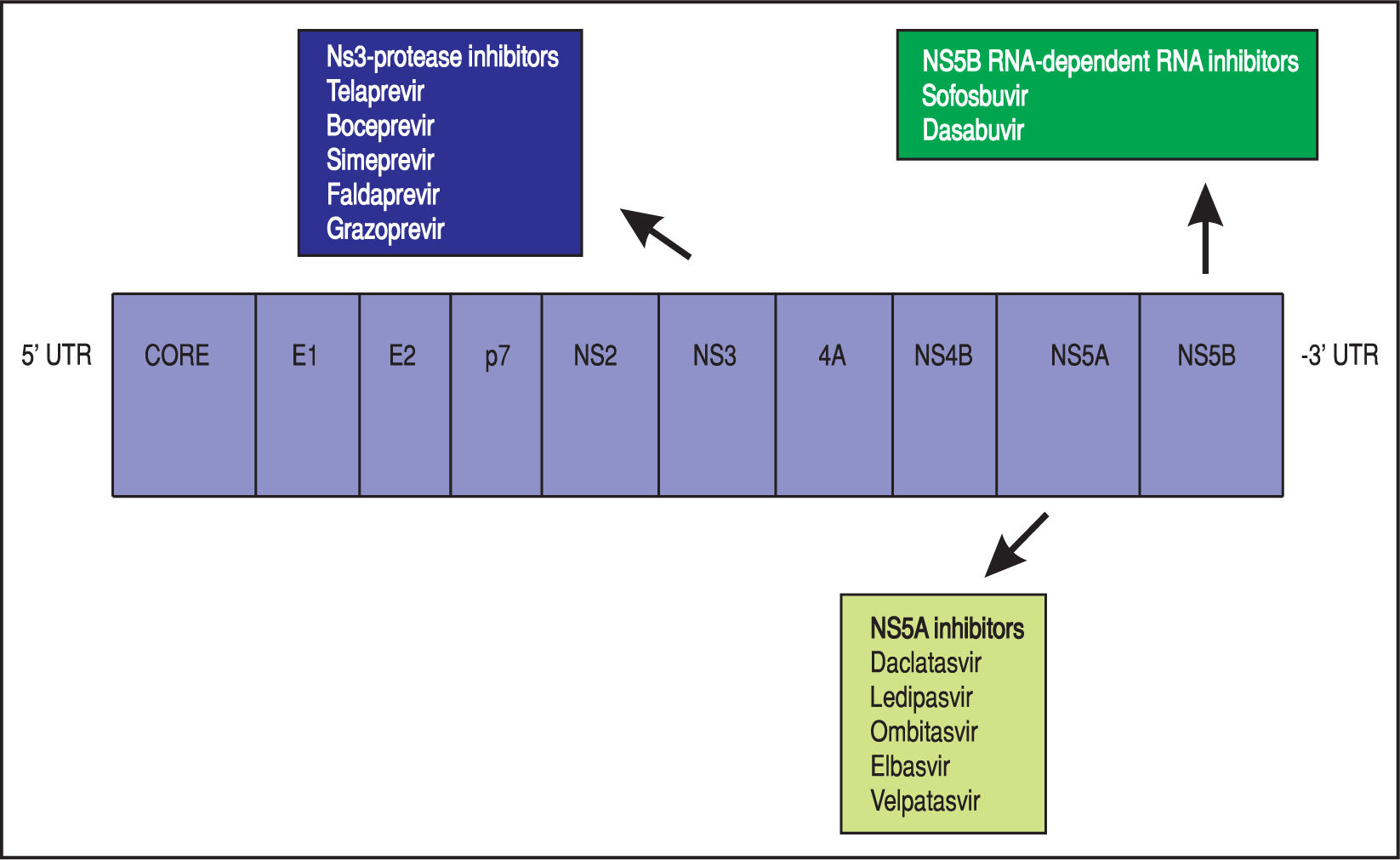
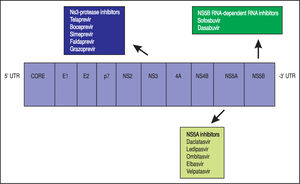
Theoretically, a randomised-placebo controlled trial is the ideal design to clarify how antiviral treatment improves extra-hepatic outcomes of HCV. On the other hand, critical events commonly take time to develop, as they occur at a low incidence in an average-risk population. A great sample size with a long follow-up would be required; in addition, randomisation to placebo can hardly be considered as ethically acceptable nowadays for HCV-infected patients, as safe and effective drugs for anti viral therapy of HCV are currently available. The mechanisms underlying such relationships remain unclear; it has been suggested that HCV promotes atherogenesis through direct and indirect mechanisms. Clinical trials are in progress to assess whether the clearance of HCV RNA from serum by direct-acting antiviral drugs (DAAs) (Figure 2) (instead of IFN-based therapies) reduces the frequency of cardiovascular disease in dialysis patients and chronic kidney disease in the adult general population.
Discussion and ConclusionsMany epidemiological and experimental data have been recently accumulated suggesting that active HCV infection is linked to an increased incidence or progression of CKD in the adult general population. Also, it appears that a greater CV risk exists in patients on maintenance dialysis with active HCV. However, despite the growing evidence, it has not been definitively established whether causal relationships occur. The evidence reported above suggest that patients with HCV should be screened for CKD even in the absence of conventional risk factors for CKD. We urgently need randomized, double-blind, placebo-controlled trials regarding the impact of the treatment for liver disease in HCV-posi-tive patients on the incidence and progression of CKD in the general population. Clinical trials are ongoing to assess whether the clearance of HCV RNA from serum by direct-acting antiviral drugs reduces all-cause or disease-specific (cardiovascular) mortality among patients on maintenance dialysis.
Abbreviations- •
CDC: Centers for Disease Control and Prevention.
- •
CI: confidence intervals.
- •
CKD: chronic kidney disease.
- •
CV: cardiovascular.
- •
DAAs: direct-acting antiviral agents.
- •
eGFR: estimated glomerular filtration rate.
- •
ESRD: end-stage renal disease.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HCV RNA: hepatitis C virus viremia.
- •
HD: haemodialysis.
- •
HIV: human immunodeficiency virus.
- •
HR: hazard ratio.
- •
HD: haemodialysis.
- •
I2: ratio of true heterogeneity to total variation in observed effects.
- •
NA: not available.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steato-hepatitis.
- •
RCT: randomized clinical trial.
- •
SVR: sustained virological response.