Hepatocellular carcinoma (HCC) typically originates from HBV or HCV associated liver cirrhosis. Primary Sjögren’s syndrome (pSS) is a kind of autoimmune disease. A sixty-two year old female patient with mild liver damage was diagnosed with pSS after excluding viral, alcoholic and drug-induced hepatitis according to serum immunological detection and liver biopsy. But when she was hospitalized for a second time two years later, a CT scan revealed liver neoplasm. Surgery confirmed HCC and liver cirrhosis by pathology. The elevated level of AFP recovered to normal after tumorectomy. In conclusion, HCC might be a candidate outcome in patients with pSS; it is the doctors’ responsibility to keep this kind of patient under surveillance.
Hepatocellular carcinoma (HCC) is common in patients with liver cirrhosis, and its incidence is rising worldwide. The reported cumulative incidence rates of HCC were 1.3 and 14.9% per person-year, respectively.1,2 Additionally, approximately 1–4% of patients with chronic hepatitis C in Western countries3–6 develop HCC, while about 8% of these patients in Asia7 develop to HCC. A common cause of HCC development is HBV infection. Other causes of HCC include alcoholic or nonalcoholic fatty liver disease, primary biliary cirrhosis (PBC) and autoimmune hepatitis (AIH) associated liver cirrhosis.
Although patients with AIH can develop to HCC, the incidence, approximately less than 0.2% per year, is quite low,8 but the risk of HCC among AIH patients with cirrhosis is up to 1.9% per year.9 Primary Sjögren’s syndrome (pSS), similar to AIH, is an autoimmune disease; and HCC due to an autoimmune disease other than AIH is rare. In this present report, we demonstrate a patient diagnosed with pSS during her first hospitalization, which increasingly developed to HCC within two years, and discuss the associated clinical significance.
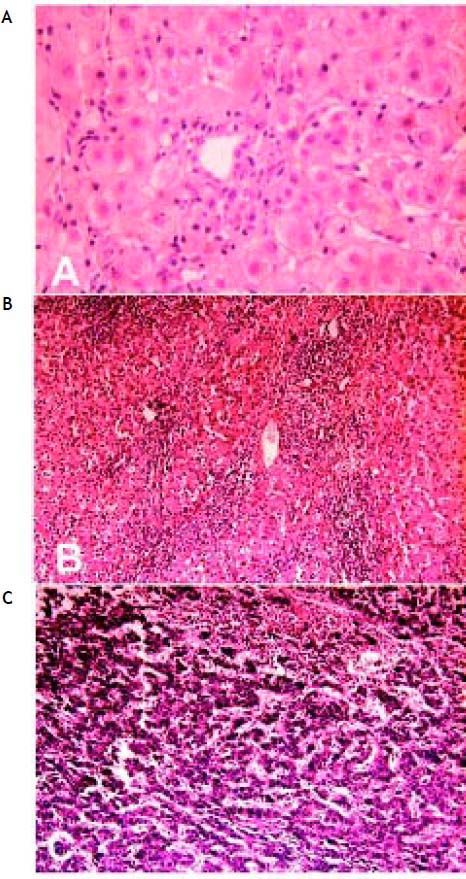
Case ReportFirst hospitalization: diagnosis of pSSThe general clinical features of this patient have been summarized in table 1. This patient was first hospitalized in the Department of Gynecology at our hospital because of hypogastrium discomfort and hydrops in the pelvic cavity. Liver function testing showed that the patient had elevated serum levels of aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γ-GT) and immunoglobulin (Ig). The patient was then transferred to the Department of Gastroenterology. Further sera testing showed that alanine transarninase (ALT) and activated partial thromboplastin time (APTT) were normal, and the markers for hepatitis virus A (anti-HAV antibody Ig M), B (HBsAg, HBsAb, HBeAg, HBeAb, HBcAb and preS1Ag), C (anti-HCV antibody IgG), D (anti-HDV antibody IgG, IgM and HDV Ag) and E (anti-HEV antibody Ig M) as well as the syphilis virus and HIV infection were all negative. Antinuclear antibody (ANA), anti-smooth muscle antibody (SMA), anti-mitochondrial antibody (AMA), ribonucleoprotein (RNP), anti-double stranded-DNA antibody and liver-kidney microso-mes1 (LKM-1) were all negative as well. Serum Epstein-Barr virus, cytomegalovirus and adenovirus DNA were undetectable by polymerase chain reaction. Type B ultrasound and computed tomography (CT) scan were carried out one after another for the liver, gallbladder and bile duct system, and nothing abnormal was found except for a small gallstone in the gallbladder. Although the patient was treated with diammonium glycyrrhizinate and ursodeoxycholic acid (UDCA, Ursofalk) for recovery of the liver damage, the origin of the liver injury remained uncertain and the diagnosis was indeterminate. Thus, a liver biopsy was performed although the H&E stained liver tissues only presented a few infiltrated lymphocytes, and other pathological alterations including steatosis, ballooned hepatocytes, fibrosis or bile duct lesions were not observed (Figure 1A). A detailed clinical history was then re-inquired. The patient did not have a fever, but complained that she had been suffering from eye and mouth pruritus, intermittent tooth-pellet falling-off and dysphagia for ten years, and had to drink water every now and then. Physical examination showed gingival atrophy and angiote-lectasis on both cheeks. Local skin depigmentation was found near the metacarpophalangeal joints and on the extensor aspect of her elbow joints. A Schirmer test was positive. Further immunological detection showed that rheumatoid factor (RF) was fifteen times that of the normal baseline, and both SS-A and SS-B antibodies were positive. The serum level of C4 was low while gammaglobulin was higher than normal. The patient’s laboratory detection results are summarized in table 2. Thus, the patient was considered to have a diagnosis of primary Sjögren’s Syndrome with liver involvement.
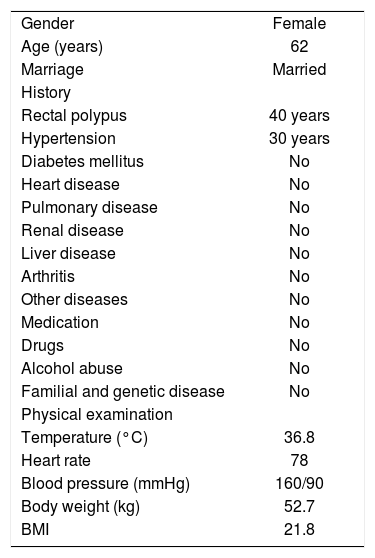
General features of the patient at fist hospitalization.
| Gender | Female |
| Age (years) | 62 |
| Marriage | Married |
| History | |
| Rectal polypus | 40 years |
| Hypertension | 30 years |
| Diabetes mellitus | No |
| Heart disease | No |
| Pulmonary disease | No |
| Renal disease | No |
| Liver disease | No |
| Arthritis | No |
| Other diseases | No |
| Medication | No |
| Drugs | No |
| Alcohol abuse | No |
| Familial and genetic disease | No |
| Physical examination | |
| Temperature (°C) | 36.8 |
| Heart rate | 78 |
| Blood pressure (mmHg) | 160/90 |
| Body weight (kg) | 52.7 |
| BMI | 21.8 |
Liver pathology pre- and post-operation at first and second hospitalization. A. Liver biopsy shows only a few lymphocytes infiltration (H&E, x 200) at first hospitalization. B. Postoperative pathology shows liver cirrhosis (H&E, x 100) and C. postoperative pathology shows hepatocellular carcinoma (H&E, x 100) at second hospitalization.
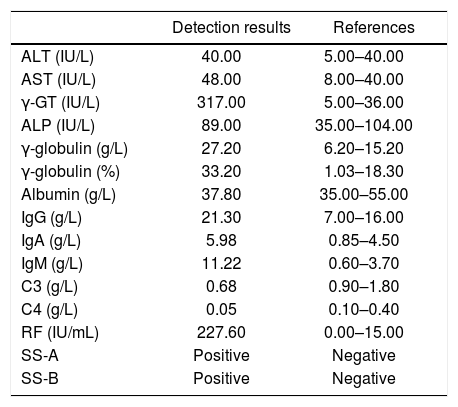
The laboratory detections of the patient at diagnosis of pSS.
| Detection results | References | |
|---|---|---|
| ALT (IU/L) | 40.00 | 5.00–40.00 |
| AST (IU/L) | 48.00 | 8.00–40.00 |
| γ-GT (IU/L) | 317.00 | 5.00–36.00 |
| ALP (IU/L) | 89.00 | 35.00–104.00 |
| γ-globulin (g/L) | 27.20 | 6.20–15.20 |
| γ-globulin (%) | 33.20 | 1.03–18.30 |
| Albumin (g/L) | 37.80 | 35.00–55.00 |
| IgG (g/L) | 21.30 | 7.00–16.00 |
| IgA (g/L) | 5.98 | 0.85–4.50 |
| IgM (g/L) | 11.22 | 0.60–3.70 |
| C3 (g/L) | 0.68 | 0.90–1.80 |
| C4 (g/L) | 0.05 | 0.10–0.40 |
| RF (IU/mL) | 227.60 | 0.00–15.00 |
| SS-A | Positive | Negative |
| SS-B | Positive | Negative |
Marked are the abnormal parameters. ALT: alanine transarninase. AST: aspartate aminotransferase. γ-GT: γ-glutamyl transpeptidase. ALP: alkaline phosphatase. Ig: immunoglobulin. RF: rheumatoid factor.
The patient was then mainly treated by administration of Prednisone at a dose of 20 mg per day and UDCA at a dose of 500 mg per day, and her liver function recovered to normal. The patient went home with a persistent treatment of Prednisone at a dose of 5 mg per day and UDCA of 250 mg per day.
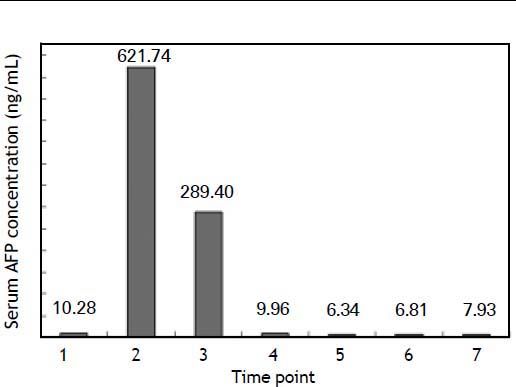
Second hospitalization: HCC presentationThe patient was hospitalized for the second time two years later because of emaciation and dark face for two months. She had stopped taking Prednisone and UDCA, but did not remember the exact stopping time. Biochemistry detection showed much lower γ-GT, ALP and RF levels than those at her first hospitalization. Thyroid function was normal. The serum level of alpha fetoprotein (AFP) was found to be very high this time (Figure 2), but all hepatitis virus markers were still negative. Liver cancer was considered and a contrast-enhanced CT scan confirmed this suspicion.
Serum AFP variations of the patient. Time points: 1, first hospitalization. 2, second hospitalization before operation. 3–7, two days, two months, six months, twelve months and twenty-one months after operation, respectively. The reference range of serum AFP level is under 8.00 ng/mL according to our laboratory.
The patient was then transferred to the Division of Hepatobiliary Surgery. Liver cirrhosis, liver neoplasm (3 cm x 2 cm x 3 cm, at right liver), a mildly enlarged spleen and cholecystolithiasis were found during the operation. Tumorectomy, splenectomy and cholecystectomy were performed. Postoperative pathology showed typical characterization of liver cirrhosis and HCC (Figures 1B and 1C).
Postoperative chemotherapyDuring the nine months after the operation, the patient underwent chemotherapy with Tegafur and/ or Oxaliplatin three times, and combined treatment for mild chemical liver damage with glycyrrhizinate, glutathione or Tiopronin. Serum AFP was maintained under the baseline level.
Latest hospitalizationThe latest hospitalization of the patient was eighteen months after her operation. The patient found dark stools for two days, and vomited bloody fluid (400 mL) one time. When she presented at the hospital, her blood pressure was 110/60 mmHg and her heart rate was 76 beats per minute. Laboratory testing demonstrated anemia and hypoalbuminemia.γ-GT and APTT were mildly elevated. AFP was normal. The cause of the upper gastrointestinal hemorrhage was ascribed to gastroesophageal venous bleeding, for which the gastric acid inhibitors Omeprazole (Losec) and Somatostatin (Stilamin) were used via continuous venous injection until stool testing returned to normal. After treatment for ten days, the patient regained normal functioning, and was permitted to be discharged from the hospital.
The alterations of serum AFP levels are shown in figure 2.
DiscussionHCC is common in the Chinese population, and the predominant causes are chronic hepatitis virus infection, alcoholic liver cirrhosis, and even nonalcoholic steatohepatitis (NASH). PBC and AIH, which are autoimmune liver diseases, may also develop to HCC in patients with a cirrhotic history.10 In a recent large study cohort,9 the overall estimated HCC risk in AIH patients was less than 0.2%; however, the prevalence rose to 1.9% when cirrhosis occurred; and the mean interval between diagnosis of AIH and HCC was ten years. According to another study, decompensate cirrhosis and HCC are common long-term outcomes of AIH patients without transplantation.11 Therefore, surveillance of patients with AIH is recommended.
pSS is an autoimmune disease that mainly attacks the exocrine glands, but any part of the body, such as liver, lung, kidney and gastrointestinal tract can be involved, and the common complication of pSS is lymphoma.12,13 The incidence of pSS is quite low, and 90% of the patients are female and older than 40 years. The typical symptoms include dry eye and dry mouth, but no two patients present in the same way.12
The diagnosis of pSS has different criteria, but eye and mouth symptoms, positive Schirmer test, and SS antibodies, RF, ANA and lower lip biopsy are significant supportive aspects. To get a definite diagnosis, patients must have either a positive lip biopsy or a positive serum marker.12 As for the patient we reported here, she was transferred to our department because of the detection of abnormal serum liver function. HBV and HCV infection and NASH were excluded according to serum detections and clinical and pathological characteristics.14 AIH and PBC were considered but finally excluded according to the diagnostic scoring system and criteria.15–17 Clinical history inquiry revealed that she had dry eyes and mouth, tooth-pellet falling-off and dysphagia for ten years, and following detection demonstrated a positive Schirmer test, elevated RF level and hypergammaglobulinemia. More importantly, both SS-A and SS-B antibodies were positive, which is in accordance with a recent report,18 and their co-existence is highly suggestive of pSS.19 Thus, the diagnosis of pSS with liver involvement could be established for the patient. Actually, previous studies have reported a prevalence of liver affection in patients with pSS from 6% to 58%. In a recent study that enrolled 95 patients with pSS, 44% of the patients had abnormal hepatic biochemistries, such as elevated abnormal levels of ALT, AST or ALP and bilirubin; 50% of these patients had no definitive causes, and pSS was considered the underlying cause of the liver involvement.20
The exact pathogenesis of pSS is still unclear, although genetic predisposition and viral or bacterial infection may play important roles. It is inferred that a virus or bacterial infection causes dormant gene expression and immune system attack. In such a case, salivary and lacrimal glands would be infiltrated by activated T cells; this tissue damage could be related to the anomalous interaction between activated lymphocytes and different epithelial tissues including the liver and the activated B cells, which produce SS-A and SS-B autoantibodies.12
In a recent study,18 in which pSS patients were followed up over the long term, 89% of patients presented with systemic complications including pulmonary, renal, articular, and muscular involvements as well as neuropathies and lymphoma, but liver involvement was not indicated. Another retrospective study reported a hepatoma in 1320 pSS patients, but it was not described in detail.13 To date, the long-term outcomes of pSS have been variable, and so far, typical liver cirrhosis and HCC in pSS patients have not been documented. In this report, the patient demonstrated clinical and laboratory characteristics of pSS at her first hospitalization. Two years later, the patient developed typical liver cirrhosis and carcinoma, but with no clues associated with the common causes, such as chronic viral infection and/or alcohol abuse, were found during her several hospitalizations. A connection between the development of liver cancer and pSS might exist. For pSS, symptoms could be ameliorated after treatment, but it could not be cured. The persistent occult origin of pSS led to a liver lesion in this patient. Actually, her slightly increased serum AFP level at the beginning was a vague but significant indication. It is certain that obvious elevated serum AFP levels are strong evidence of HCC, but some patients with mildly increased AFP might also eventually develop HCC, indicating that monitoring of AFP variation is important and necessary in the case of chronic liver disease. HCC usually arises from chronic liver disease, especially cirrhosis. Therefore, there must have been some occult events which kept occurring within the liver in this patient during the two years following her first hospitalization. Liver damage is usually induced by immune responses and direct toxicants, but the patient did not drink, smoke or taking any special medications or drugs. Increased levels of IgG, gamma globulin and RF and a decreased level of C4 were detected in this patient, suggesting a disordered immune system. Thus, immune response-induced injuries cannot be excluded. According to a recent study, Th17 cells and regulatory T cells were found in the salivary glands of pSS patients; type 1 and 2 B effector cells, B-cell modulating factors and B-cell-activating factor play prominent roles and interrelate in pSS pathogenesis.21 Therefore, complex immune responses occur when pSS occurs. It is reported that a low C4 level is associated with a high risk of lymphoma and death,22 and hypergammaglobulinemia is identified as a prognostic factor for systemic involvement, which reflects chronic B cell activation in pSS.18 The two markers also were presented in this patient. We suppose that the causes which incited pSS in this patient resulted in a permanent immune response, which might have activated the cytokines network. Some cytokines, TGF beta in particular, activate fibro-productive cells, lead to extra-cellular matrix synthesis and deposition, and finally result in fibrosis and cirrhosis. Both the postoperative pathology and the upper gastrointestinal hemorrhage testified to the presence of
In summary, patients with chronic hepatitis or mild liver damage, no matter what the known or unknown causes, should be monitored by testing liver function and serum AFP level, and with periodic image scanning in order to find HCC as early as possible. Primary SS patients with liver involvement might develop liver cirrhosis and HCC. For these patients, it is the doctors’ responsibility to provide sufficient surveillance.
Conflict of InterestThe authors state that they have no conflict of interest.
Abbreviations- •
APTT: activated partial thromboplastin time.
- •
ALT: alanine transarninase.
- •
AFP: alpha-Fetoprotein.
- •
AMA: anti-mitochondrial antibody.
- •
ANA: antinuclear antibody.
- •
SMA: anti-smooth muscle antibody.
- •
AST: aspartate aminotransferase.
- •
AIH: autoimmune hepatitis.
- •
CT: computed tomography.
- •
HCC: hepatocellular carcinoma.
- •
Ig: immunoglobulin.
- •
LKM-1: liver-kidney microsomes1.
- •
NASH: nonalcoholic steatohepatitis.
- •
PBC: primary biliary cirrhosis.
- •
pSS: primary Sjögren syndrome.
- •
RF: rheumatoid factor.
- •
RNP: ribonucleoprotein.
- •
γ-GT: γ-glutamyl transpeptidase.