To determine the prevalence of minimal hepatic encephalopathy(MHE) in patients with liver cirrhosis (LC) due to hepatitis C virus (HCV) infection and to evaluate the impact of sustained viral response (SVR) on MHE.
Materials and methodsWe performed a prospective study using MHE screening and follow-up on patients with HCV and LC. The patients were evaluated at the beginning of treatment and 24 weeks after treatment.
Results64 patients were included. 51.6% were male, the median age was 62 years, Child-Pugh classification A/B/C 93.8%/4.7%/1.6% and median MELD was 8.3. Prior hydropic decompensation was present in 11 patients. Median values of liver stiffness, as measured by transient elastography (TE) were 22.8kPa. Indirect signs of portal hypertension (PH) were present in 53.1% of patients, with a mean of 11.9mmHg among the ones with a measurement of the hepatic venous pressure gradient. The prevalence of MHE before treatment was 26.6%. After treatment, 98.4% of patients achieved SVR. The presence of MHE at 24 weeks post-treatment had an statistically significant association with the presence of pre-treatment MHE (80% vs. 21.6%; p<0.01), higher MELD scores at 24-weeks post-treatment (9.8 vs. 8; p=0.02), higher Child-Pugh scores at 24-weeks post-treatment (p=0.04), higher baseline INR levels (1.4 vs. 1.1; p<0.001) and with the presence of indirect signs of PH (100% vs. 47.1%; p=0.02). During follow-up, those patients without MHE at 24 weeks post-treatment had a higher probability of experiencing an improvement in post-treatment TE (80.9% vs. 40%, p=0.04).
ConclusionWe found that SVR may lead to MHE resolution in a considerable proportion of patients, which has potential implications for disease prognosis.
Minimal hepatic encephalopathy (MHE) is primarily characterized by a subtle impairment of neuro-cognitive abilities which is not readily detectible via standard mental status testing or neurological examination [1]. MHE is the earliest stage of hepatic encephalopathy (HE) and is a clinically relevant condition that affects the quality of life and job performance of patients with liver cirrhosis [2].
The actual prevalence of MHE is unknown and varies among different studies depending on the criteria used for diagnosis and the study populations. In cirrhotic patients with good liver function (Child-Pugh class A), MHE prevalence is low (<15%), while in those with decompensated or advanced cirrhosis (Child-Pugh class B or C), MHE may affect half of the patients [3]. MHE is not restricted to liver cirrhosis (LC); patients with other liver-related conditions such as portal vein thrombosis or portosystemic shunt can also present with MHE. It is also notable that MHE appears to be more frequent in patients with hepatitis C virus (HCV) infection, irrespectively of the stage of liver fibrosis. HCV-positive patients display abnormalities in verbal learning, attention span, executive function and memory when they are evaluated with suitable neuropsychological tests [4].
As MHE-associated cognitive deficits are difficult to detect through routine physical or neurological examinations, specific neurophysiological tests are needed for diagnosis. There are several diagnostic tests available for the detection of MHE, but there is not a universal or ‘gold standard’ assessment. The 2 most widely used tests are the critical flicker frequency (CFF) and the psychometric hepatic encephalopathy score (PHES) test battery, along with its variants [5].
The PHES is a selection of 5 psychometric test batteries copyrighted and validated in Italian, German and Spanish patient cohorts. PHES assesses attention span, motor speed and accuracy, visual orientation and visual-spatial construction [1]. PHES has been shown to be influenced by age, education level and cultural issues. In addition, performing this battery can be time consuming and prone to bias from disturbance, mood and interaction with the tester.
The CFF has the advantage of not being dependent on age, gender, cultural or educational factors and not showing a learning effect [6]. However, the CFF depends significantly on the experimental setting (the color and luminance of the stimuli, the visual angle, etc.). CFF showed a moderate pooled sensitivity of 61% yet a reasonable specificity of 79% [7].
Several studies have shown quick improvement in liver function and other parameters in cirrhotic patients treated with anti-HCV direct-acting antivirals (DAA), which are molecules targeting specific nonstructural proteins of the virus, allowing interferon-free treatment [8–13]. To date, patients with HCV infection and patients with LC have been reported as more susceptible to MHE, although it remains unknown whether the natural course of MHE may be altered after interferon-free DAA treatment. Therefore, our objective was to prospectively explore the potential role of HCV clearance in the course of MHE among patients with LC and HCV infection after DAA treatment.
2Materials and methodsThe inclusion criteria for this study were (i) LC and (ii) HCV infection with an indication for DAA treatment (paritaprevir/ritonavir+ombitasvir+dasabuvir, ledipasvir+sofosbuvir, daclatasvir+sofosbuvir and elbasvir/grazoprevir). A total of 181 consecutive outpatients with LC due to HCV infection were treated with DAA from May 2015 until March 2017 in our tertiary care hospital. Of these patients, 64 met inclusion criteria and were selected for our study before initiating treatment. All of them had a baseline evaluation immediately before starting treatment. Fifty-six of them were prospectively followed-up and evaluated at two time points; at baseline and at 24 weeks after treatment. LC was diagnosed on a clinical basis using semiology, laboratory parameters, ultrasound-Doppler assessments and liver stiffness measurements by TE using FibroScan® (Echosens, Paris, France) (cut-off in 14kPa). Liver function was assessed using the model for end-stage liver disease (MELD) and Child-Pugh scores. All patients were offered hepatic venous pressure gradient (HVPG) measurements, but only 27 patients accepted. HVPG≥10mm Hg was defined as clinically significant portal hypertension (CSPH). HCV infection was diagnosed by the presence of serum anti-HCV antibodies and detectable HCV RNA. Sustained viral response (SVR) was defined as undetectable HCV RNA 24 weeks after the end of antiviral treatment and was analyzed by intention to treat. The exclusion criteria were overt HE, acute gastrointestinal hemorrhage, hepatorenal syndrome or spontaneous bacterial peritonitis in the 7 days prior to inclusion, significant non-hepatic diseases such as decompensated heart, respiratory or renal failure, decompensated or poorly controlled diabetes mellitus, and anamnestic neurological diseases such as Alzheimer's disease or Parkinson's disease. Likewise, unwillingness to comply with study requirements, obvious alcohol abuse, ophthalmologic disorders and red-green visual blindness were exclusion criteria. Patients on medication including psychoactive drugs such as antidepressants or sedatives were also excluded.
The study protocol was approved by the Ethics Committee of the Hospital Universitario Puerta de Hierro-Majadahonda (PI-48-16, 2016) and written informed consent was obtained from all the patients prior to inclusion.
The PHES is comprised of 5 paper-pencil tests (Number Connection Tests A and B, the Digit Symbol coding Test, the Serial Dotting Test and the Line Drawing Test). The Serial Dotting Test consists of 10 rows of 10 circles, and the subject is timed on how quickly he or she can place a dot in the center of each circle. The Line Drawing Test requires the subject to draw a continuous line between two parallel (winding) lines, and scores include completion time and errors. The on-line software developed by the Spanish Network for Hepatic Encephalopathy was used (available at http://www.redeh.org), and its results were adjusted for age and educational level [14]. The CFF was measured using a HEPAtonorm analyzer (Accelab GmbH, Kusterdingen, Germany). This test measures the frequency at which the patient perceives that a fused light becomes a flickering light. The device causes a stepwise decrease in frequency from 60 to 25Hz. After a brief instruction and training period, the flicker frequencies were measured 10 times and the mean value and standard deviation (SD) were calculated. CFF was measured in a quiet, semi-darkened room to avoid interferences. Both tests were performed by the same investigator in a quiet room. Patients were classified as having MHE when the PHES score was below −4 points or the CFF score was below the cut-off value (39Hz) [15,16].
Data analysis was performed using STATA 14 (Stata Corporation, College Station, Texas) for Macintosh. Continuous variables were displayed as means and SD, whereas discrete variables were expressed as percentages. Comparisons between groups were made using the Mann–Whitney U test, the Student's t test, the Chi-square test or the Fisher's exact test (when expected values in any of the cells of a contingency table were less than 5) for categorical data. Correlations between psychometric tests and CFF were calculated by using Spearman–Rho rank correlation. p-values below 0.05 were considered as statistically significant.
3ResultsSixty-four patients were included in this study (patient characteristics in Table 1).
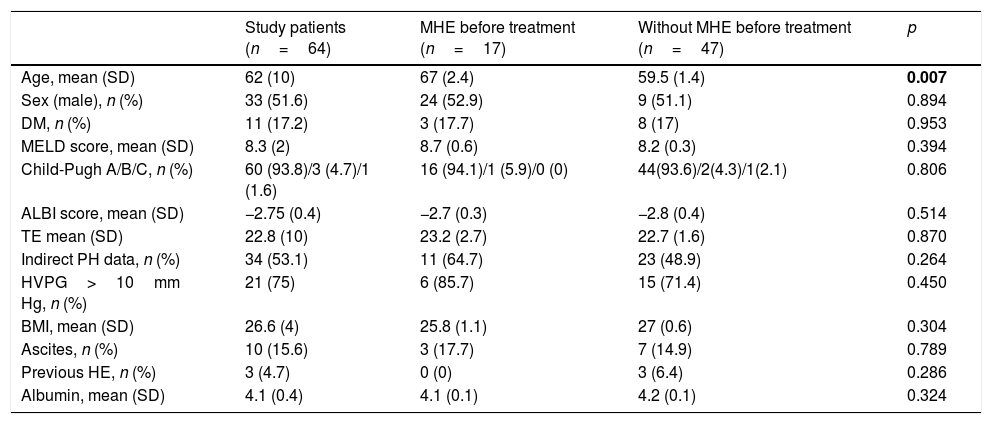
Baseline characteristics of the study patients (n=64).
| Study patients (n=64) | MHE before treatment (n=17) | Without MHE before treatment (n=47) | p | |
|---|---|---|---|---|
| Age, mean (SD) | 62 (10) | 67 (2.4) | 59.5 (1.4) | 0.007 |
| Sex (male), n (%) | 33 (51.6) | 24 (52.9) | 9 (51.1) | 0.894 |
| DM, n (%) | 11 (17.2) | 3 (17.7) | 8 (17) | 0.953 |
| MELD score, mean (SD) | 8.3 (2) | 8.7 (0.6) | 8.2 (0.3) | 0.394 |
| Child-Pugh A/B/C, n (%) | 60 (93.8)/3 (4.7)/1 (1.6) | 16 (94.1)/1 (5.9)/0 (0) | 44(93.6)/2(4.3)/1(2.1) | 0.806 |
| ALBI score, mean (SD) | −2.75 (0.4) | −2.7 (0.3) | −2.8 (0.4) | 0.514 |
| TE mean (SD) | 22.8 (10) | 23.2 (2.7) | 22.7 (1.6) | 0.870 |
| Indirect PH data, n (%) | 34 (53.1) | 11 (64.7) | 23 (48.9) | 0.264 |
| HVPG>10mm Hg, n (%) | 21 (75) | 6 (85.7) | 15 (71.4) | 0.450 |
| BMI, mean (SD) | 26.6 (4) | 25.8 (1.1) | 27 (0.6) | 0.304 |
| Ascites, n (%) | 10 (15.6) | 3 (17.7) | 7 (14.9) | 0.789 |
| Previous HE, n (%) | 3 (4.7) | 0 (0) | 3 (6.4) | 0.286 |
| Albumin, mean (SD) | 4.1 (0.4) | 4.1 (0.1) | 4.2 (0.1) | 0.324 |
Values in bold are stadistically signficant.
A slight majority were male (33 patients, 51.6%) and the mean age was 62 years (SD 10). The distribution of Child-Pugh scores was as follows: 60 patients were class A (93.8%), 3 patients were class B (4.7%) and 1 patient was class C (1.6%). The median MELD score was 8.3 (SD 2). Previous hepatic decompensation (ascites) was present in 11 patients (17.2%). Three patients (4.7%) had a previous episode of HE grade I almost one year prior to initiation of antiviral treatment, at the time of assessments asymptomatic. The median value of liver stiffness was 22.8kPa (SD 10). Thirty-four patients (53.1%) had indirect signs of portal hypertension, e.g., platelet count <100000103/μL, splenomegaly by ultrasound or esophageal varices in gastroscopy. Among the 27 patients for whom measurement of the HVPG was available, the mean value was 11.9mmHg (SD 3.7) and 21 of them had CSPH.
MHE was diagnosed in 17 patients (26.6%) before treatment and its frequency varied significantly according to age (p<0.01). No association was found between the presence of MHE and TE or the value of HVPG (Table 1).
After treatment, 62 patients (96.9%) achieved SVR (patient characteristics are included in Table 2). MHE at 24 weeks post-treatment was evaluated in a total of 56 patients (87.5%). This assessment was not possible in 8 patients (12.5%) due to the following reasons: 2 patients (3.1%) died during follow-up (one patient died due to hepatocellular carcinoma 20 weeks after treatment; the other patient died from non-liver disease during treatment), 4 patients (6.3%) were lost to follow-up and 2 patients (3.1%) were treated with antidepressants. Of the patients who died, only one of them had MHE before treatment.
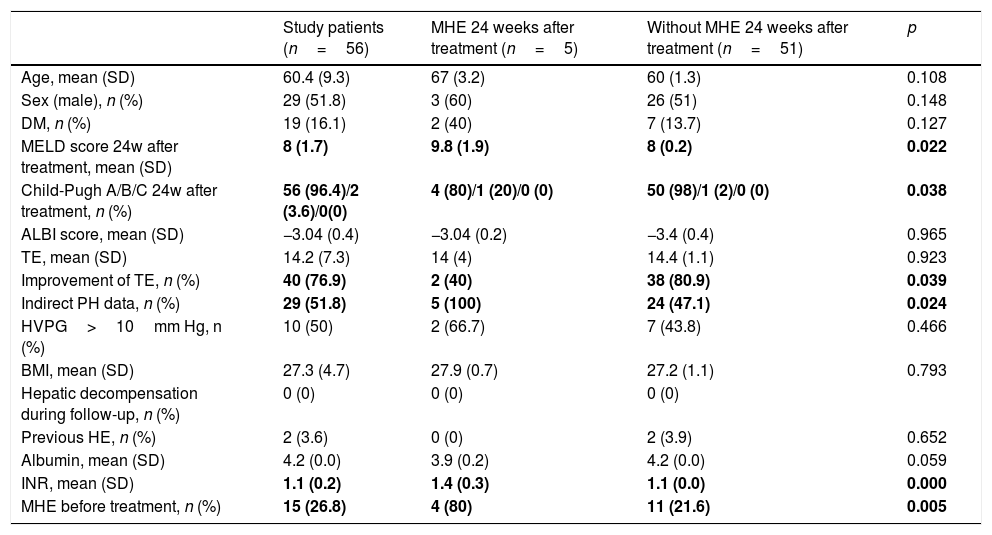
Characteristics of the study patients after achieve SVR (n=56).
| Study patients (n=56) | MHE 24 weeks after treatment (n=5) | Without MHE 24 weeks after treatment (n=51) | p | |
|---|---|---|---|---|
| Age, mean (SD) | 60.4 (9.3) | 67 (3.2) | 60 (1.3) | 0.108 |
| Sex (male), n (%) | 29 (51.8) | 3 (60) | 26 (51) | 0.148 |
| DM, n (%) | 19 (16.1) | 2 (40) | 7 (13.7) | 0.127 |
| MELD score 24w after treatment, mean (SD) | 8 (1.7) | 9.8 (1.9) | 8 (0.2) | 0.022 |
| Child-Pugh A/B/C 24w after treatment, n (%) | 56 (96.4)/2 (3.6)/0(0) | 4 (80)/1 (20)/0 (0) | 50 (98)/1 (2)/0 (0) | 0.038 |
| ALBI score, mean (SD) | −3.04 (0.4) | −3.04 (0.2) | −3.4 (0.4) | 0.965 |
| TE, mean (SD) | 14.2 (7.3) | 14 (4) | 14.4 (1.1) | 0.923 |
| Improvement of TE, n (%) | 40 (76.9) | 2 (40) | 38 (80.9) | 0.039 |
| Indirect PH data, n (%) | 29 (51.8) | 5 (100) | 24 (47.1) | 0.024 |
| HVPG>10mm Hg, n (%) | 10 (50) | 2 (66.7) | 7 (43.8) | 0.466 |
| BMI, mean (SD) | 27.3 (4.7) | 27.9 (0.7) | 27.2 (1.1) | 0.793 |
| Hepatic decompensation during follow-up, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| Previous HE, n (%) | 2 (3.6) | 0 (0) | 2 (3.9) | 0.652 |
| Albumin, mean (SD) | 4.2 (0.0) | 3.9 (0.2) | 4.2 (0.0) | 0.059 |
| INR, mean (SD) | 1.1 (0.2) | 1.4 (0.3) | 1.1 (0.0) | 0.000 |
| MHE before treatment, n (%) | 15 (26.8) | 4 (80) | 11 (21.6) | 0.005 |
Values in bold are stadistically signficant.
At 24 weeks post-treatment, MHE was diagnosed in 5 patients (8.9%), 4 of which had MHE before treatment. The presence of MHE at 24 weeks post-treatment had a statistically significant association with the presence of pre-treatment MHE (80% vs. 21.6%; p<0.01), higher MELD scores at 24-weeks post-treatment (9.8 vs. 8, p=0.02), higher Child-Pugh scores at 24-weeks post-treatment (5 score vs. 5.6 score, p=0.004), higher baseline INR levels (1.4 vs. 1.1; p<0.001) and with the presence of indirect signs of PH (100% vs. 47.1%; p=0.02). In addition, those patients without MHE at 24 weeks post-treatment had a higher probability of experiencing an improvement in post-treatment TE (80.9% vs. 40%, p=0.04) (Table 2).
Fifteen of the 17 patients with pre-treatment MHE were evaluated at 24 weeks post-treatment. Of them, 11 patients experienced MHE resolution (73.3%; p=0.01) (Table 3).
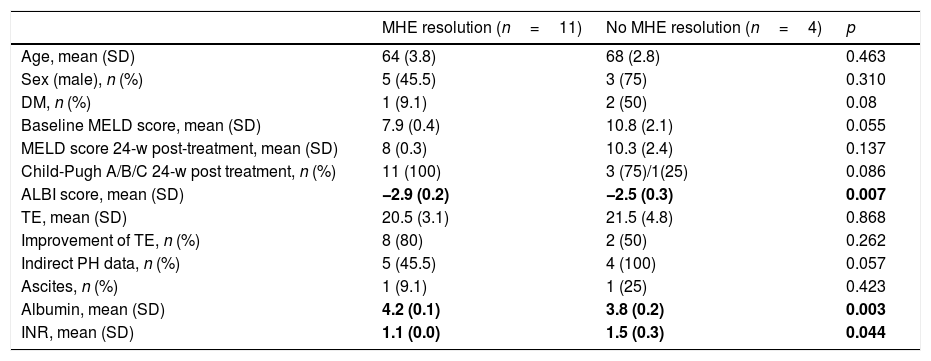
Characteristics of the study patients according to MHE resolution after SVR (n=15).
| MHE resolution (n=11) | No MHE resolution (n=4) | p | |
|---|---|---|---|
| Age, mean (SD) | 64 (3.8) | 68 (2.8) | 0.463 |
| Sex (male), n (%) | 5 (45.5) | 3 (75) | 0.310 |
| DM, n (%) | 1 (9.1) | 2 (50) | 0.08 |
| Baseline MELD score, mean (SD) | 7.9 (0.4) | 10.8 (2.1) | 0.055 |
| MELD score 24-w post-treatment, mean (SD) | 8 (0.3) | 10.3 (2.4) | 0.137 |
| Child-Pugh A/B/C 24-w post treatment, n (%) | 11 (100) | 3 (75)/1(25) | 0.086 |
| ALBI score, mean (SD) | −2.9 (0.2) | −2.5 (0.3) | 0.007 |
| TE, mean (SD) | 20.5 (3.1) | 21.5 (4.8) | 0.868 |
| Improvement of TE, n (%) | 8 (80) | 2 (50) | 0.262 |
| Indirect PH data, n (%) | 5 (45.5) | 4 (100) | 0.057 |
| Ascites, n (%) | 1 (9.1) | 1 (25) | 0.423 |
| Albumin, mean (SD) | 4.2 (0.1) | 3.8 (0.2) | 0.003 |
| INR, mean (SD) | 1.1 (0.0) | 1.5 (0.3) | 0.044 |
Values in bold are stadistically signficant.
The patients with MHE resolution after treatment, in comparison with those who did not, had higher baseline albumin levels (4.2 vs. 3.8g/dL, p<0.01), lower baseline INR levels (1.1 vs. 1.5; p<0.05) and lower ALBI score (−2.9 vs. −2.5; p<0.01). Although the baseline and 24-weeks post-treatment MELD scores were lower in patients with MHE resolution, this difference was not statistically significant (7.9 vs. 10.8; p=0.06 and 8 vs. 10.3; p=0.1). Despite not finding any association between pre-treatment MHE and pre-treatment CSPH (p>0.05), we found that an improvement of HVPG 24 weeks after treatment was more frequent in patients without pre-treatment MHE (28.6% vs. 70%, p=0.03).
Remarkably, we did not find any correlation between the results of CFF and PHES tests either before (r=0.02, p=0.86) or 24 weeks after treatment (r=−0.02, p=0.88).
4DiscussionTo the best of our knowledge, this is the first study assessing MHE outcomes after SVR. In our cohort, 73.3% of patients experienced MHE resolution, which suggests that MHE may improve and mirror liver function amelioration after viral clearance. In the present study, some factors related to the severity of pre-treatment liver disease were associated with the presence of MHE at 24 weeks post-treatment (MHE before treatment, MELD and Child Pugh scores, INR levels and indirect signs of PH). Similarly, improvement of TE after SVR was associated with the absence of MHE after treatment. These findings are consistent with previous published literature and may have prognostic implications. As previously discussed, MHE could help in predicting survival and outcomes of patients with MELD scores of 10 or higher [17]. Although frequently overlooked, MHE is of outmost importance because of its relationship with falls, impairment of motor vehicle driving abilities, overt HE, and even survival [17–20]. Patients who recover from MHE would then be expected to improve their quality of life and to experience a delay in the progression of liver disease, yet further studies are needed to support this hypothesis.
Regarding age, no association between age and post-treatment MHE or MHE resolution (p=0.11 and p=0.9, respectively) was found. Moreover, we were unable to locate any reported association between age and MHE in current literature. The apparent association between age and pre-treatment MHE might be spurious and influenced by a limited sample size.
The frequency of MHE was lower in our study than in other previous studies (27% vs. 30–50%) [21]. However, the high proportion of Child Pugh class A patients in our patient cohort (93.8%) may account for this finding. PHES and CFF tests are widely accepted for detecting MHE and have shown high reproducibility and little bias due to training effect or daytime variability [5]. Furthermore, we performed the follow-up tests 9–12 months after the pre-treatment visit, which should be sufficient amount of time to avoid training effect, and always in the morning. Therefore, our results should be minimally influenced by these weak yet potential sources of bias. However, no correlation was detected in our research between both tests. In fact, PHES and CFF are considered to be complementary tests because they explore different pathways and mechanisms of MHE, with continuous and dichotomous variables assessing either psychometric or neurophysiological factors, respectively. Although there is some controversy regarding the most appropriate method to detect MHE, some experts have advocated using PHES and CFF simultaneously as a reasonable approach to raise sensitivity [22]. Our results support such recommendations.
This real-life exploratory study has some inherent limitations. First, our work is based on single-center recruitment of consecutive outpatients who need to meet very restrictive inclusion and exclusion criteria for assessing MHE. This precluded from obtaining a sample size with greater statistical power; some patients could not perform the tests due to visual impairment, inability to understand the fundamentals or were on treatments with anti-depressants or sedatives. In this setting, obtaining a significant number of HVPG measurements is also very difficult. Secondly, there was no control group, for instance patients without HCV infection or without cirrhosis. We were unable to obtain a control group without SVR due to the high effectiveness of DAA treatment. In addition, patients with more advanced liver disease were underrepresented in the study. These patients are known to have poorer outcomes with regards to MHE [21]. Therefore, the population with a more advanced disease could benefit the most from treatment with DAA when it comes to MHE, although our results suggest that HE resolution may be more probable at earlier stages of liver disease. Finally, long term follow-up is unavailable at the moment even though it would be valuable for assessing quality of life and liver disease outcomes.
Treatment of HCV with IFN-free DAA has led to a revolution which has resulted in high SVR figures in a wide range of populations, including many cirrhotic patients. In this proof-of-concept prospective study, which included HCV-infected cirrhotic patients with MHE, we found that SVR may lead to MHE resolution in a considerable proportion of patients, which may have potential implications for disease prognosis. Patients with more compensated liver disease may have a greater probability of experiencing MHE resolution. In addition, we recommend performing both PHES and CFF tests to assess MHE due to their poor individual performance. New studies in this scarcely studied field are encouraged.
AbbreviationsMHE minimal hepatic encephalopathy hepatic encephalopathy liver cirrhosis hepatitis C virus critical flicker frequency psychometric hepatic encephalopathy score International Society for Hepatic Encephalopathy and Nitrogen Metabolism direct-acting antivirals transient elastography model for end-stage liver disease hepatic venous pressure gradient clinically Significant Portal Hypertension sustained viral response
None declared.
Conflict of interestThe authors have no conflicts of interest to declare.