Background and aims. Most portal vein thromboses (PVT) in cirrhotics are discovered incidentally. While case series demonstrate improved portal vein patency with anti-coagulation, there is little information on impact of PVT on morbidity and mortality. This study aimed to compare morbidity and mortality in cirrho-tics with untreated PVT with those without PVT.
Material and methods. Cirrhotics evaluated for orthotopic liver transplant in a single large transplant center were prospectively followed. Subjects had contrast CT or MRI at initial evaluation and serial imaging every 6 months until transplantation, removal from the list or death. Univariate and multivariate Cox regression analysis were used to assess associations between new PVT and factors of interest.
Results. Of the 290 prospectively followed cirrhotics who met inclusion criteria, PVT was detected in 70 (24.1%)-47 had PVT at the time of initial evaluation and 23 developed one during the pre-transplant study period. A third of the patients with PVT had re-canalization or spontaneous resolution of thrombus while awaiting transplantation. There was no difference in the pre or post-transplant mortality between cirrhotics with and without PVT.
Conclusion. Cirrhotics with untreated PVT fared equally well as those without PVT before and after transplantation. Further studies with larger numbers of patients are needed to determine if anticoagulation therapy truly improves outcomes in cirrhotics with portal vein thrombosis.
With the routine use of ultrasound Doppler imaging, portal vein thrombosis (PVT) is increasingly being diagnosed in asymptomatic patients with cirrhosis. Although PVT is uncommon (prevalence < 1%) in patients with well-compensated cirrhosis,1 the prevalence increases with worsening Child Pugh scores and is more common (between 4.5 and 35%) in patients being evaluated for orthotopic liver transplantation (OLT).2
While anti-coagulation is recommended for acute symptomatic PVT in patients without cirrhosis, there are no good data to suggest that anticoagulation improves outcomes in symptomatic or asymptomatic cirrhotics with PVT.
Guidelines from the American Association for the Study of Liver Diseases have not laid out firm recommendations on anticoagulation for PVT in the setting of cirrhosis.3 The guidelines suggest that anti-coagulation should be considered if there is evidence to suggest the presence of a known pro-thrombotic condition or in cases of associated superior mesenteric vein thrombosis. For the vast majority of patients who do not have the above, norecommendations have been made either for or against anti-coagulation. A recent publication showed that anti-coagulation with low molecular weight heparin in cirrhotics without portal vein thrombosis may prevent portal vein thrombosis and decrease hepatic decompensation.4 However, the benefit of anti-coagulation in preventing hepatic decompensation appeared to be out of proportion to that achieved by prevention of portal vein thrombosis. However, the study did not monitor anti-Xa levels and the doses appear to be sub-therapeutic. While anti-coagulation was shown to prevent PVT in the above study, there is still no good data to show that it improves morbidity or mortality in cirrhotic patients who have known PVT.4.–6
Many physicians are reluctant to anticoagulate at full doses, patients with cirrhosis due to an increased risk of bleeding in the presence of varices and thrombocytopenia. On the other hand, there is concern that untreated PVT may progress to involve the superior mesenteric vein, making patients ineligible for OLT.
The primary aim of this study was to compare clinical outcomes in prospectively followed cohort of patients with cirrhosis and incidentally detected, untreated PVT with those of patients who had cirrhosis but no PVT. All patients were candidates for orthotopic liver transplantation (OLT). The secondary aims were to:
- •
Estimate the incidence of PVT in patients with cirrhosis who were evaluated for and prospectively followed by serial imaging for OLT.
- •
Identify risk factors for new PVT.
This study prospectively followed a cohort of consecutive adult patients with cirrhosis who were evaluated for liver transplantation at Cleveland Clinic between 07/2004 and 06/2009. The study was approved by the Institutional Review Board.
All patients underwent a liver vascular ultrasound (US) as well as a second imaging in the form of either computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen with contrast at the time of liver transplant evaluation. All patients then underwent serial CT scan or MRI every 6 months until transplantation, removal from the transplant list or death. MELD (Model for End Stage Liver Disease) and Child Pugh scores were calculated at the time of listing and whenever PVT developed. Additional data were collected for each patient from our enterprise electronic health record (Epic systems, Verona, WI) including demographics, clinical history, etiology of cirrhosis, imaging at the time of listing and subsequent imaging (CT scan/ vascular US/MRI abdomen), treatment including anticoagulation, risk factors (hypercoagulable state, AFP, presence of HCC), and extent of PVT. Mortality was evaluated separately by querying the Social Security database. In order to study the impact of PVT on patients awaiting OLT, only patients who had a minimum follow-up of 6 months after transplant evaluation and before transplantation or death were included in this study. Pre-transplant events were censored at 18 months after entry into the study and post transplant events were censored at 6 months post transplant.
PVT was categorized as occlusive or partially occlusive thrombosis of the main portal vein.
Patients were excluded if they:
- •
Had an initial thrombosis that included the superior mesenteric vein.
- •
Hepatocellular carcinoma detected within 3 months of PVT diagnosis.
- •
Follow-up of less than 6 months after being listed for OLT.
- •
Received anti-coagulation for any indication.
Descriptive statistics were computed for all factors; these included means, standard deviations and percentiles for the continuous variables and frequencies for the categorical factors. Differences between the demographics and clinical characteristics of patients with established PVT, new PVT and no PVT were determined by Pearson’s χ2 tests for categorical variables and ANOVA or Kruskal-Wallis test for continuous variables. Time-to-event analysis was performed to study pre- and post-OLT survival. Time of follow-up was defined as the number of months from the transplant listing to last follow up visit, or date of mortality or removal from transplant list. Kaplan-Meier plots were constructed and log-rank tests were used to compare PVT groups. Multivariable Cox regression was performed in order to adjust for other demographic and clinical factors. PVT was forced into the model, and an automated stepwise selection method was performed on 1,000 bootstrap samples to choose the final model. A p < 0.05 was considered statistically significant, and all analyses were done using SAS version 9.2 software (The SAS Institute Inc, Cary, NC) and R version 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
ResultsBaseline characteristicsBetween 07/2004 and 06/2009, 902 cirrhotic patients were evaluated for OLT. Of those, 290 had follow up of over 6 months and therefore were included in the study (Figure 1). PVT was detected in 70 patients (24.1%). Forty seven had PVT at baseline (group 1) and 23 developed new PVT (group 2). The remaining 220 patients never developed PVT (group 3) during the study period (Figure 2). PVT was not a contraindication for OLT in any of the patients. There were no significant differences in baseline characteristics between the three groups, except for ascites, which was more common in the group with PVT (groups 1 and 2) (Table 1).
Portal vein thrombosis (PVT) in patients evaluated for liver transplant.
| Factor | Group 1: PVT @ baseline (n = 47) | Group 2: new PVT (n = 23) | Group 3: no PVT (n = 220) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 59.0 ± 8.3 | 57.8 ± 9.2 | 55.8 ± 9.1 | 0.073 |
| Male | 29 (61.7) | 13 (56.5) | 144 (65.5) | 0.65 |
| Caucasian | 40 (85.1) | 20 (87.0) | 184 (83.6) | 0.9 |
| Baseline | ||||
| Etiology | 0.2 | |||
| Viral | 10 (21.3) | 6 (26.1) | 62 (28.3) | |
| NASH | 9 (19.1) | 3 (13.0) | 35 (16.0) | |
| Alcohol | 4 (8.5) | 1 (4.4) | 37 (16.9) | |
| Cholestatic | 3 (6.4) | 2 (8.7) | 24 (11.0) | |
| Metabolic | 2 (4.3) | 1 (4.4) | 1 (0.46) | |
| Other | 14 (29.8) | 6 (26.1) | 37 (16.9) | |
| Viral/Alcohol | 5 (10.6) | 4 (17.4) | 23 (10.5) | |
| Any Alcohol | 9 (19.1) | 5 (21.7) | 60 (27.3) | 0.46 |
| Creatinine | 1.2 ± 0.79 | 1.3 ± 1.2 | 1.02 ± 0.66 | 0.1 |
| INR | 1.2 ± 0.18 | 1.3 ± 0.3 | 1.3 ± 0.31 | 0.76 |
| Bilirubin | 3.2 ± 2.5 | 4.1 ± 5.5 | 3.3 ± 3.5 | 0.56 |
| Albumin | 3.8 ± 4.5 | 2.9 ± 0.5 | 3.1 ± 0.57 | 0.074 |
| Platelets | 92.0 ([54.0, 126.0)] | 72.0 ([55.0, 88.0)] | 95.0 [59.0, 138.0] | 0.2 |
| Ascites | 28 (59.6)* | 12 (52.2)* | 42 (19.2) | <0.001 |
| MELD | 14.4 ± 5.0 | 15.3 ± 6.7 | 13.8 ± 4.5 | 0.29 |
| FU time | ||||
| Months from evaluation to last follow up | 25.2 ± 19.9 | 28.3 ± 18.0 | 27.4 ± 17.5 | 0.7 |
| Months from OLT to last follow up | 18.2 ± 16.5 | 20.9 ± 11.2 | 19.4 ± 14.4 | 0.84 |
Platelets and AFP values are presented as Median [P25, P75] and p-values correspond to Kruskal-Wallis tests. All other continuous variables presented as Mean ± SD and p-values and correspond to ANOVA. Categorical variables presented as N (%) and p-values correspond to Pearson’s χ2 tests. p-value < 0.05 is considered statistically significant.
There were 243 patients who did not have PVT at baseline-23 of these patients developed PVT during the 3,043 person-months of follow up. Thus, the incidence of new PVT was 9.1 per 100 person-years of follow up, with a cumulative incidence of 8.4% at 12 months and 17.3% at 24 months.
Risk factors associated with the development of new PVT were studied. On multivariate analysis, the presence of ascites and worsening renal function were the only predictors that reached statistical significance, while prior endoscopic treatment for esophageal varices did not increase risk (32, 26, 34%, respectively in groups 1, 2, and 3). After adjusting for creatinine, the patients with ascites at the time of evaluation were 4.7 times more likely to develop PVT than those without ascites (p < 0.001). In addition, for each 1 mg/dL increase in creatinine at time of evaluation, the hazard of developing PVT increased by 60% (p = 0.006).
Impact of PVT on cirrhosisThe overall incidence of GI bleeding after initial evaluation and while awaiting transplantation was comparable between the three groups (21.3, 17.4 and 11.4% respectively, p = 0.17) (Table 2). While there was a trend towards increased GI bleeding in the patients with PVT, the difference did not reach statistical significance.
Clinical outcomes of cirrhotic patients with and without portal vein thrombosis (PVT).
| Factor | Group 1: baseline PVT (n = 47) | Group 2: new PVT (n = 23) | Group 3: no PVT (n = 220) | p-value |
|---|---|---|---|---|
| Follow-up | ||||
| Transplanted | 26 (55.3) | 15 (65.2) | 146 (67.0) | 0.32 |
| HCC on follow up | 1 (2.1) | 0 (0.0) | 7 (3.2) | 0.65 |
| UGIB | 10 (21.3) | 4 (17.4) | 25 (11.4) | 0.17 |
| Ascites during follow up | 20 (42.6) | 11 (47.8) | 76 (34.7) | 0.32 |
| SBP during follow up | 2 (4.3) | 1 (4.3) | 26 (11.9) | 0.19 |
| Encephalopathy on follow up | 8 (17.4) | 3 (13.0) | 44 (20.0) | 0.69 |
| Banding | < 0.001 | |||
| Before PVT | 16 (32.0) | 6 (26.1) | 76 (34.1) | |
| After PVT | 9 (18.0) | 1 (4.4) | NA | |
| No endoscopic therapy | 25 (50.0) | 16 (69.6) | 147 (65.9) | |
| Re-Canalization (partial & complete) | 14 (29.8)*† | 8(34.8) | 0(0.0) | < 0.001 |
| Extension of Thrombus | < 0.001 | |||
| Yes | 3 (6.0) | 0 (0.0) | 0 (0.0) | |
| No | 44 (94.0) | 23 (100) | 220 (100.0) | |
| Deceased | 17 (36.2) | 7 (30.4) | 64 (29.2) | 0.64 |
| Mortality in relation to OLT | 0.89 | |||
| Alive | 30 (63.8) | 16 (69.6) | 155 (71.4) | |
| Died Pre-OLT | 12 (25.5) | 5 (21.7) | 42 (19.4) | |
| Died Post-OLT | 5 (10.6) | 2 (8.7) | 20 (9.2) | |
| Cause of death | 0.078 | |||
| Severe liver disease | 5 (29.4) | 3 (42.9) | 16 (27.6) | |
| Not-related to liver disease | 6 (35.3) | 3 (42.9) | 14 (24.1) | |
| Sepsis | 4 (23.5) | 0 (0.0) | 27 (46.6) | |
| Multi-organ damage | 0 (0.0) | 0 (0.0) | 1 (1.7) | |
| Other | 2 (11.8) | 1 (14.3) | 0 (0.0) | |
To study the impact of a new PVT on the severity of liver disease in the form on the MELD scores, we compared the progression of MELD scores between the patients in groups 2 and 3. MELD scores increased by a mean of 4.8 while awaiting transplant and this was similar in patients with and without PVT.
Progression of PVT during follow upNo patient developed clinically demonstrable mesenteric ischemia from a de novo or progression of existing thrombus into the superior mesenteric vein.
Nearly half of all PVTs (45%) were considered occlusive. There was no difference in the outcomes between occlusive and non-occlusive PVT.
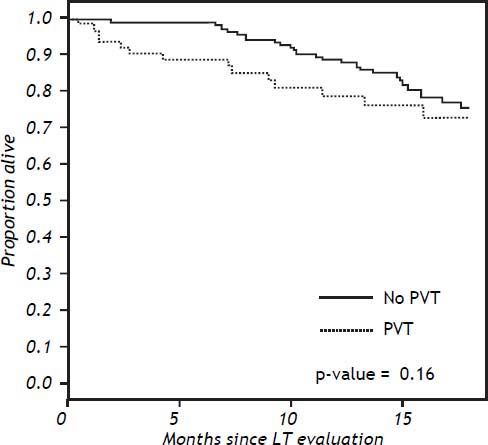
Effect of PVT on pre- and post-OLT mortalityTwelve (25.5%), 5 (21.7%) and 42 (19.4%) in groups 1, 2 and 3 respectively, died before OLT. On multivariable analysis, factors affecting mortality included MELD score and the presence of ascites at baseline. There was no difference in mortality while awaiting OLT between the patients with and without PVT on univariable or multivariable analysis (p = 0.38) (Figure 2).
Twenty six (55%), 15 (65%) and 146 (67%) patients in the three groups, respectively, underwent OLT (Figure 1). Of the 41 with PVT who underwent OLT, 25 (61%) required thrombectomy at the time of transplantation, 10 (24%) had thrombosis that resolved on its own and the others did not require specific surgical intervention at the time of transplant for PVT. There was no difference in rates of rejection or biliary complications between the two groups. Overall, post-OLT mortality in the respective groups during the study period was 10.6%, 8.7% and 9.2% ( p > 0.05). There was no difference in the 60-day or 6 month post transplant mortality between the patients with and without PVT (Figure 3).
DiscussionOur cohort of 290 cirrhotic patients listed for liver transplantation and followed up with serial imaging revealed that the incidence of new PVT in patients awaiting transplant was 9.1% per year of follow up. The high incidence of thrombosis is one of the interesting findings of the study. Approximately 30% of patients who were diagnosed to have PVT in this study did not have one at baseline but developed one during serial follow up imaging every 6 months. We therefore believe that our data is more accurate compared to studies where serial surveillance imaging studies were not performed. These cases represent true PVT since the overwhelming majority of subjects had imaging with contrast (and not a liver vascular ultrasound which may over-estimate the incidence of PVT). Unlike findings in prior studies, the etiology of liver disease or prior endoscopic therapy of varices by banding was not a risk factor for PVT (none of the patients had sclerotherapy).7 The presence of ascites as well as worsening renal function were associated with an increased risk of new PVT, although we were unable to conclude if this association was causal or a consequence of worsening liver disease. Considering that the MELD was similar between the three groups, worsening liver disease is unlikely to be the sole explanation. Stadlbauer, et al.8 showed that a reduction in portal pressure lead to improvement of renal perfusion through positive effects on renal blood flow auto-regulation. Therefore, it is possible that increases in portal venous pressure related to PVT will be associated with decreased renal perfusion, manifesting as worsening of creatinine.
There was no statistically significant difference in survival on the transplant list between patients with and without PVT. They were just as likely to survive 60 days and 6 months post-transplant as the patients without PVT. All of the above suggests that PVT may not have a significant effect on the natural history of cirrhosis in patients whose liver disease is advanced enough to be listed for liver transplantation.
Engelsbe, et al.9 found that there was no difference in patients with and without PVT in terms of transplant rates or waiting list mortality (HR = 0.90, P = 0.23). In contrast to our findings, they reported a higher post-transplant mortality in patients with PVT (HR = 1.32; p = 0.02). However, the authors used a multi-center database, which has inherent limitations in that it is retrospective, and often with incomplete data.
Data favoring anti-coagulation of PVT is based on retrospective data and looks at re-canalization of the portal vein as the primary outcome. In a study from Spain,10 anti-coagulation lead to re-canalization of the portal vein in about 40% of patients with acute PVT and no cirrhosis. This re-canalization rate is similar to the 30% rate in our untreated patients with PVT (group 1).
In a study by Francoz, et al.,11 anticoagulation was associated with a higher rate of complete or partial re-canalization of the splanchnic vein in cirrhotic patients who received anticoagulation as compared to those who were not anticoagulated (42 vs. 0%, p = 0.002). The authors also mentioned that post-transplant survival was higher in the patients with partial or no PVT than in the cirrhotics with complete PVT (83 vs. 50%, p = 0.04). However, the end-point included deaths up to two years after transplant, and it is unlikely that the presence of a PVT before transplant could account for deaths that far out from the surgery. Again, since our study included only patients on the transplant list, it is unclear if their liver disease had advanced such that anti-coagulation was unlikely to help at that point.
Many experts have previously highlighted the lack of prospective studies evaluating the course of PVT in cirrhosis.3,12 We believe that our study design has several strengths and answers the questions they raised. Firstly, we prospectively followed three cohorts of cirrhotics from the time of listing, and our follow up was long enough to measure outcomes. Secondly, we carefully excluded patients where portal vein thrombosis may behave differently from our intended study population, such as patients with HCC, where the incidence of PVT is higher and the mechanism of thrombus formation may be secondary to the hypercoagulable state related to the malignancy rather than a low flow state.12 Thirdly, all our patients had imaging at the time of entry to the study and follow up every 6 months to assess for development or progression of PVT. This is because most cirrhotics remain asymptomatic when they develop PVT. Because of the serial surveillance, we were able to identify a higher incidence of PVT than previously described.
Limitations of the study included absence of routine testing for a hypercoagulable state routinely in all patients as done in other studies. Also, this was a single center study and the sample size may be too small to draw firm conclusions regarding survival (type 2 error).
In conclusion, cirrhotic patients evaluated for liver transplantation with untreated PVT appear to fare qually well as patients without PVT both before and after liver transplantation. Most are asymptomatic, and some patients have partial re-canalization of PVT without anticoagulation. The thrombus was succesfully managed surgically and there are no differences in post transplant outcomes. Our results suggest that anticoagulation or clot lysis may not be warranted in patients with cirrhosis and isolated PVT who are candidates for liver transplantation. Prospective randomized controlled trials with adequate sample size are needed to further corroborate the findings.
Financial DisclosuresNone.
Potential ConflictsThere are no potential conflicts of interest.
Author Contributions- •
BVJ: study concept and design; acquisition, analysis and interpretation of data; drafting of the manuscript.
- •
AA, RK: acquisition, analysis and interpretation of data; drafting of the manuscript.
- •
RL: statistical analysis.
- •
AAtreja: analysis and interpretation of data, drafting of the manuscript.
- •
CM, NNZ: study concept and design, critical revision of the manuscript for important intellectual content.
- •
WDC: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision.