Introduction. Liver retransplantation (LReTx) is the therapeutic option for hepatic graft failure. Survival after LReTx is poorer than after primary liver transplantation. Given the organ shortage, it is essential to optimize the use of this resource.
Objective. To evaluate rates, indications and patient survival after LReTx and identify factors associated with mortality following LReTx.
Material and methods. We conducted a retrospective cohort study of all adults undergoing LReTx based on registry data from the Liver Transplantation Group (Complexo Hospitalar Santa Casa de Porto Alegre), southern Brazil.
Results. Between June 16, 1991 and July 19, 2011, 824 patients underwent 866 liver transplants. Forty-two procedures corresponded to LReTx (4.8% of all liver transplants performed). Thirty-eight patients who underwent a single LReTx procedure were included in this study. The leading indication for LReTx was hepatic artery thrombosis (HAT) (31.6%), followed by primary nonfunction (PNF) (18.4%). The main indication for early LReTx was PNF (58.3%) and for late LReTx was HAT (38.5%). During the follow-up period, 26 patients (68.4%) died after LReTx. Patient survival at 1 and 3 years after LReTx was 44.7% and 44.7%, respectively. Patients infected with hepatitis C virus, serum albumin < 2.5 g/dL and receiving mechanical ventilation immediately before LReTx had a significantly lower survival rate than the other patients.
Conclusion. Considering the increased mortality when the graft loss is delayed, it is necessary to define the minimum acceptable results to indicate LReTx and identify the patients who would most benefit from this treatment.
When a liver graft fails after transplantation, liver retransplantation (LReTx) is the only therapeutic option that offers a chance for long-term survival. Overall, according to studies conducted in Europe and the United States, LReTx is generally associated with poorer survival1.–4 and higher costs.5,6 Furthermore -and very important- patients listed for LReTx compete for the same number of organs with those candidates for first-time liver transplant, and offering more than one transplant opportunity to the same patient, while others die on the waiting list, has been the subject of considerable controversy.3,7.–9 Therefore, LReTx represents a clinical dilemma.
LReTx may occur at any time after the initial transplant, but, in general, it is divided into two groups: early or late LReTx.6,10 Early LReTx is performed soon after the first transplant, usually within the first week or month. The most common diagnoses for allograft failure requiring early LReTx include primary nonfunction (PNF), hepatic artery thrombosis (HAT), and other technical issues. Given the shortage of donors, it is increasingly common to use grafts from the so-called expanded criteria donors, a practice that has been associated with a higher incidence of ischemic lesions and the occurrence of primary graft dysfunction.11 Thus, primary dysfunction/nonfunction is one of the major causes of early LReTx,12,13 which has then been viewed as an ethically required practice. Late LReTx, occurring months or years after the initial transplant, is usually performed for recurrent disease, late technical problems, and more rarely, chronic rejection.14.–16 Recurrence of primary disease in the graft, especially hepatitis C virus (HCV) infection, has become the most common indication for late LReTx.12,14,16 There is evidence that morbidity and mortality are higher among HCV-infected patients than among uninfected individuals when undergoing LReTx, which makes the procedure particularly controversial in this context.17
The factors responsible for the poor outcome of retransplanted patients are still poorly understood.7 Apparently, it is due to a combination of factors related to the recipient, donor, and random perioperative and postoperative events.7,13 Thus, prognostic models have been developed for risk stratification of candidates for LReTx.1,11,18,19 Nevertheless, to date, none has been routinely incorporated into clinical practice.13 One of the best-known models was developed by Rosen, et al.,11 and is used to estimate the survival of patients undergoing retransplantation from the second week after the first transplant. Its calculation includes recipient age, total bilirubin, creatinine, and interval between first transplantation and retransplantation. Patients undergoing LReTx with a score > 20.5 have a poor prognosis (survival of 54% at 90 days and 42% at 1 year), while retransplanted patients with a score ≤ 16 have shown survival of 82and 75% at 90 days and 1 year, respectively.11 The Model for End-stage Liver Disease (MELD)20 has been validated as a predictor of survival in several cohorts of patients with varying levels of liver disease severity, and also in patients of geographically and temporally diverse origins, such as the Brazilian population.21
The MELD score was adopted by the Brazilian National Transplant System as the basis for deceased donor liver allocation in 2006.22 The current MELD-based allocation system appears to serve primary and retransplantation candidates equitably.23 An analysis of all wait-list registrants in the United States between 2000 and 2006, which included 2,081 retransplant candidates, showed that mortality was comparable between primary and retransplant candidates within a range of MELD scores where most transplantations took place. However, in the very low MELD score ranges, retransplant candidates had a higher mortality than primary liver transplantation candidates with the same score.23 It seems that the MELD score represents a good predictor of survival after LReTx.24.–26 In the study by Maggi, et al.,26 separation of patients into three groups based on their MELD scores (< 16, 16–24, > 25) showed a statistically significant difference in global survival (log-rank test, p = 0.02) of patients with different MELD scores. Patients with higher scores had the worst survival.
The main objective of this study was to evaluate rates, indications, postoperative mortality, and patient survival after LReTx in a cohort of patients from southern Brazil and identify factors associated with mortality following LReTx. We also examined the ability of Rosen and MELD scores to predict survival in this scenario.
Material and MethodsThis is a retrospective study covering a 20–year period, from June 16, 1991 to July 19, 2011. Among all patients undergoing liver transplantation from deceased donors at a tertiary care hospital, Complexo Hospitalar Santa Casa de Porto Alegre, Brazil, we identified those undergoing LReTx. The only exclusion criterion was patients who received a second LReTx. Information was obtained from the prospectively collected database of the Liver Transplantation Group at Complexo Hospitalar Santa Casa de Porto Alegre. The study was approved by the Institutional Review Board of the institution. A terms and conditions for collection and proper use of institutional data, including confidentiality, was completed and signed by the authors for all patients included in the study sample.
Surgical technique and immunosuppressionThe same surgical team performed all LReTx procedures using standard techniques. Since August 1997, the most commonly used technique has been hepatectomy with preservation of the inferior vena cava (the “piggyback” technique), which is the technique of choice. The decision to transfuse was based on clinical and hemodynamic criteria. Blood loss was counteracted by transfusion of red blood cells (RBC), with the aim to maintain hemoglobin between 8.0 and 10.0 g/dL. Other blood products were administered when excessive blood loss could not be controlled by standard procedures. Platelet concentrates were administered if the platelet count decreased to 40 × 109/L. A cell saver system was used in all surgeries. All transfused blood products were ABO-Rh compatible. Crystalloid and colloid solutions were used for volume replacement. Anesthesia was induced and maintained with an intravenous combination of propofol, fentanyl, and rocuronium. Hemodynamic monitoring consisted of an arterial line and a pulmonary artery catheter. Organ procurement was performed according to standard techniques.27,28 The University of Wisconsin solution was utilized for cold perfusion in all but 10 patients, in whom a histidine-tryptophan-ketoglutarate solution was used (in two patients the solution used was not reported).
Immunosuppressive treatment was given according to the protocol at the time of transplantation. Briefly, on the day of transplantation, patients were started on a rapidly tapering steroid course. After LReTx, patients received either cyclosporine or tacrolimus (in the last 13 years) and prednisone. Some patients were also prescribed mycophenolate sodium or mycophenolate mofetil.
Data analysisThe following variables from recipients were analyzed: age, gender, skin color (white or nonwhite) as surrogate for race, primary etiology of end-stage liver disease, diabetes mellitus (yes or no), indications for LReTx, and laboratory results for creatinine [mg/dL], international normalized ratio (INR), total serum bilirubin [mg/dL], serum sodium [mEq/L], and albumin [g/dL], available immediately before the LReTx procedure. MELD,29 MELD-Na30 and Rosen11 scores were calculated based on laboratory values obtained at the time of LReTx. The Rosen score was calculated only for 28 patients who underwent LReTx at intervals exceeding 15 days in relation to primary liver transplant. Other variables related to the recipient included: preoperative requirement for dialysis (yes or no), ventilatory support (yes or no), time interval between the first and second transplant (≤ 30 and > 30 days), retransplantation eras (pre-MELD [June 16, 1991–June 30, 2006] or post-MELD era [July 1, 2006–July 19, 2011]), and period of retransplantation according to the experience of the group (first decade [June 16, 1991–June 30, 2001] or second decade [July 1, 2001–July 19, 2011]).
Donor-related characteristics and variables included: age (< 40 or ≥ 40 years), gender, and cause of brain death. Variables related to operative parameters were categorized into cold ischemia time ( ≥ 8 or < 8 h), RBC transfusion (≥ 9 or < 9 units), and platelet transfusion (yes or no).
The following variables related to donor-recipient matching were evaluated: gender (identical, malefemale, or female-male), ABO blood group compatibility (identical, compatible, or incompatible), and age.
Patients were followed up for at least 12 months after LReTx in order to determine the primary endpoint (death). Patient survival was calculated from the day of retransplantation until the date of death or until the end of the study, according to the status of the patient at follow-up.
Statistical analysisAll statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as absolute and relative frequencies, mean ± standard deviation, and median (minimum to maximum). Actuarial survival analysis was performed using the Kaplan-Meier method. Subgroup survival curves were compared using the log-rank test. For survival analysis, continuous variables were dichotomized. In this analysis, p < 0.05 was considered to be statistically significant.
ResultsBetween June 16, 1991 and July 19, 2011, 824 patients underwent 866 liver transplants with the Liver Transplantation Group at Complexo Hospitalar Santa Casa de Porto Alegre. Of these, 42 procedures corresponded to LReTx, accounting for 4.8% of all liver transplants performed. Two patients received three grafts each and were excluded from the analysis. Then, 38 patients who underwent a single LReTx procedure were included in this study.
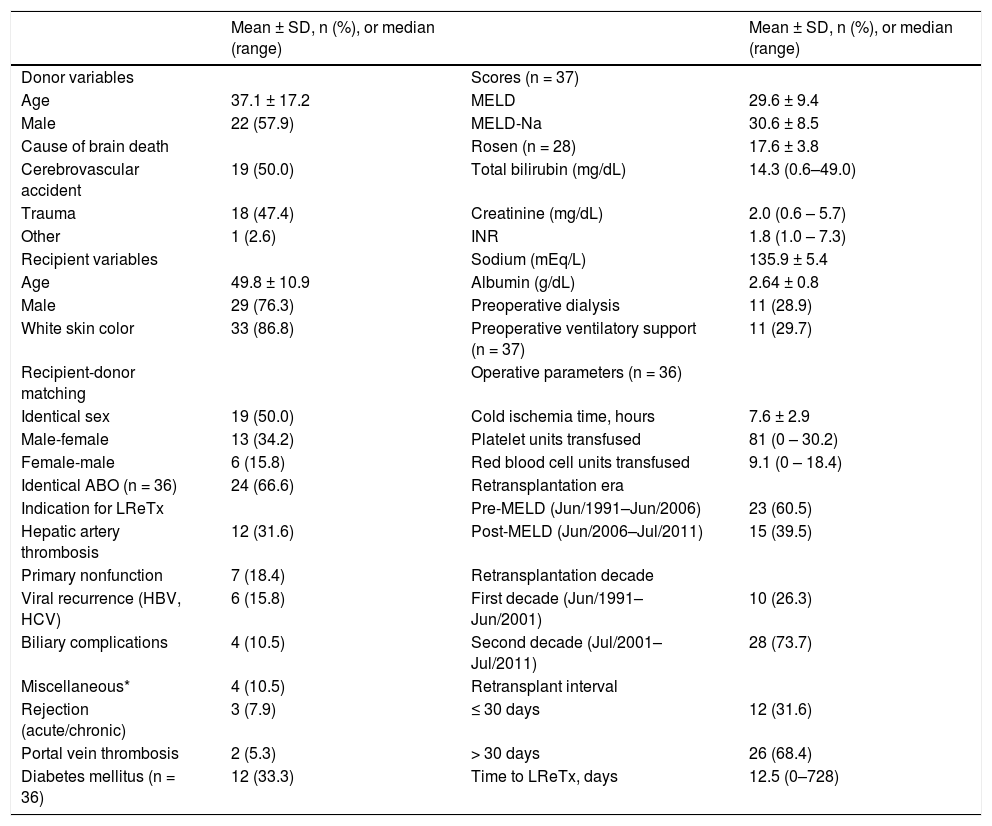
As shown in table 1, most donors and recipients were male and of white skin color. The mean age of recipients was 49.79 years (range, 16–69 years), and mean age of donors was 37.1 years (range, 7–66 years). Half of the cases showed identical recipientdonor sex matching and 66.67% of cases, an identical ABO. The leading indication for LReTx was HAT (31.6%), followed by PNF (18.4%). The overall mean of pre-LReTx MELD, MELD-Na and Rosen scores was 29.57 ± 9.41 (range, 13–45), 30.65 ± 8.48 (range, 14–43), and 17.62 ± 3.80 (range, 10.3–26.1), respectively. About 30% of patients were on dialysis prior to LReTx, and about 30% were on ventilatory support. Most patients underwent LReTx in the pre-MELD era, in the second decade of operation of the Liver Transplantation Group, and 30 days after the first procedure (Table 1).
Characteristics of the cohort of patients who underwent liver retransplantation (LReTx) in southern Brazil between June 1991 and July 2011 (n = 38).
| Mean ± SD, n (%), or median (range) | Mean ± SD, n (%), or median (range) | ||
|---|---|---|---|
| Donor variables | Scores (n = 37) | ||
| Age | 37.1 ± 17.2 | MELD | 29.6 ± 9.4 |
| Male | 22 (57.9) | MELD-Na | 30.6 ± 8.5 |
| Cause of brain death | Rosen (n = 28) | 17.6 ± 3.8 | |
| Cerebrovascular accident | 19 (50.0) | Total bilirubin (mg/dL) | 14.3 (0.6–49.0) |
| Trauma | 18 (47.4) | Creatinine (mg/dL) | 2.0 (0.6 – 5.7) |
| Other | 1 (2.6) | INR | 1.8 (1.0 – 7.3) |
| Recipient variables | Sodium (mEq/L) | 135.9 ± 5.4 | |
| Age | 49.8 ± 10.9 | Albumin (g/dL) | 2.64 ± 0.8 |
| Male | 29 (76.3) | Preoperative dialysis | 11 (28.9) |
| White skin color | 33 (86.8) | Preoperative ventilatory support (n = 37) | 11 (29.7) |
| Recipient-donor matching | Operative parameters (n = 36) | ||
| Identical sex | 19 (50.0) | Cold ischemia time, hours | 7.6 ± 2.9 |
| Male-female | 13 (34.2) | Platelet units transfused | 81 (0 – 30.2) |
| Female-male | 6 (15.8) | Red blood cell units transfused | 9.1 (0 – 18.4) |
| Identical ABO (n = 36) | 24 (66.6) | Retransplantation era | |
| Indication for LReTx | Pre-MELD (Jun/1991–Jun/2006) | 23 (60.5) | |
| Hepatic artery thrombosis | 12 (31.6) | Post-MELD (Jun/2006–Jul/2011) | 15 (39.5) |
| Primary nonfunction | 7 (18.4) | Retransplantation decade | |
| Viral recurrence (HBV, HCV) | 6 (15.8) | First decade (Jun/1991–Jun/2001) | 10 (26.3) |
| Biliary complications | 4 (10.5) | Second decade (Jul/2001–Jul/2011) | 28 (73.7) |
| Miscellaneous* | 4 (10.5) | Retransplant interval | |
| Rejection (acute/chronic) | 3 (7.9) | ≤ 30 days | 12 (31.6) |
| Portal vein thrombosis | 2 (5.3) | > 30 days | 26 (68.4) |
| Diabetes mellitus (n = 36) | 12 (33.3) | Time to LReTx, days | 12.5 (0–728) |
HBV: hepatitis B virus. HCV: hepatitis C virus. INR: international normalized ratio. MELD: Model for End-stage Liver Disease.
Miscellaneous includes one patient with Budd-Chiari syndrome, one patient with drug-induced acute liver failure, one patient with combined hepatic artery thrombosis and biliary complications, and one patient with an unknown cause. Data expressed as mean ± standard deviation (SD), median (minimum and maximum), or n (%) as appropriate.
Indications for primary liver transplantation and LReTx are shown in tables 2 and 3. The leading indications for liver transplantation were HCV-related cirrhosis (34.2%) and hepatocellular carcinoma (18.4%) (Table 2). The main indication for early LReTx was early PNF (58.3%) and for late LReTx was HAT (38.5%). Recurrent hepatitis accounted for 23.1% of all cases of late LReTx (Table 3).
Primary etiology of end-stage liver disease in retransplanted patients (n = 38).
| Primary diagnosis | n (%) |
|---|---|
| HCV-related cirrhosis | 13 (34.2) |
| Hepatocellular carcinoma | 7 (18.4) |
| Alcoholic cirrhosis | 6 (15.8) |
| HBV-related cirrhosis | 4 (10.5) |
| Other causes of cirrhosis* | 4 (10.5) |
| Autoimmune cirrhosis | 2 (5.3) |
| Combined liver-kidney transplantation | 2 (5.3) |
HBV: hepatitis B virus. HCV: hepatitis C virus.
Indications for liver retransplantation according to the time interval after primary liver transplantation
| Indications for retransplantation | Early (≤ 30 days) n = 12 (%) | Late (> 30 days) n = 26 (%) |
|---|---|---|
| Hepatic artery thrombosis | 2 (16.7) | 10 (38.5) |
| Primary graft dysfunction* | 7 (58.3) | 0 |
| Biliary complications | 0 | 4 (15.4) |
| Recurrent disease | 0 | 6 (23.1) |
| Miscellaneous** | 1 (8.3) | 3 (11.5) |
| Rejection (acute/chronic) | 1 (8.3) | 2 (7.7) |
| Portal vein thrombosis | 1 (8.3) | 1 (3.8) |
Mean follow-up was 30.42 months (range, 0–133.7 months). During the follow-up period, 26 of 38 retransplanted patients (68.4%) died after LReTx, 21 of them during hospitalization for retransplantation (55.3%). Patient survival at 30 and 90 days and at 1, 3 and 5 years after LReTx was 52.6, 47.4, 44.7, 44.7, and 41.3%, respectively (Figure 1). The main cause of death was septic shock (61.54%), followed by hypovolemic shock (19.2%), multiple organ system failure (15.4%), and liver failure (3.8%).
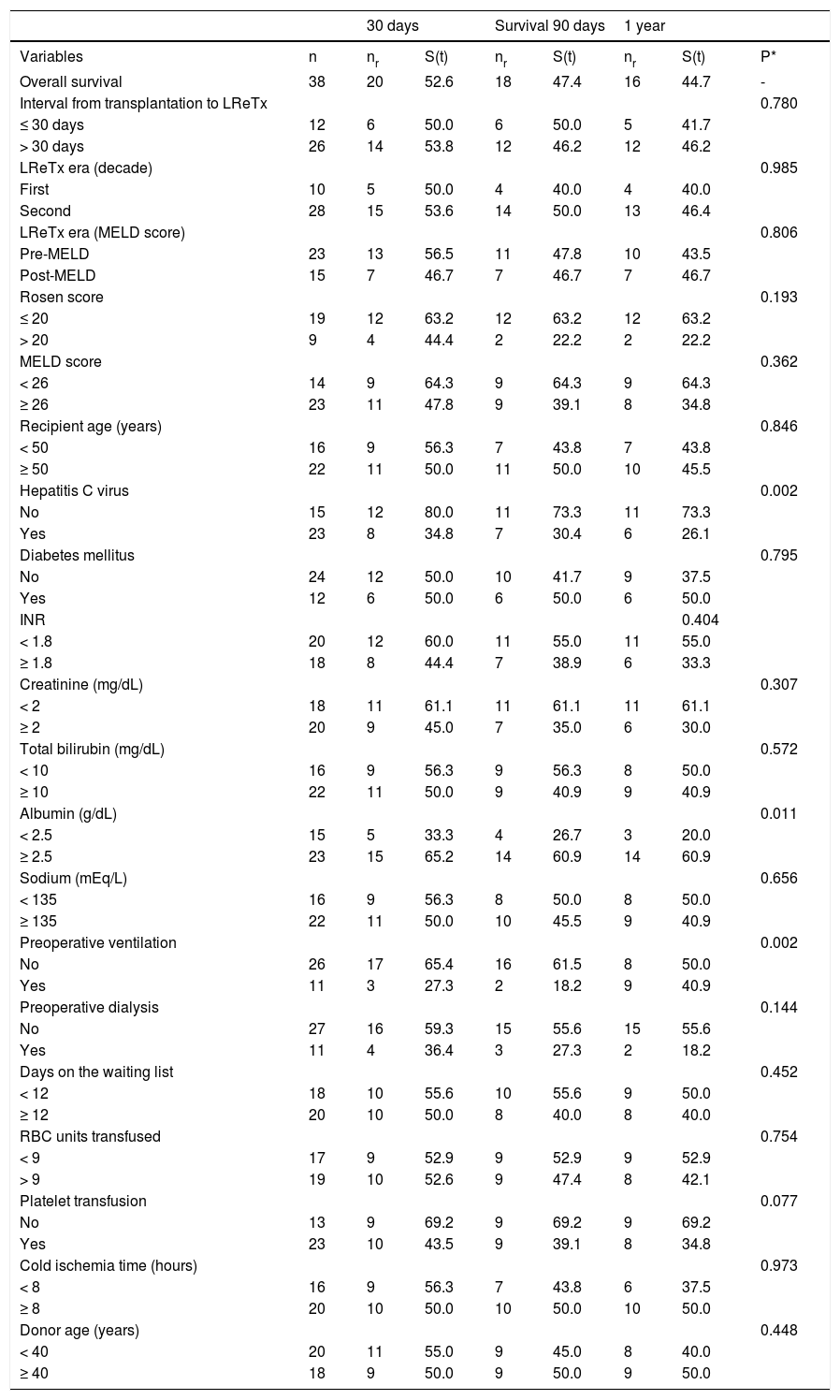
The univariate analysis of factors correlated with 30-day, 90-day and 1-year survival is shown in table 4. Patients infected with HCV, serum albumin < 2.5 g/dL and receiving mechanical ventilation immediately before LReTx had a significantly lower survival rate than the other patients. The following variables, analyzed and categorized as indicated in parentheses, had no impact on post-LReTx survival: urgency of LReTx (interval between transplantation and retransplantation ≤ 30 or > 30 days), LReTx era (classified as pre- or post-MELD and first or second decade of service experience), Rosen score (≤ 20 or > 20), MELD score (< 26 or ≥ 26), age of the recipient (< 50 or ≥ 50 years), diabetes mellitus before LReTx (yes or no), recipient INR (< 1.8 or ≥ 1.8), creatinine (< 2 or ≥ 2 mg/dL), total serum bilirubin (< 10 or ≥ 10 mg/dL), serum sodium (< 135 or ≥ 135 mEq/L), need for dialysis immediately before LReTx (yes or no), time spent on the waiting list for LReTx (< 12 or ≥ 12 days), packed RBC transfused during the perioperative period (< 9 or ≥ 9 units), platelet units transfused during the perioperative period (yes or no), cold ischemia time (< 8 or ≥ 8 h), and age of the donor (< 40 or ≥ 40 years).
Univariate analysis of variables analyzed for first liver retransplantation (LReTx) in relation to survival prospects (Kaplan Meier method).
| 30 days | Survival 90 days | 1 year | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | nr | S(t) | nr | S(t) | nr | S(t) | P* |
| Overall survival | 38 | 20 | 52.6 | 18 | 47.4 | 16 | 44.7 | - |
| Interval from transplantation to LReTx | 0.780 | |||||||
| ≤ 30 days | 12 | 6 | 50.0 | 6 | 50.0 | 5 | 41.7 | |
| > 30 days | 26 | 14 | 53.8 | 12 | 46.2 | 12 | 46.2 | |
| LReTx era (decade) | 0.985 | |||||||
| First | 10 | 5 | 50.0 | 4 | 40.0 | 4 | 40.0 | |
| Second | 28 | 15 | 53.6 | 14 | 50.0 | 13 | 46.4 | |
| LReTx era (MELD score) | 0.806 | |||||||
| Pre-MELD | 23 | 13 | 56.5 | 11 | 47.8 | 10 | 43.5 | |
| Post-MELD | 15 | 7 | 46.7 | 7 | 46.7 | 7 | 46.7 | |
| Rosen score | 0.193 | |||||||
| ≤ 20 | 19 | 12 | 63.2 | 12 | 63.2 | 12 | 63.2 | |
| > 20 | 9 | 4 | 44.4 | 2 | 22.2 | 2 | 22.2 | |
| MELD score | 0.362 | |||||||
| < 26 | 14 | 9 | 64.3 | 9 | 64.3 | 9 | 64.3 | |
| ≥ 26 | 23 | 11 | 47.8 | 9 | 39.1 | 8 | 34.8 | |
| Recipient age (years) | 0.846 | |||||||
| < 50 | 16 | 9 | 56.3 | 7 | 43.8 | 7 | 43.8 | |
| ≥ 50 | 22 | 11 | 50.0 | 11 | 50.0 | 10 | 45.5 | |
| Hepatitis C virus | 0.002 | |||||||
| No | 15 | 12 | 80.0 | 11 | 73.3 | 11 | 73.3 | |
| Yes | 23 | 8 | 34.8 | 7 | 30.4 | 6 | 26.1 | |
| Diabetes mellitus | 0.795 | |||||||
| No | 24 | 12 | 50.0 | 10 | 41.7 | 9 | 37.5 | |
| Yes | 12 | 6 | 50.0 | 6 | 50.0 | 6 | 50.0 | |
| INR | 0.404 | |||||||
| < 1.8 | 20 | 12 | 60.0 | 11 | 55.0 | 11 | 55.0 | |
| ≥ 1.8 | 18 | 8 | 44.4 | 7 | 38.9 | 6 | 33.3 | |
| Creatinine (mg/dL) | 0.307 | |||||||
| < 2 | 18 | 11 | 61.1 | 11 | 61.1 | 11 | 61.1 | |
| ≥ 2 | 20 | 9 | 45.0 | 7 | 35.0 | 6 | 30.0 | |
| Total bilirubin (mg/dL) | 0.572 | |||||||
| < 10 | 16 | 9 | 56.3 | 9 | 56.3 | 8 | 50.0 | |
| ≥ 10 | 22 | 11 | 50.0 | 9 | 40.9 | 9 | 40.9 | |
| Albumin (g/dL) | 0.011 | |||||||
| < 2.5 | 15 | 5 | 33.3 | 4 | 26.7 | 3 | 20.0 | |
| ≥ 2.5 | 23 | 15 | 65.2 | 14 | 60.9 | 14 | 60.9 | |
| Sodium (mEq/L) | 0.656 | |||||||
| < 135 | 16 | 9 | 56.3 | 8 | 50.0 | 8 | 50.0 | |
| ≥ 135 | 22 | 11 | 50.0 | 10 | 45.5 | 9 | 40.9 | |
| Preoperative ventilation | 0.002 | |||||||
| No | 26 | 17 | 65.4 | 16 | 61.5 | 8 | 50.0 | |
| Yes | 11 | 3 | 27.3 | 2 | 18.2 | 9 | 40.9 | |
| Preoperative dialysis | 0.144 | |||||||
| No | 27 | 16 | 59.3 | 15 | 55.6 | 15 | 55.6 | |
| Yes | 11 | 4 | 36.4 | 3 | 27.3 | 2 | 18.2 | |
| Days on the waiting list | 0.452 | |||||||
| < 12 | 18 | 10 | 55.6 | 10 | 55.6 | 9 | 50.0 | |
| ≥ 12 | 20 | 10 | 50.0 | 8 | 40.0 | 8 | 40.0 | |
| RBC units transfused | 0.754 | |||||||
| < 9 | 17 | 9 | 52.9 | 9 | 52.9 | 9 | 52.9 | |
| > 9 | 19 | 10 | 52.6 | 9 | 47.4 | 8 | 42.1 | |
| Platelet transfusion | 0.077 | |||||||
| No | 13 | 9 | 69.2 | 9 | 69.2 | 9 | 69.2 | |
| Yes | 23 | 10 | 43.5 | 9 | 39.1 | 8 | 34.8 | |
| Cold ischemia time (hours) | 0.973 | |||||||
| < 8 | 16 | 9 | 56.3 | 7 | 43.8 | 6 | 37.5 | |
| ≥ 8 | 20 | 10 | 50.0 | 10 | 50.0 | 10 | 50.0 | |
| Donor age (years) | 0.448 | |||||||
| < 40 | 20 | 11 | 55.0 | 9 | 45.0 | 8 | 40.0 | |
| ≥ 40 | 18 | 9 | 50.0 | 9 | 50.0 | 9 | 50.0 | |
INR: international normalized ratio. MELD: Model for End-stage Liver Disease; nr: absolute number of surviving individuals. RBC: red blood cells. S(t): survival according to time period (expressed as %).
In the United States, indications for LReTx accounted for 8% of all wait-listed cases5 and 10% of all procedures performed in 2005.31 In Brazil, the rates of wait-listed patients or patients undergoing LReTx are unknown, and the only record available is that, of 1,252 liver transplants performed between 2001 and 2005 in the State of São Paulo, 7.8% were retransplantations.32 In the cohort analyzed in this study, based on the experience of a single center in southern Brazil over a 20-year period, the rate of LReTx was 4.8%. This rate is probably underestimated because it includes only patients who actually underwent LReTx and may be explained, among other reasons, by the lack of well-defined criteria for placement of patients on the waiting list for retransplantation and by the death of wait-listed patients. In Brazil, before the introduction of the MELD score in 2006 for allocation of deceased donor livers, the basis for organ allocation was strictly time spent by candidates on the waiting list, respecting ABO compatibility. Patients who required retransplantation acquired high-urgency status if placed on the waiting list within 48 h of primary liver transplant, a period which is often too short for making consistent decisions. After implementation of the MELD system for organ allocation, patients with PNF or HAT are considered to be in a high-urgency situation if placed on the waiting list within 7 days of transplantation, and the high-urgency status may be extended in both cases for another 7 days if no retransplantation occurs. After this period, the urgency request is withdrawn and, for patients with PNF, the graft should be allocated according to the calculated MELD score, while patients with HAT are assigned a score of 40.
The analysis of the indications for LReTx revealed that HAT was the most common indication, followed by PNF of the graft (31.6 and 18.4% of cases, respectively). HAT is a relatively rare complication following liver transplantation in adults. In a consecutive series of 1,192 transplants performed in adults between 1988 and 2000, the incidence of HAT was 2.5%.33 However, because of its severe consequences, HAT has become one of the most frequent indications for retransplantation.8,34 HAT is classified as early when diagnosed within 30 days of transplantation, or late when detected after this period.35 The clinical presentation depends on time of onset and occurrence of side branches. In early HAT, necrosis of the biliary tract is a common occurrence, followed by uncontrolled sepsis and even death. Late HAT may be asymptomatic, but in many cases it manifests as biliary complications, such as necrosis and abscess, as well as liver graft ischemia.36,37 In our study, late HAT accounted for most cases, and LReTx was performed during a widely varying period of time after transplantation (from 97 to 916 days), representing the main indication (38.5%) for late LReTx. A similar result was observed in Valencia, Spain, where HAT was the main cause of late LReTx (32.7%).16 A study conducted in England to assess retransplantation in cases of late HAT suggested that presence of multidrug-resistant bacteria-positive cultures, increased MELD score (> 12) and use of antibiotic therapy at the time of retransplantation were risk factors for post-retransplantation death.38 In our study, the small number of cases of late HAT (n = 10) hindered the identification of risk factors for post-retransplantation death.
Graft function is influenced by several factors, including cold ischemia time, reperfusion, and characteristics of the recipient and donor,39,40 and its dysfunction is therefore considered to be of multifactorial origin. Although there is no consensus on the definition of graft dysfunction, the primary nonfunctioning of the graft is considered a severe form of reperfusion injury that results in its irreversible loss, in the absence of detectable technical or immunological problems.39 In a center in the United States, over a 20-year period, 2.2% of liver transplants had PNF among 2,341 transplants performed in 2,130 patients.41 In addition, PNF occurred more often in the retransplant setting, with female donor, donor age, donor days in the intensive care unit, cold ischemia time, and operating room time being accounted as significant factors for PNF.41 A study using the United Network Organ Sharing (UNOS) database,42 assessing patients listed as Status 1 (life expectancy < 7 days without a transplant), reported that, among patients listed for PNF within 7 days of liver transplantation, there was no significant association between survival and LReTx (i.e., the probability of survival of patients with PNF without retransplantation in 7 days was approximately 86%, higher than that predicted by UNOS technical standards), and the MELD score was not a predictor of wait-list mortality. At our institution, PNF was an indication in 18.4% of LReTx cases, a rate similar to that described in a Spanish center.14 In agreement with previous reports in the literature.39,43 PNF was also the most common indication for early retransplantation (58.3%) at our center.
Postoperative mortality is higher in LReTx.44 In our series, 26 of 38 retransplanted patients (68.4%) died after LReTx, 21 of them during hospitalization for retransplantation (55.3%). The main causes of postoperative mortality were sepsis (61.54%) and hemorrhage (19.2%). Studies conducted in Italy13 and Germany44 showed sepsis mortality rates of 40% and 26% in post-LReTx, respectively. Therefore, in spite of the prophylactic measures taken at our center, such as rational use of antimicrobial agents, early enteral supply, early discharge from mechanical ventilation, and intensive physical therapy, continuous surveillance of patients during all phases of the liver retransplantation process is vital. Furthermore, it is worth mentioning that the systematic investigation of infection in recipients, both before and after the procedure, is of crucial relevance.
Survival is markedly lower in retransplanted patients. The study of the University of Pittsburgh was one of the first to assess post-LReTx outcomes by analyzing the outcome of 418 patients who underwent LReTx over a six-year period.3 Survival at 1 and 5 years following LReTx was 62 and 47%, respectively. Two recent studies, one conducted in Spain (n = 79)34 and the other in the United Kingdom (n = 196),8 reported 1- and 5-year survival rates after LReTx of 66 and 42% and of 66 and 57%, respectively. In a multicenter Italian study (n = 187),26 overall survivals of patients who underwent LReTx were 65, 48 and 38% at 1, 5 and 10 years, respectively, with no statistically significant difference in relation to graft survival after the first and second LReTx. At our institution, actuarial patient survival at 1 and 5 years after LReTx was 44.7 and 41.3%, respectively. These rates were markedly lower than those observed in patients who underwent -approximately in the same time period, at the same center and with the same team- primary transplantation, which were 81% in the first year and 61% after a mean follow-up of 14 years.45
In the present study, due to the small number of cases, only Kaplan-Meier survival curves were generated and evaluated for significance using the logrank test for patient survival. Survival after LReTx was significantly lower in recipients infected with HCV (p = 0.002), with serum albumin < 2.5 g/dL (p = 0.011) and requiring mechanical ventilation immediately before retransplantation (p = 0.002) when compared to recipients without these characteristics. Of 23 HCV-infected retransplanted patients, 17 (73.9%) died during hospitalization for retransplantation. Therefore, lower survival of HCV-infected patients observed in this series appears to be directly related to the clinical status of patients at the time of LReTx, which plays an important role in postoperative outcomes.11,18,46 The impaired clinical status in these patients immediately before LReTx is also evident by two other factors associated with lower survival: serum albumin < 2.5 g/dL and need for mechanical ventilation, a prognostic factor for survival.3,7,19 In contrast to the findings of other studies, in our analysis serum creatinine,3,7,19,46 rate of bilirubin,3,18 age of the donor3 and recipient,3,11,12,18 urgency of retransplantation,8 Rosen score > 20,11 and MELD score > 2611 -among other factors- were not associated with lower post-retransplantation survival, probably due to the small number of patients analyzed (e.g. only 9 patients had a Rosen score > 20).
This study has biases that deserve mention, including the small number of patients who actually underwent LReTx and the lack of a national or institutional protocol with clear criteria for inclusion in and exclusion from the transplantation list. Nevertheless, these data reflect the reality of a tertiary referral center for liver transplantation in southern Brazil, which presents good results in patients undergoing primary transplantation.45
In conclusion, the most common cause for LReTx was late HAT. The actuarial survival rates of retransplanted patients were 44.7% at 1 year and 41.3% at 5 years, which were lower than the results of primary transplantation at our center. Sepsis was the most common cause of death after LReTx and most deaths occurred soon after the procedure. Neither Rosen and MELD scores nor recipient and donor age had an impact on patient survival after LReTx, which may be perceived as a reflection of the small sample size. However, patients infected with HCV, with serum albumin < 2.5 g/dL and receiving mechanical ventilation immediately before LReTx had a significantly lower survival, which seems to be directly related to the patient’s clinical status at the moment of LReTx. Considering the increased mortality after LReTx, in addition to the prophylactic measures taken at our center to prevent infections and the systematic investigation of infections in the recipients, an adequate selection of candidates is mandatory to achieve better results, especially when the recipients are infected with HCV.
Abbreviations- •
HAT: hepatic artery thrombosis.
- •
HCV: hepatitis C virus.
- •
INR: international normalized ratio.
- •
LReTx: liver retransplantation.
- •
MELD: Model for End-stage Liver Disease.
- •
PNF: primary nonfunction.
- •
RBC: red blood cell.
- •
SPSS: Statistical Package for the Social Sciences.
- •
UNOS: United Network Organ Sharing.
The authors would like to thank Dr. Mário Wagner for his assistance with the statistical analysis.
Financial SupportThe study did not receive financial support or grants.