Introduction. High activity antiretroviral therapy (HAART) has allowed people infected with human immunodeficiency virus (HIV) to live longer. In the course of time, hepatocellular carcinoma (HCC) began to be found in these patients. Investigations have suggested that, as it has been described for other tumors, HIV infection raises the risk of developing HCC. However, convincing evidence is still required. Our aim was to quantify the incidence of HCC in hepatitis C cirrhotic patients with and without human immunodeficiency virus infection in the HAART era.
Material and methods. This prospective cohort study was conducted in hepatitis C cirrhotic patients with and without HIV co-infection, between june 1, 1999 and May 21, 2010. Ultrasound screening for HCC was performed every 6 to 12 months to all the patients until January 15, 2011. Incidence rate and cumulative incidence (Kaplan-Meier) were calculated.
Results. One hundred and forty eight patients (69 hepatitis C virus mono-infected and 79 HIV/hepatitis C virus co-infected) were followed for a median time of 43 months, with a total follow-up of 555 person-years (324 for co-infected and 231 for mono-infected patients). Twelve patients developed HCC (5 co-infected and 7 mono-infected). The incidence of HCC in co-infected patients and mono-infected patients was 1.54 (95% confidence interval = 0.5 to 3.6) and 3.03 (95% confidence interval = 1.22 to 6.23) cases per 100 person-year respectively (log-rank p = 0.3225).
Conclusion. In the HAART era, HIV co-infection is not associated with a higher incidence of HCC in hepatitis C cirrhotic patients.
Hepatocellular carcinoma (HCC), the most common primary cancer of the liver, results in significant morbidity and mortality. Since the introduction of highly active antiretroviral therapy (HAART) in 1996, the survival of human immunodeficiency virus (HIV) infected patients has improved considerably, therefore chronic hepatitis C virus (HCV) infection has become an increasingly important problem and the epidemiology of HCC has been altered.1,2
Within HIV positive populations in different countries, relative proportions of HCV co-infection vary widely according to the prevalence of different high-risk behaviours. In Argentina HCV/HIV co-infection was observed in 30% of the HIV infections.3
The effect of HIV infection in the progression of HCV morbidity is not fully understood, however evidence suggests that progression to end-stage liver disease occurs faster in co-infected patients. Thus, HIV/HCV co-infection increases the progression to cirrhosis, but whether it also increases the incidence of HCC remains to be elucidated.4–8
HIV-infected patients are at an increased risk of non-Hodgkin lymphoma, Kaposi sarcoma, and cervical cancer, all of which were considered to be acquired immunodeficiency syndrome (AIDS)-related events in the 1993 revised classification system for HIV infection. This increased risk seems to be partly related to oncogenic virus co-infections (i.e., Epstein-Barr virus, human herpes virus 8 and human papilloma virus) and to cellular immune-depression. Some investigators have suggested that, as it has been described for other tumours, HIV infection raises the risk of developing HCC, because they found a higher incidence of liver cancer among HIV-infected patients than in the general population; however more than 80% cases of HCC are preceded by cirrhosis, and the higher risk has been essentially attributed to hepatitis B virus (HBV)/HCV co-infections and alcohol abuse.7,9–16
Convincing evidence about the incidence of HCC in cirrhotic patients with and without HIV infection is still required. The aim of this study was to determine the incidence of HCC in HCV infected cirrhotic patients with and without HIV infection in the HAART era.
Material and MethodsStudy designThis prospective cohort study was conducted in HCV cirrhotic patients with and without HIV coinfection. All patients were attended at the liver unit of a public community hospital between June 1, 1999 and May 21, 2010. HCV cirrhotic patients were eligible for this study if they had at least 6 months of follow-up with 2 follow-up visits, and 2 evaluations by ultrasonography (US).
Chronic HCV infection was defined by detection of plasma anti-HCV antibody and HCV RNA. HIV infection was defined by detection of plasma anti-HIV antibody and confirmed by Western blot. Cirrhosis was diagnosed by histological examination or by combination of clinical, biochemical, esophagogastroduodenoscopy and US imaging data. All patients were tested for HIV and HBV at baseline visit and at regular intervals.
Follow-up began on the date of the first US and ended either when an event occurred, the patient died, or if no event occurred until January 15, 2011. All patients had a baseline visit and afterwards they were seen at least every 6 months and underwent US screening for HCC every 6 to 12 months. HIV positive and negative patients received similar care during follow-up. Both were mainly managed by hepatologists, and a group of infectologists provided care to HIV-infected patients.
Demographic (age and sex) and clinical variables (laboratory values pertinent to liver function, Child-Turcotte-Pugh scores, alcohol consumption, CD4 cell counts, plasma HIV-1 RNA, HCV RNA, and HCV treatment) at baseline and at the time of cancer diagnosis were evaluated to analyze the characteristics of the cancer patients and the general cohort. At each visit clinical events, laboratory data, consumption of alcohol, HAART regimen, anti-HCV treatment and liver transplant criteria were reviewed.
Every nodule smaller than 1 centimetre found at US was followed up at three months intervals with US, whereas lesions equal or larger than 1 cm were further investigated with either histological analysis, computed tomography scan, or dynamic contrast enhanced magnetic resonance imaging. HCC diagnosis recommendations have been changed since 1999, so the requirements for definite diagnosis of HCC were changed throughout follow-up.
Between 1999 and 2001 confirmation diagnosis was reached via histological analysis. Later on between 2001 and 2005, HCC diagnosis was made via histological analysis or via computed tomography scan or magnetic resonance imaging, in lesions larger than 2 cm showing early arterial phase contrast enhancement plus early venous phase contrast washout, with coincident findings in at least two imaging techniques, or using a single imaging technique in the presence of a plasma alpha-fetoprotein level equal to or greater than 400 ng/mL. Finally in nodules which were found between 2005 and 2011, HCC diagnosis was made via histological analysis or via computed tomography scan or magnetic resonance imaging, in focal lesions of 1-2 centimetres showing arterial hyper-vascularization with washout in the portal/venous phase, with coincident findings in two dynamic studies, whereas for nodules larger than 2 cm only one dynamic imaging technique with typical features of HCC or plasma alphafetoprotein greater than 200 ng/mL were required for diagnosis.17–19
Statistical analysisQualitative variables are described with frequencies and percentages. Quantitative variables are described with median and interquartile range (IQR). Comparisons between categorical variables were made by the chi-square of the Fisher test when appropriate. Comparisons between continuous variables were made using the Mann-Whitney U test. Time to event was calculated as days from baseline visit (First US) to endpoint diagnosis (HCC). The cumulative incidence for HCC was calculated by the Kaplan-Meier method, and was stratified by HIV coinfection status. The cumulative incidence between HCV and HIV/HCV co-infected patients was compared using the Log-Rank test. For all tests we considered as statistically significant a bilateral p value < 0.05. All analyses were carried out in Statistix 8.0.
ResultsIn the 11-year period from June 1, 1999 to May 21, 2010, one hundred and ninety one HCV cirrhotic patients were identified. To exclude non incident cases of HCC, 11 patients (One HIV/HCV co-infected and ten HCV mono-infected) with pre-existing HCC were not included. One hundred and eighty patients began follow-up, and then 32 were excluded because their follow-up was shorter than 6 months. Finally 148 patients were included in the analysis, 69 (46.6%) HCV mono-infected and 79 (53.4%) HIV/ HCV co-infected patients.
The baseline characteristics of HIV positive and negative patients are displayed in table 1. In comparison with HIV negative patients, HIV positive patients were younger [median age: 40 years old (Interquartile range-IQR = 38-43) vs. 49 years old (IQR = 43-54.5)], displayed higher serum HCV viral load levels at the time of the inclusion in the study [median HCV RNA level: 5.9 log10 IU/mL. (IQR = 5.6-5.9 log10 IU/mL) vs. 5.7 log10 IU/mL. (IQR = 5.15-5.9 log10 IU/mL)]. And the percentage of daily alcohol consumption (more than 60 grams/day) was significantly higher (62.3% vs. 31.9%, p < 0.05). Anti-HCV Therapy was performed in a lower percentage of co-infected patients than mono-infected (38% vs. 62%, p < 0.05). There was no other significant difference between both groups.
Baseline characteristics of HIV-HCV co-infected, and HCV mono-infected patients.
| HIV/HCV (n:79) | HCV (n:69) | P value | |
|---|---|---|---|
| Male sex [No. (%)] | 62 (78.5) | 45 (65.2) | 0.0721 |
| Median age [years (IQR)] | 40 (38-43) | 49 (43-54.5) | 0.0000* |
| Alcohol Consumption [No.(%)]† | 49 (62.3) | 22 (31.9) | 0.0002* |
| Genotype 1 [No. (%)] | 47 (59.5) | 46 (66.7) | 0.5216 |
| Median HCV RNA [Log10 IU/ml (IQR)]‡ | 5.9 (5.6-5.9) | 5.7 (5.2-5.9) | 0.0064* |
| HCV Treatment [No. (%)] | 30 (38) | 43 (62.3) | 0.0031* |
| Sustained virological response [No. (%)]§ | 4 (17.4) | 9 (25) | 0.4916 |
| Child-Turcotte-Pugh Score A [No. (%)] | 54 (68.4) | 60 (87) | 0.8435 |
HIV: human immunodeficiency virus. HCV: hepatitis C virus. IQR: interquartile range.
Almost all HIV/HCV co-infected patients (89.9%) were on HAART treatment at inclusion, and the median of CD4+ T cell was 332/µL (IQR 227 to 500). Most patients had relatively preserved liver function at inclusion (Child A: 68.4% of HIV-HCV co-infected and 87% of mono-infected patients).
Patients were followed up for a median time of 43 months (IQR: 23.3-58.8), HIV/HCV co-infected patients during 45 months (IQR: 25 to 65), and HCV mono-infected patients during 37 months (IQR: 20 to 53), p = 0.0907. Total follow up of 555 person-years (324 person/years for HIV/HCV co-infected patients and 231 for HCV mono-infected patients). Sixteen patients (Twelve HCV-HIV co-infected and 4 monoinfected patients) died during the study period, and 23 patients (fifteen co-infected and 8 mono-infected patients) were lost to follow-up.
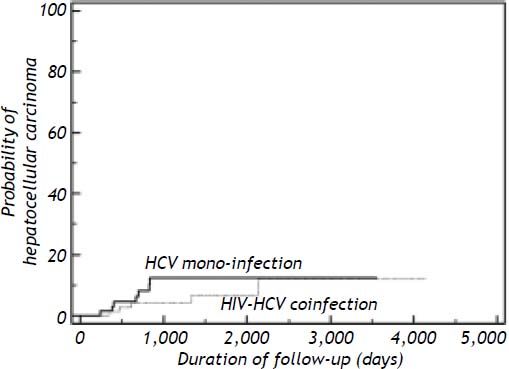
A new development of HCC was seen in 12 patients (5 co-infected and 7 mono-infected patients), resulting in a total frequency of 6.3% in HIV/HCV co-infected patients and 10.1% in HCV mono-infected patients. The density of incidence of HCC in HIV-HCV co-infected patients and HCV mono-infected patients was 1.54 cases per 100 person-year (95% confidence interval = 0.5 to 3.6) and 3.03 cases per 100 person-year (95% confidence interval = 1.22 to 6.23) (log-rank p = 0.3225) respectively. The incidence ratio of HCC for HCV mono-infected relative to HIV-HCV co-infected patients was 1.97 (Figure 1).
Kaplan-Meier cumulative risk of hepatocellular carcinoma among patients with HIV/HCV co-infection (n = 79) and patients with HCV mono-infection (n = 69). Difference was not statistically significant, log-rank test p = 0.3225. HCV: hepatitis C virus. HIV: human immunodeficiency virus.
The characteristics of patients who developed HCC are displayed in table 2. The median age at HCC diagnosis was 42 years old (IQR 34.5-50.5) in HIV/HCV co-infected patients and 64 years old (IQR 50-68) in HCV mono-infected patients ( p 0.0147). Alcohol consumption higher than 60 g/day was present in 4 HIV/HCV co-infected and 1 monoinfected patients (p = 0.0357). Median levels of plasma alpha-fetoprotein were not statistically different between HIV positive and negative patients (p = 0.4606). Almost all patients had relatively preserved liver function at inclusion (Child A: 80% of HIV-HCV co-infected and 85.7% of mono-infected patients). Genotype 1 was the most prevalent HCV genotype in the two groups, 20% of co-infected patients and 57.1% of mono-infected patients received anti-HCV treatment. Among HIV positive patients the median observation time to develop of HCC was 20.3 months (IQR14-58), and in mono-infected patients was 22.4 months (IQR 13-27). Four co-infected patients were on HAART treatment at inclusion, and the median CD4 cell count was 368 cells/µL (IQR 229 to 471). The only patient without HAART at inclusion had 349 CD4+ T cell/µL. One co-infected patient and 3 mono-infected patients died.
Characteristics of patients with hepatocellular carcinoma.
| HIV/HCV (n:5) | HCV (n:7) | P values | |
|---|---|---|---|
| Male sex [No. (%)] | 5 (100) | 5 (71.4) | 0.1904 |
| Median age [years (IQR)] | 42 (34.5-50.5) | 64 (50-68) | 0.0147* |
| Alcohol consumption [No.(%)]† | 4 (80) | 1 (14.3) | 0.0357* |
| Median observation time [months (IQR)] | 20.3 (14-58) | 22.4 (13-27) | 0.6261 |
| Genotype 1 [No. (%)] | 4 (80) | 4 (57.1) | 0.4076 |
| Median HCV RNA [Log10 lU/ml (IQR)]‡ | 5.8 (5.4-5.9) | 5.6 (4.9-5.9) | 0.6255 |
| HCV treatment [No. (%)] | 1 (20) | 4 (57.1) | 0.1982 |
| Sustained virological response [No. (%)]§ | 1 (100) | 1 (25) | 0.1709 |
| Median α-fetoprotein [lU/ml. (IQR)] | 5.5 (1.8-195) | 20 (6.5-64) | 0.4606 |
| Child-Turcotte-Pugh Score A [No. (%)] | 4 (80) | 6 (85.7) | 0.4279 |
HIV: human immunodeficiency virus. HCV: hepatitis C virus. IQR: Interquartile range.
All HCV mono-infected patients with HCC were evaluated for Liver Transplantation (LT). LT was performed in only one patient, 3 patients dropped out of list, 1 patient did not accept the therapy and there are no data of two patients. Three HIV-HCV co-infected patients were evaluated for LT. One patient underwent curative radiofrequency ablation and another one was refused LT by the LT unit because of his HIV condition. There are no data of one patient. Two patients were not evaluated for LT, one had active alcoholism, and another one died before the evaluation.
DiscussionEnd stage liver disease is currently a leading cause of death in HIV infected patients, and HCC represents a quarter of liver-related deaths. In France, from 1995 to 2005, the proportion of AIDS-related deaths decreased from 92% to 35% and liver related deaths increased (by 11-fold) in HIV-infected adults. Moreover, Weber et al., in the Data Collection on Adverse Events of Anti-HIV Drugs study (The D:A:D Study), found that liver-related death was the most frequent cause of non-AIDS-related death in HIV-infected persons.2,20 Furthermore, in the HAART era the epidemiology of AIDS/non-AIDS defining cancer is changing. For example, in the United States, Long et al. found that, between 1996 and 2005, AIDS defining cancer rates decreased from 12.5 to 3.5 cases/1,000 person-years (P = < 0.001 for trend) while non-AIDS defining cancer rates increased from 3.9 to 7.1 cases/1,000 person-years (P = 0.13 for trend).21
Among HCV infected patients, between 8-24% develop cirrhosis over two or three decades following the onset of the infection and 1%-4% of them will develop HCC annually. As HIV infected patients live longer and are exposed to the effects of mild and prolonged immune deficiency, hepatitis viruses can be expected to manifest their oncogenic potential increasingly.22–25
Until now, the impact of HIV infection on the incidence of liver cancer in HCV cirrhotic patients has not been clearly established. This prospective study performed in the HAART era, showed that HIV coinfection is not associated with a higher incidence of HCC in HCV cirrhotic patients.
Most studies in these populations are case-control studies or retrospective cohorts, with insufficient follow-up to compare the incidence of HCC, and are not adjusted by HCV-HBV infection or alcohol consumption. Furthermore, these studies are subject to several sources of error. For example in one cohort the authors concede that up to 50% of the apparent HCV group was never tested for HIV. Finally, cirrhosis appears to be the most important risk factor and precedes HCC in more than 80% of cases; for this reason, HCC studies which include non-cirrhotic patients, should consider both cirrhotic and non-cirrhotic groups in the incidence rate analysis. In the present study only cirrhotic patients were included.26
All studies proffer convincing evidence that HCC incidence in HIV patients is higher than in HIV negative patients nearly exclusively in association with HCV or HBV infection, and cite increased longevity as the principal cause of this phenomenon. To our knowledge up to now, there are no prospective studies that have found higher incidence of HCC in HIV and viral hepatitis co-infection compared to isolated HCV infection, as one might expect if HIV accelerates the disease progression of viral hepatitis towards HCC.
However, recent studies show that HIV-induced immunodeficiency is independently associated with a higher risk of HCC. One of the studies shows that patients with CD4 less than 500 cells/µ, are associated with a higher risk of HCC, whereas the cumulative duration with immunodeficiency is not.27 Moreover, Clifford, et al., in a case-control study nested in the Swiss HIV Cohort Study, found a specific association between HCC risk and Low CD4 cell count in the year preceding HCC diagnosis.25 It should be considered that both studies were retrospective and including patients with HBV and HCV infection. Nevertheless, though studies on HCV natural history have shown that HIV-related immune suppression worsens the risk of cirrhosis and liverrelated death, they have failed to identify a direct effect of HIV-related immunodeficiency on HCC risk.7,28
On the other hand, there are several studies showing no difference in the incidence of HCC between HIV positive and negative patients. Kramer, et al., in a retrospective cohort performed between 1991 and 2000, could not find a higher incidence of HCC in the co-infected group. Another retrospective cohort study conducted in the setting of decompensated cirrhosis, found a higher incidence of HCC in HCV mono-infected subjects than in co-infected.7,29
Moreover a large retrospective cohort study of McGuinnis, et al. compared the incidence of HCC between 14018 HIV positive and 28036 age, sex and location-matched HIV-negative controls. A higher age-matched incidence of HCC was clearly demonstrated in the HIV positive group (incidence rate ratio 1.68) but when adjusted for HCV infection and/or alcohol consumption the incidence rate ratios were similar, suggesting that HIV co-infection confers no additional risk of HCC compared to HCV infection alone. Equally important, in a prospective trial including 384 HCV-infected patients (141 HIV co-infected), only six patients developed HCC (One HIV/ HCV co-infected patient and five HCV mono-infected). The incidence rate of HCC was 390 cases per 100,000 per year. Finally in 2009, a meta-analysis performed by Deng, et al. identifies no difference between HIV/HCV co-infected and HCV mono-infected patients with regard to the development of liver cancer (Odds ratio 0.76).28,30–31
In the present study, HIV/HCV co-infected patients were younger than mono-infected at HCC diagnosis. Since HCV-related HCC typically develops in the setting of cirrhosis, the early age at HCC diagnosis in HIV-infected subjects could be attributed to their young age at the time of acquisition of HCV infection. In addition, a more rapid course of HCV-related liver disease in HIV co-infected subjects could also have played a role. This observation was made in three previous studies.32–34
Excessive alcohol consumption is a well-known factor in morbidity and mortality resulting for chronic liver diseases, and it has been associated with an increased risk of HCC.35 Daily alcohol consumption was evaluated in the present study and the proportion was higher in HIV/HCV co-infected patients. Furthermore 80% of HIV/HCV co-infected patients with HCC, drank more than 60 grams/day.
Some reports have emphasized a more aggressive course of HCC in HIV infected patients with respect to HCC seen in HIV-negative individuals.33,34 This study was performed in the setting of cirrhotic patients who were participating of a surveillance program, therefore HCC was found mostly in earlier stages, so there was no difference between HIV positive and negative patients.
Although HIV-positive status was previously an absolute contraindication for LT, the latter is now becoming more commonplace following several transplant series which have demonstrated similar survival outcomes in MELD-score matched HIV positive and negative recipients.36–39 In this cohort all HCV mono-infected patients and 3/5 co-infected patients with HCC were evaluated for LT.
This study has three major strengths. First the study was a prospective cohort. Second, the study cohort was matched with a similar cohort with equivalent severity of liver disease and third all patients had HCV infection confirmed by anti-HCV antibodies and HCV RNA. However some limitations should be pointed out. Persons included were restricted to patients being treated for HIV infection in the hospital. Data about when infection was acquired was not available in most cases. Finally HCC is a condition with a typical long latency, so we need a longer follow up to detect significant differences on incidence.
ConclusionIt can be asserted that in the HAART era HIV co infection is not associated with a higher incidence of HCC in HCV cirrhotic patients.
AcknowledgmentThe authors thank Marilin Tauterys and Maria Laura Pacheco for their technical assistance.
Abbreviations- •
AIDS: acquired immunodeficiency syndrome.
- •
HAART: high activity antiretroviral therapy.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
IQR: interquartile range.
- •
LT: liver transplantation.
- •
US: ultrasonography.