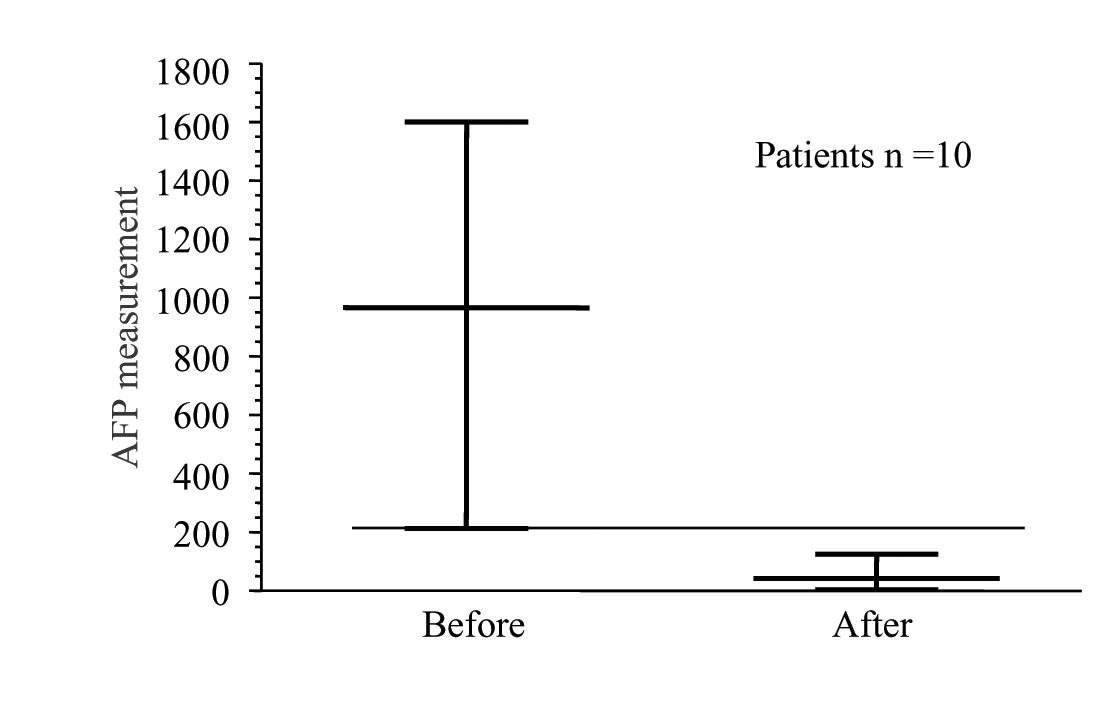
Background: Percutaneous ethanol injection has been successfully used for hepatocelular carcinomas (HCC) smaller than 5 cm in size. For larger lesions large volume ethanol injection has not been well explored. Aim: Evaluate the results of intraoperative Ultrasonographic-guided large volume ethanol injection for HCC larger than 4 cm in size. Patients and methods: Ten patients were candidates for this treatment between June 1999 and July 2003. A retrospective review of the clinical files was performed. Absolute ethanol, average of 100 mL (range 80-120 mL) was administered intraoperatively. Follow-up evaluation included alpha-fetoprotein (AFP) and ultrasound or computed tomography. Results: There were six women and four men, the median age was 62 years (range 56-80). The median lesion size was 8 cm (range 4-15 cm). Hepatitis C liver cirrhosis was the most common associated chronic liver disease (70%). A significant reduction of AFP levels after treatment was observed (Initial 966 ng/dL, post treatment levels: 42 ng/dL) US and CT scan showed tumor necrosis. Morbidity was 40%. No operative mortality was recorded. The one and four year survival rate was 60% and 20%. Conclusion: Intraoperative US-guided large volume ethanol injection is a safe palliative therapy for cirrhotic patients with HCC lesions greater than 5 cm in size. The impact on survival should be compared in a controlled double blind study.
Percutaneous ethanol injection (PEI) is considered the most effective form of direct ablation therapy for HCC.1-3 It has become one of the most widely used procedures for treating Hepatocellular Carcinoma (HCC) in patients with cirrhosis.3-5 It was first advocated by Sugiura and associates in 1983.6 The main mechanism whereby ethanol causes cell necrosis remains speculative but it is well known that it causes denaturation of proteins and dehydration of the cytoplasm by the diffusion of ethanol within the neoplastic cells. The result is coagulative necrosis, as well as necrosis of the vascular endothelium leading to platelet aggregation and small vessel thrombosis followed by tissue ischemia.7
PEI has traditionally been restricted to the treatment of single small lesions 5 cm or less in size.8-10 However, there has been a recent trend, toward treating larger tumors using higher doses of ethanol or a combination therapy using transcatheter arterial embolization associated to PEI.7,10 PEI is usually performed in the radiology suite but can also be performed in the operating room under general anesthesia. The benefits are obvious because a large dosage can be instilled, under anesthesia, spilling of ethanol into the peritoneum or extravasation of ethanol into the capsular surface is recognized, a Pringle maneuver can be performed, which may decrease the blood flow to a hypervascular HCC and bleeding can be easily diagnosed and controlled.8
The aim of this study was to evaluate the results of intraoperative US-guided large volume ethanol injection for HCC lesions greater than 5 cm in size.
Patients and methodsA retrospective review of the clinical files of patients diagnosed and treated at the Department of Gastroenterology of the Instituto Nacional de Cancerologia, Mexico City with histologically proven HCC treated from June 1999 to July 2003 was performed. Patients with unresectable lesions who underwent US-guided intraoperative large volume ethanol injection were included for analysis. The time onset of symptoms was estimated and the presence of any previous risk factors for development HCC were reviewed. Ordinary blood and liver function tests were obtained, measurement of alpha-protein (AFP) level, and serologic studies for viral hepatitis (hepatitis C virus antibody and hepatitis B surface antigen) were evaluated. Patients were diagnosed with either ultrasonography (US), computed tomography (CT) and in selected cases with magnetic resonance imaging (MRI). Definitive diagnosis of HCC was established by histopathologic criteria.
The selection criteria for this treatment included patients with preoperative detection with US or CT scan of a lesion larger than 5 cm, that were not considered for liver resection because of liver cirrhosis or bad clinical conditions or when the lesion was considered unresectable during operative evaluation. Tumor size ranged from 5-15 cm (median 8 cm). Patients with more than three lesions, portal vein thrombosis, ascites, extrahepatic metastases or with obstructive jaundice were not considered for ethanol injection. These patients had no other alternative treatment. All patients gave informed consent in order to participate in the study before they received the treatment.
Technique. The procedure was performed under general anesthesia, with endotracheal intubation and mechanical ventilation. Laparotomy, laparoscopy or thoracotomy were used to evaluate the lesions. The indication for each approach was made depending on the location of the lesion, presence or not of cirrhosis and of previous abdominal scars. During surgery, exploration consisted of careful inspection and bimanual palpation of the liver tissue with and without a Pringle maneuver as well as careful abdominal cavity contents examination. Intraoperative ultrasound (IOU) was performed and direct scanning of the liver was accomplished with a 7.5 mHz intraoperative probes. Normal saline solution was applied to enhance sound transmission. A systemic evaluation of the entire liver was performed by a radiologist after surgical mobilization of the liver in both sagital and transverse planes. The liver was scanned starting from the left lobe where the transducer was placed at the inferior, lateral and superior portions. Examination included demonstration of the hepatic veins, inferior vena cava and the right and left portal veins. The lesions known from preoperative studies were identified and their relationship to hepatic and portal veins established. Resection was evaluated by defining the relationship of the lesion to vascular structures such as portal and hepatic veins and inferior vena cava. When unsuspected palpable or non-palpable lesions were detected ultrasound-guided biopsies were performed.
To calculate the volume of ethanol required to ablate the lesion we used the formula for a sphere: V = 4/3 π (R + 0.5 cm)3, where V is the total volume of injected ethanol and R is the radius of the tumor (in centimeters).7 This formula is used only as a reference, because it was designed to be used in several sessions. We performed a single session so ultrasound was added to adequate the dosage of ethanol. Absolute ethanol (99.5%) is injected into the lesion throughout the entire diameter. An average of 100 mL (range 80-120 mL) was administered depending on lesion size. One single dose should not exceed 120 mL. In one patient with a 4 cm tumor a second percutaneous injection was needed and this patient received a total dose of 80 mL. The technique required an average time of 30 minutes. No intra-operative complications were detected related to this procedure.
Statistics. We described the frequency and the distribution of each studied variable, age, the time onset of symptoms, AFP, and dose of alcohol injection were reported as median and range. Survival curve was done by the Kaplan-Meier method. The level of AFP was compared with the confidence interval at 95%.
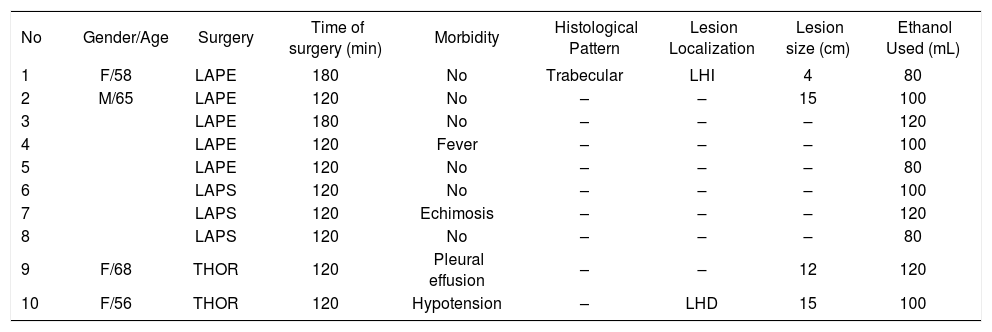
ResultsTen patients with HCC were included for analysis. Six were female and 4 male (sex ratio: 1.5:1). Median age at the time of diagnosis was 62 years old (range 56-80) (Table I). History of blood transfusion was recorded in three and alcoholism in one. Abdominal pain in the right upper quadrant was the main clinical symptom and was present in all patients, a palpable abdominal mass was present in 7 (70%), enlargement of the liver was diagnosed in 3 (30%). Seven patients had morphologic changes usually observed in chronic liver disease (70%). The median time of onset of symptoms was 4 months (range 1-12 months). Liver function tests were normal except for serum elevation of transaminases in 3 patients. Seven patients had hepatitis C liver cirrhosis, 2 had hepatitis C and B liver cirrhosis and one patient had no association with chronic liver disease. All patients were classified as Child-Pugh A. The Karnofsky score was greater than 80% in these patients. Histological examination showed a well-differentiated pattern HCC in 5 (50%), trabecular type in 2 (20%), tubular pattern in 2 (10%) and a combination of trabecular and tubular in 1 (10%).
Patients with large HCC treated by intraoperative US-guided large volume ethanol injection.
| No | Gender/Age | Surgery | Time of surgery (min) | Morbidity | Histological Pattern | Lesion Localization | Lesion size (cm) | Ethanol Used (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | F/58 | LAPE | 180 | No | Trabecular | LHI | 4 | 80 |
| 2 | M/65 | LAPE | 120 | No | – | – | 15 | 100 |
| 3 | LAPE | 180 | No | – | – | – | 120 | |
| 4 | LAPE | 120 | Fever | – | – | – | 100 | |
| 5 | LAPE | 120 | No | – | – | – | 80 | |
| 6 | LAPS | 120 | No | – | – | – | 100 | |
| 7 | LAPS | 120 | Echimosis | – | – | – | 120 | |
| 8 | LAPS | 120 | No | – | – | – | 80 | |
| 9 | F/68 | THOR | 120 | Pleural effusion | – | – | 12 | 120 |
| 10 | F/56 | THOR | 120 | Hypotension | – | LHD | 15 | 100 |
LAP: Laparotomy, LAPS: Laparoscopy, THOR: Thoracotomy
Laparotomy was performed in five patients, because a previous surgical operation was performed that included cholecystectomy in three, and liver biopsy in two. Laparoscopy was used in three patients that had anterior lesions in patients without a previous abdominal scar. Thoracotomy was performed in two cirrhotic patients because the lesions were located in segments VII and VIII of the liver, these patients were initially thought to be eligible for resection. The lesion was located in the right lobe of the liver in six patients, in the left in three, and one patient had lesions in both lobes. The size of HCC was measured by US, CT scan or during surgery, the median size of the lesions was 8 cm (range 5-15 cm).
There were no intraoperative complications. Postoperative side-effects included symptoms of ethanol intoxication in four patients that resolved spontaneously within the immediate postoperative hours. Morbidity was 40%, nevertheless all cases had minor complications. Pleural effusion was diagnosed in one patient that was operated through a thoracotomy and was resolved with a thoracic tube. One patient presented transient low grade fever that was attributed to tumor necrosis, one patient presented mild hypotension after treatment that responded to intravenous fluids. One patient showed 48 hours after treatment lumbar pain and ecchymosis on the posterior abdominal wall was diagnosed. No operative mortality was recorded in this series. Median hospital stay was two days but ranged from one to five days. The cumulative survival rate after treatment was 60 and 20% at one and four years (Figure 1).
Follow-up was performed in all patients until the end of the study or until death. Physical examination was performed and the presence of symptoms recorded. Measurement of serum AFP levels were also recorded. The median initial AFP level was 966 ng/mL and three months after treatment it was 42 ng/mL (Figure 2). Abdominal CT scan performed three and six months after treatment showed tumor necrosis in all patients, reduction in size in one case but the rest showed no modification in the size of the lesion. No recurrence of the primary lesion was diagnosed but in three patients new lesions were diagnosed in the liver or the lungs.
DiscussionSurgical resection is generally accepted as the treatment of choice for HCC. Recent advances of surgical technique, anesthesia and postoperative care have resulted in a reduction of operative morbidity and mortality even in cirrhotic patients.8 Unfortunately, it is estimated that only about 20% of HCC can be resected at the initial presentation.11 Prognosis without treatment is poor and other non-resective forms of treatment have been described.12 Mexico is not considered a high endemic area for HCC but the incidence of this neoplasm in the last 25 years has been rising steadily.13 At our Institution most of patients are diagnosed in late clinical stages or with advanced liver cirrhosis and less than 15% of the cases are candidates for liver resection.14Other forms of treatment that include systemic or arterial chemotherapy, oral tamoxifen-thalidomide, radiofrequency ablation and chemoembolization have been tried at our hospital with variable results but few patients are long-term survivors with good life quality.
Percutaneous ethanol injection is a safe treatment and has been used in this and other hospitals for small lesions usually less than 3 cm in size with excellent results.1,3,4,8Experience though a percutaneous approach for larger lesions has been scarce and it can be done ultrasound guided with similar benefits.15However, it is generally considered as time-consuming because it requires a great number of sessions and morbidity is related to the dosage of ethanol and the number of sessions. Pain has been the principal side-effect.16 US-guided intraoperative injection of large volume of ethanol offers the “theoretical” advantage of total tumoral ablation in a single session. Ultrasound is highly recommended because the amount of ethanol needed to produce tumor ablation can be determined more precisely and when a complete morphologic change of the lesion is observed the treatment is finished. This initial experience shows that this procedure can be performed with minimal and minor morbidity and without mortality. Pain was not observed because general anesthesia was used as well as postoperative medications. Tumoral necrosis was confirmed due to a consistent reduction of serum AFP levels observed as well as changes in the CT scan. Recurrence of the originally treated tumor was not found, but three patients developed satellite lesions in the liver or lung metastases at eight to 12 months after treatment. Other authors have reported a similar observation.17 Much has to be investigated in terms of prognostic factors for long term survival. We believe that solitary encapsulated and smaller than 10 cm lesions have a better response than diffuse non-encapsulated lesions.
ConclusionIntraoperative US-guided large volume ethanol injection is a safe palliative therapy for patients with HCC lesions greater than 5 cm in size. We believe that this procedure could increase survival of cirrhotic patients with unresectable HCC. However, prospective and randomized studies are needed to demonstrate this observation.