Introduction. Quantitative hepatitis B surface antigen (qHBsAg) combined with HBV DNA may be useful for predicting chronic hepatitis B (CHB) activity and nucleoside analogue (NA) response.
Material and methods. In this retrospective cohort study we evaluated qHBsAg levels according to CHB disease phase and among patients on treatment. Random effect logistic regression analysis was used to analyze qHBsAg change with time in the NA-treated cohort.
Results. 545 CHB carriers [56% M, median age 48 y (IQR 38-59), 73% Asian] had qHBsAg testing. In the untreated group (44%), 8% were classified as immune tolerant, 10% immune clearance, 40% inactive, and 43% had HBeAg- CHB and the median HBsAg levels were 4.6 (IQR 3.4-4.9), 4.0 (IQR 3.4-4.5), 2.9 (IQR 1.4-3.8), and 3.2 log IU/mL (IQR 2.6-4.0), respectively; p < 0.001. In the NA-treated group (28% entecavir, 68% tenofovir, 4% lamivudine), no significant change in qHBsAg levels occured with time. However, 19% of patients on long-term NA had sustained qHBsAg < 2 log10 IU/mL.
Conclusion. qHBsAg titers were associated with CHB phase and remained stable in those on long-term NA. A significant number of treated patients had low-level qHBsAg, of which some may be eligible for treatment discontinuation without risk of flare.
Approximately 240 million people are infected with the hepatitis B virus (HBV) and are at risk of cirrhosis, decompensated liver disease and hepatocellular carcinoma.1,2 Current laboratory tests for determining disease classification and response to therapy include serum hepatitis B DNA (HBV DNA) and liver transaminases. In recent years, quantitative hepatitis B surface antigen (qHBsAg) has emerged as a potentially important biomarker as levels have been shown to correlate with intrahepatic HBV DNA and cccDNA and thus may more accurately reflect viral transcription and in turn disease activity.3–5 Furthermore, recent studies have challenged the concept of immune tolerance in chronic hepatitis B (CHB) carriers, showing that many exhibit significant HBV-specific immune activity, highlighting the need for more accurate biomarkers to assess CHB natural history.6,7
In several large Asian cohorts, serum qHBsAg has been validated as a tool for monitoring hepatitis B disease activity and determining hepatitis B disease phase.3,4,8–10 In hepatitis B e antigen (HBeAg) positive patients, qHBsAg levels are higher compared to those with HBeAg negative CHB.4,5,8 Furthermore, the combination of low qHBsAg levels and low HBV DNA may identify CHB patients most likely to undergo spontaneous HBsAg seroconversion.9,11–14 CHB treatment guidelines recommend potent NA i.e., tenofovir disoproxil fumarate (TDF) or entecavir (ETV)15,16 as first-line therapy, but guidelines differ on duration of therapy, especially in HBeAg negative CHB car” riers due to risk of off-treatment virologic relapse.15–17 HBV DNA levels alone are not sufficient to estimate this risk as many patients achieve virologic suppression (i.e. undetectable HBV DNA levels) and yet remain at risk of virologic relapse if treatment is discontinued. qHBsAg may help in identifying those patients on NA therapy with greater chance of sustained virologic and clinical response after treatment discontinuation. In treatment naïve patients, lower baseline qHBsAg levels as well as more rapid decline in qHBsAg may predict those patients with greater likelihood of virologic response.18–20 Among patients on long-term therapy a qHBsAg cut off of ≤ 2log10 IU/mL has been proposed as an end point for treatment discontinuation, with low risk of biochemical and viral relapse.21–23
Previous studies utilizing qHBsAg testing have been performed primarily in either Asian populations with HBV genotype B and C or European cohorts with HBV genotype D, and most evaluated patients receiving firstgeneration NAs [i.e., lamivudine (LAM) and telbivudine]. We conducted the first and largest retrospective cohort study to date in North America of CHB carriers to investigate the potential role of qHBsAg in assessing disease phase and in the evaluation of response to potent second generation NA therapy.
Material and MethodsStudy populationAdult patients ( ≥18 years) with CHB (HBsAg positive for a least 6 months) who had qHBsAg testing between January 1, 2010 and November 1, 2015 were eligible for inclusion in this retrospective study cohort study. We included patients who had never received CHB treatment or who were currently receiving NA-based therapy, (i.e., LAM, telbivudine, adefovir, ETV, or TDF). Patients were excluded if they had been previously treated with pegylated interferon, if liver enzymes or HBV DNA was not available within three months of qHBsAg testing, if they discontinued NA due to non-compliance or were lost to follow-up. In our clinic, qHBsAg is a relatively new test and very few patients had baseline qHBsAg measurements prior to NA initiation, hence in those few cases their pretreatment qHBsAg values were excluded from statistical analysis. Further details regarding our CHB patient population, follow-up and electronic database has been previously published.24 CHB disease phases, treatment indication, and duration of NA therapy were determined based on serial testing and clinical follow-up according to expert guidelines.15 However, at our institution it is current standard clinical practice to continue NA therapy indefinitely for patients with both HBeAg positive and HBeAg negative CHB. This study received institutional review board approval from the University of Calgary Conjoint Health Research Review Board in accordance with ethical guidelines of the 1975 Declaration of Helsinki.
Patient demographics and laboratory values were obtained by review of electronic database. Demographic variables included age, sex and self-reported ethnicity or place of birth. Laboratory parameters included aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in addition to HBV specific testing. HBeAg and hepatitis B e antibody (HBeAb) testing was performed using commercial chemiluminescent microparticle immunoassays (CMIA; Abbott ARCHITECT®, Chicago, IL, USA). Serum HBV DNA levels were determined using kinetic PCR, either COBAS® AmpliPrep/COBAS® TaqMan HBV (Roche, Branchburg, NJ, USA) or Abbott RealTime HBV (Abbott, Chicago, IL, USA). Fibrosis was based on liver stiffness measurement using transient elastography (TE; FibroScan®; KNS Canada Inc., Toronto, ON, Canada) with the following parameters: F0-1 < 7.8, F2 7.9 - 8.7, F3 8.8 - 11.6, F4/cirrhosis ≥ 11.7.25
HBV genotyping and quantitative HBsAg testingHBV genotyping was available in 28% of patients and was determined via INNO-LiPA HBV genotyping assay (Innogenetics N.V., Ghent, Belgium). Prior to January 2014, qHBsAg titres were determined by an in-house assay performed at the National Microbiology Laboratory (VITROS Eci HBsAg, Ortho Clinical Diagnostics, Markham, ON, Canada). After January 2014 qHBsAg testing was performed in Alberta using the Abbott ARCHITECT® HBsAg assay (Chicago, IL, USA). The Abbott and VITROS assays have been found to be highly comparable with a coefficient of determination (R-squared) of 0.97 (Osiowy, C. personal communication, unpublished data).26
Statistical analysisBaseline characteristics were described using the median and interquartile range (IQR) for continuous variables and percentages for categorical variables. Patients with serial measurements of qHBsAg were compared to those with a single qHBsAg measurement using a t test for continuous variables and a Fishers Exact Test (FET) for categorical variables. qHBsAg measurements were compared by disease phase and type of treatment using one-way analysis of variance. For untreated patients who were HBeAg negative the correlation between log10 qHB-sAg and fibrosis, liver enzymes, HBV DNA (all log10 transformed) was examined and 95% confidence intervals calculated. For patients in whom serial measurements were available, a random effects (intercepts and slope) regression model was used to model changes in log10 qHBsAg over time. Patients on treatment who had at least one low value (log10 qHBsAg ≤ 2.0) were compared to those who did not have any low values. Statistical analysis was done using STATA (StataCorp, College Station, TX, USA). Two-sided P values ≤ 0.05 were considered statistically significant.
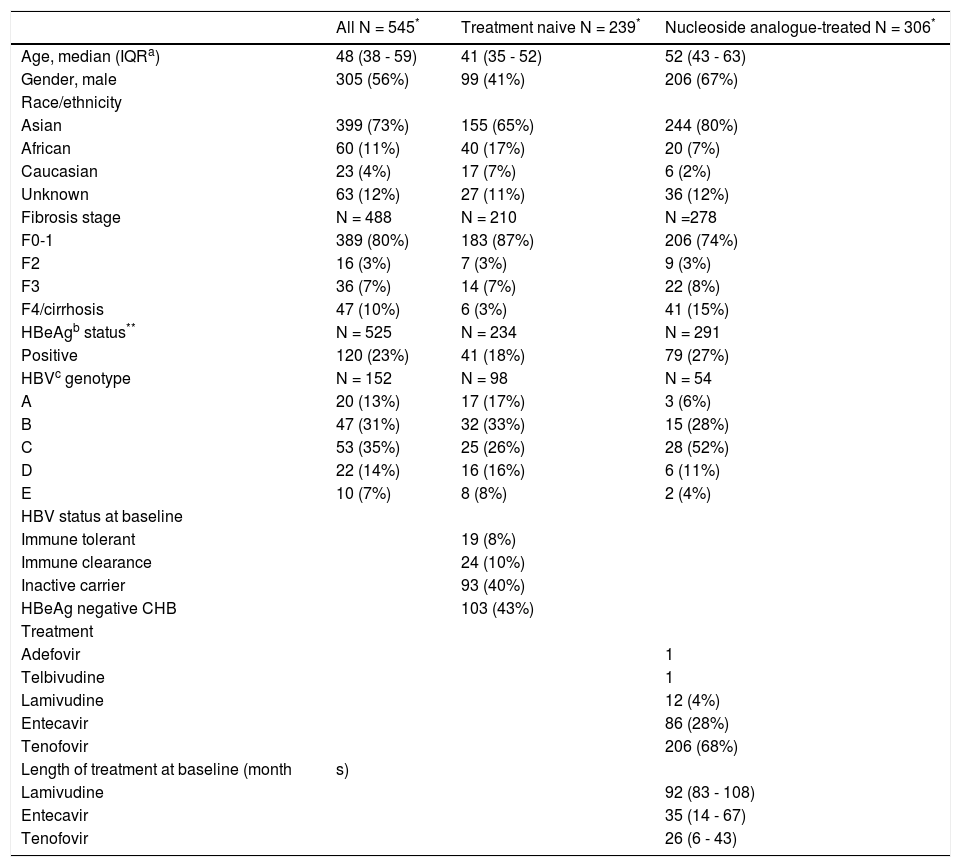
ResultsSummary of baseline characteristicsThe baseline characteristics for 545 patients enrolled in the study are presented in table 1. The median age was 48 (IQR 38-59), 56% male, 73% were Asian, 23% were HBeAg positive, 17% had advanced fibrosis or cirrhosis (F3-4). Of 152 patients with available HBV genotyping the distribution was: 13% genotype A, 31% B, 35% C, 14% D and 7% genotype E. Thirteen patients (2%) were HIV/HBV coinfected. Overall, 44% of patients were treatment naïve, of which 8% were immune tolerant, 10% were immune clearance, 40% were inactive carriers and 43% had HBeAg negative CHB, based on serial lab tests. Of NA treated patients (n = 306, 28% received ETV, 68% TDF, and 4% LAM) (Table 1). The median length of therapy at the time of qHBsAg measurement was 92 months (IQR 83-108) for those receiving LAM, 35 months (IQR 14-67) for those on ETV and 26 months (IQR 6-43) for TDF treated patients. Serial qHBsAg values was available in 41% of patients. Compared to CHB patients with a single qHBsAg measurement, those with serial measurements were older (p = 0.014) and were more likely to be on treatment (p < 0.001), but otherwise were not significantly different at baseline.
Characteristics of study participants (n = 545).
| All N = 545* | Treatment naive N = 239* | Nucleoside analogue-treated N = 306* | |
|---|---|---|---|
| Age, median (IQRa) | 48 (38 - 59) | 41 (35 - 52) | 52 (43 - 63) |
| Gender, male | 305 (56%) | 99 (41%) | 206 (67%) |
| Race/ethnicity | |||
| Asian | 399 (73%) | 155 (65%) | 244 (80%) |
| African | 60 (11%) | 40 (17%) | 20 (7%) |
| Caucasian | 23 (4%) | 17 (7%) | 6 (2%) |
| Unknown | 63 (12%) | 27 (11%) | 36 (12%) |
| Fibrosis stage | N = 488 | N = 210 | N =278 |
| F0-1 | 389 (80%) | 183 (87%) | 206 (74%) |
| F2 | 16 (3%) | 7 (3%) | 9 (3%) |
| F3 | 36 (7%) | 14 (7%) | 22 (8%) |
| F4/cirrhosis | 47 (10%) | 6 (3%) | 41 (15%) |
| HBeAgb status** | N = 525 | N = 234 | N = 291 |
| Positive | 120 (23%) | 41 (18%) | 79 (27%) |
| HBVc genotype | N = 152 | N = 98 | N = 54 |
| A | 20 (13%) | 17 (17%) | 3 (6%) |
| B | 47 (31%) | 32 (33%) | 15 (28%) |
| C | 53 (35%) | 25 (26%) | 28 (52%) |
| D | 22 (14%) | 16 (16%) | 6 (11%) |
| E | 10 (7%) | 8 (8%) | 2 (4%) |
| HBV status at baseline | |||
| Immune tolerant | 19 (8%) | ||
| Immune clearance | 24 (10%) | ||
| Inactive carrier | 93 (40%) | ||
| HBeAg negative CHB | 103 (43%) | ||
| Treatment | |||
| Adefovir | 1 | ||
| Telbivudine | 1 | ||
| Lamivudine | 12 (4%) | ||
| Entecavir | 86 (28%) | ||
| Tenofovir | 206 (68%) | ||
| Length of treatment at baseline (month | s) | ||
| Lamivudine | 92 (83 - 108) | ||
| Entecavir | 35 (14 - 67) | ||
| Tenofovir | 26 (6 - 43) |
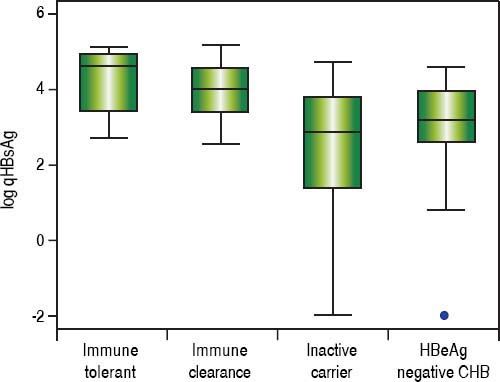
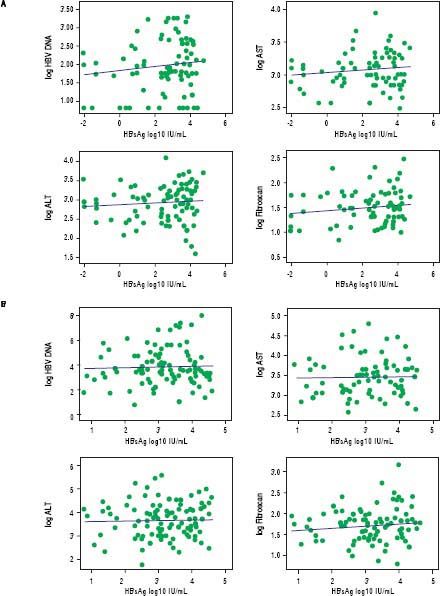
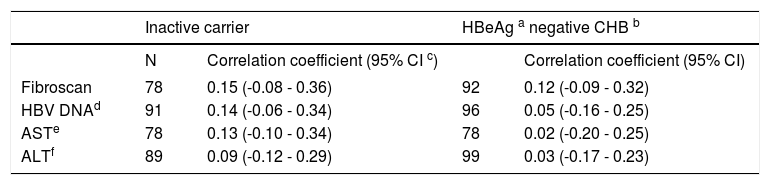
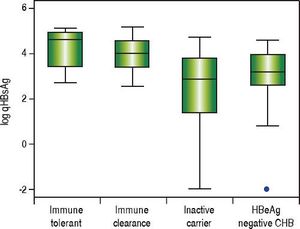
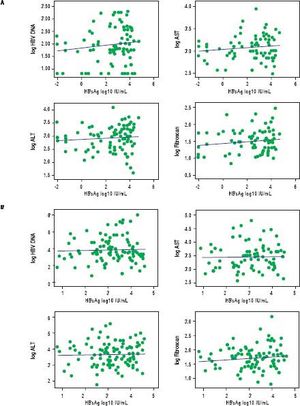
In treatment naïve CHB carriers, the median qHBsAg level was 4.6 log IU/mL (IQR 3.4-4.9) in immune tolerant, 4.0 log IU/mL (IQR 3.4-4.5) in immune clearance, 2.9 log IU/mL (IQR 1.4-3.8) in inactive carriers, and 3.2 log IU/mL (IQR 2.7-3.9) in HBeAg negative CHB (p < 0.001, for each comparison) (Figure 1). There was no evidence of a correlation between qHBsAg and fibrosis (determined by liver stiffness measurement using transient elastography), HBV DNA, ALT or AST in either inactive CHB carriers or those with HBeAg negative CHB (Table 2, Figure 2).
Correlation between quantitative HBsAg and transient elastography, HBV DNA, and liver enzymes in hepatitis B e antigen negative, treatment naïve patients with chronic hepatitis B.
| Inactive carrier | HBeAg a negative CHB b | |||
|---|---|---|---|---|
| N | Correlation coefficient (95% CI c) | Correlation coefficient (95% CI) | ||
| Fibroscan | 78 | 0.15 (-0.08 - 0.36) | 92 | 0.12 (-0.09 - 0.32) |
| HBV DNAd | 91 | 0.14 (-0.06 - 0.34) | 96 | 0.05 (-0.16 - 0.25) |
| ASTe | 78 | 0.13 (-0.10 - 0.34) | 78 | 0.02 (-0.20 - 0.25) |
| ALTf | 89 | 0.09 (-0.12 - 0.29) | 99 | 0.03 (-0.17 - 0.23) |
No significant correlation was found between quantitative HBsAg levels and liver stiffness measurement by FibroScan®, HBV DNA, and liver enzymes in inactive CHB carriers (A) or in hepatitis B eAg negative CHB (B). Outlying values of qHBsAg log10 ≤ 0 were omitted from correlation analysis. HBV DNA: hepatitis B DNA. AST: aspartate aminotransferase. ALT: alanine aminotransferase.
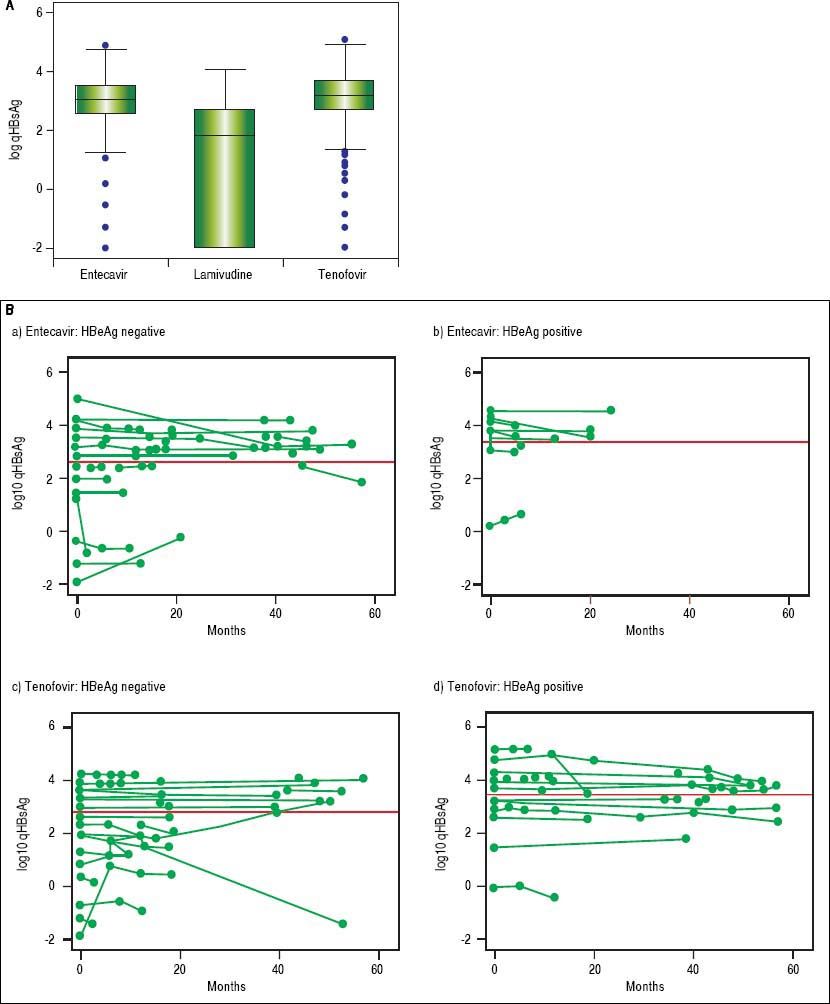
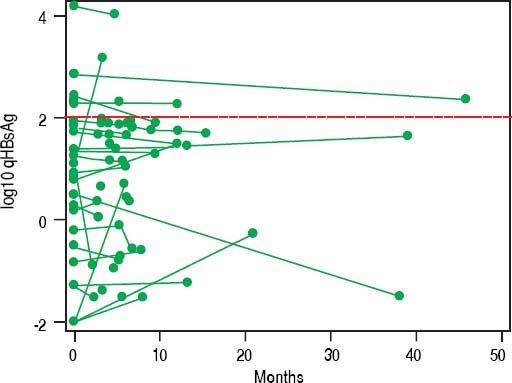
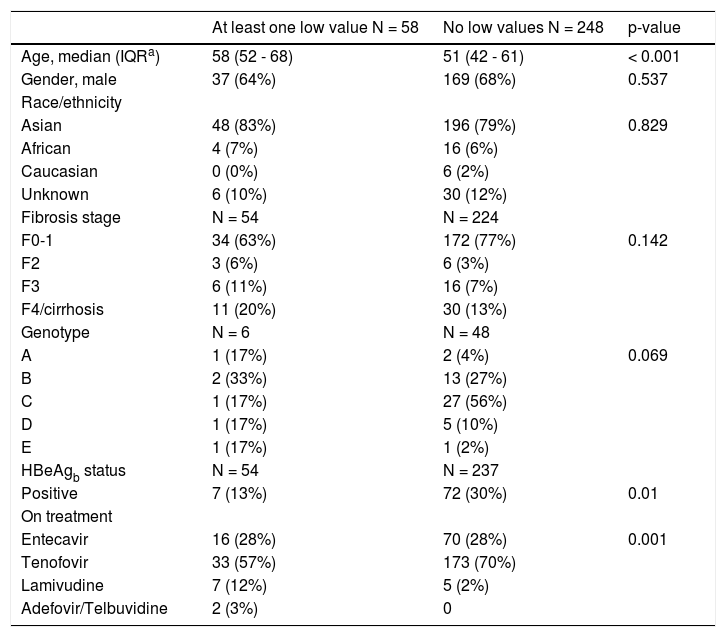
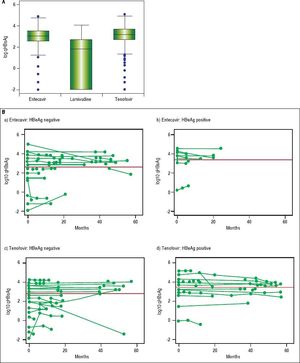
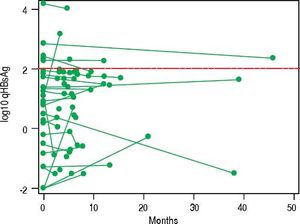
In NA treated patients the median qHBsAg levels were 1.8 log (IQR -2.0-2.7), 3.1 log (IQR 2.5-3.5) and 3.2 log IU/mL (IQR 2.7-3.7) for LAM, ETV and TDF treated groups respectively (Figure 3A). There was a statistically significant difference between the NA treated groups (p < 0.001, for each comparison). A mixed effects linear regression model was used to examine the effect of treatment on qHBsAg over time accounting for HBeAg status (Figure 3B). Treatment and HBeAg status were included as fixed effects and random intercepts and slopes (for time) were determined. In HBeAg positive patients treated with TDF, we observed a significantly higher mean intercept of 3.38 (95% CI 3.00-3.76) compared to those treated with ETV, but after accounting for random slopes there was no evidence of a significant decrease of log10 qHBsAg over time (p = 0.177). In patients with serial measurements, the trajectory of log10 qHBsAg values over time showed that those with low level qHBsAg titers remained persistently < 2 log10 for the duration of observation (Figure 4). CHB carriers on long-term treatment with qHBsAg level < 2 log10 were older (p < 0.001) and more likely to be HBeAg negative (p = 0.01) (Table 3).
Comparison of baseline characteristics of nucleoside analogue treated patients with one quantitative HBsAg < 2log10 to those with no low values.
| At least one low value N = 58 | No low values N = 248 | p-value | |
|---|---|---|---|
| Age, median (IQRa) | 58 (52 - 68) | 51 (42 - 61) | < 0.001 |
| Gender, male | 37 (64%) | 169 (68%) | 0.537 |
| Race/ethnicity | |||
| Asian | 48 (83%) | 196 (79%) | 0.829 |
| African | 4 (7%) | 16 (6%) | |
| Caucasian | 0 (0%) | 6 (2%) | |
| Unknown | 6 (10%) | 30 (12%) | |
| Fibrosis stage | N = 54 | N = 224 | |
| F0-1 | 34 (63%) | 172 (77%) | 0.142 |
| F2 | 3 (6%) | 6 (3%) | |
| F3 | 6 (11%) | 16 (7%) | |
| F4/cirrhosis | 11 (20%) | 30 (13%) | |
| Genotype | N = 6 | N = 48 | |
| A | 1 (17%) | 2 (4%) | 0.069 |
| B | 2 (33%) | 13 (27%) | |
| C | 1 (17%) | 27 (56%) | |
| D | 1 (17%) | 5 (10%) | |
| E | 1 (17%) | 1 (2%) | |
| HBeAgb status | N = 54 | N = 237 | |
| Positive | 7 (13%) | 72 (30%) | 0.01 |
| On treatment | |||
| Entecavir | 16 (28%) | 70 (28%) | 0.001 |
| Tenofovir | 33 (57%) | 173 (70%) | |
| Lamivudine | 7 (12%) | 5 (2%) | |
| Adefovir/Telbuvidine | 2 (3%) | 0 |
In this large retrospective cohort study, we present follow-up data and qHBsAg monitoring in over 500 treatment naïve and CHB carriers on long-term NA therapy in North America. In treatment naïve patients we found qHBsAg levels differ significantly by disease phase. In patients on long term NA we show that qHBsAg levels remained relatively static, when controlling for HBeAg status. In patients treated with first-generation nucleoside analog (i.e., LAM) there was significantly lower qHBsAg levels compared to ETV or TDF treated patients, likely due to the longer median duration of LAM therapy (92 vs. 35 and 26 months). Further, a significant proportion of individuals on long term NA had undetectable HBV DNA and sustained low levels of qHBsAg and may therefore be eligible for treatment discontinuation without risk of virologic relapse.
Our study is in agreement with others showing that in treatment naïve CHB patients, qHBsAg levels correspond to disease phase.4,5,8,27 but we did not find a consistent correlation between qHBsAg and HBV DNA. Correlation between qHBsAg and HBV DNA appears to be present only in early phases of disease, in acute HBV infection and in HBeAg positive disease.4,5,8 HBsAg is produced by three distinct pathways:
- •
It forms the envelope protein for mature HBV virions.
- •
It is secreted as sub-viral particles, and
- •
It is produced from HBV DNA integration into the host genome.27
qHBsAg assays do not differentiate between these different types of HBsAg. Differential production of mature HBV virions to other forms of HBsAg in different phases of disease may explain the apparent disconnect between HBsAg levels and HBV DNA. Thus, in HBeAg negative disease with low-level HBV DNA, higher qHBsAg levels may be due to more transcriptionally active HBV cccD-NA, and production of subviral non-infectious HBsAg particles rather than viral replication. Thus, the clinical value of qHBsAg measurement in treatment-naïve patients appears to be in predicting those most likely to remain inactive carriers with low risk of subsequent virologic flares and/or undergo HBsAg seroconversion. The parameters of qHBsAg ≤ 1,000 IU/mL in addition to HBV DNA ≤ 2,000 copies/mL have been shown to have reasonable sensitivity and specificity for prediction of HBsAg seroconversion.9,10 Alternatively, a very low qHBsAg level (≤ 100 to ≤ 200 IU/mL) also appears to be predictive of HBsAg sereconversion.9,12–14 Additional studies validating these parameters in larger multi-ethnic cohorts are an area for future research.
We also found that in both HBeAg positive and HBeAg negative patients on long-term, suppressive NA therapy qHBsAg levels remained relatively static over time. These findings are consistent with previous studies that have also shown the majority of qHBsAg decline to occur within the first year of starting NA therapy, and then decline slowly thereafter.28–32 There are few studies comparing the antiviral efficacy, especially utilizing qHBsAg titers, in TDF vs. ETV treated patients. In our study we found equivalent qHBsAg kinetics, but lower levels in those on LAM, likely due to the longer duration of LAM therapy, a first generation NA that was available before TDF or ETV in our clinic. Additionally, a large proportion of our treated patients had very low qHBsAg levels and may therefore be eligible for treatment discontinuation. Current guidelines recommend indefinite NA therapy for HBeAg negative patients and prolonged consolidation therapy (at least one year after HBeAg seroconversion) for HBeAg positive patients.15,33 Thus, qHBsAg may be a novel marker, in combination with HBV DNA tests, to identify patients eligible for earlier discontinuation of NA therapy, with low risk of subsequent relapse. Currently available qHBsAg assays are also less expensive than HBV DNA testing and are available in high-throughput capacity, but is not yet approved nor widely utilized in most health jurisdictions in North America.34
Previous studies of patients on long term NA have shown a number of variables such as age and specific HBV genotypes (i.e., Genotype C) to be associated with risk of post-treatment relapse. Lower baseline qHBsAg, lower end-of-treatment qHBsAg and greater decline in qHBsAg have also all been shown to be associated with decreased risk of relapse and increased chance of HBsAg seroconversion.18–21,28,35–39 The optimal timing for qHBsAg testing and exact cut-off remain to be identified in large prospective studies. A recent study from Wang, et al. (2016) examined 117 Taiwanese patients with HBV genotype B or C who were treated with ETV.37 They found that end of therapy qHBsAg was associated with sustained virologic response (which they defined as undetectable HBV DNA at 12 months). Among those patients with qHBsAg ≤ 100 IU/mL the rate of clinical relapse was 9.1% and the rate of sustained virologic response was 45.5%. Similar findings have been shown in HIV/HBV coinfected African patients with genotype E who were treated with TDF/emtricitabine or LAM.35 For HBV monoinfected patients treated with TDF these specific parameters have not yet been rigorously evaluated. Marcellin, et al. (2014) examined predictors of HBsAg loss in those patients treated with TDF as part of a large randomized controlled trial.28 They found that a more robust decline in qHBsAg ( > 1 log decline at 2 years) was associated with subsequent HBsAg loss (negative predictive value ~95%). A conservative strategy based on the currently available evidence would be to identify HBeAg negative patients on therapy with qHBsAg level ≤ 2 log10 IU/mL, combined with undetectable HBV DNA, to consider for treatment discontinuation with close clinical follow-up to monitor for virologic or clinical relapse. In our cohort, 47 (15%) patients met these criteria. Importantly, in those with serial testing, once the qHBsAg level fell below 2 log10 IU/mL it remained below that cut-off for the duration of observation. Future prospective follow-up after discontinuation of NA is an area of future investigation in our clinic.
Our study is limited in that we were unable to evaluate predictors of HBsAg seroconversion in either treatment naïve or NA treated patients, as this was a very rare occurrence in our population (only one patient with inactive CHB had HBsAg loss during the follow-up period). Furthermore, given our retrospective design we are unable to determine causality for qHBsAg kinetics, and given the variability in timing between qHBsAg testing we rely on a random effect regression analysis to standardize values over time.
In conclusion, we report the largest study to date in North America evaluating the utility of qHBsAg testing during long-term follow-up of CHB. We found in a multiethnic cohort of either untreated or treated patients on potent antivirals that qHBsAg testing is a useful complementary biomarker along with HBV DNA, for assessing CHB disease phase, eligibility for therapy, as well as following virological response to potent NA treatment. New CHB treatment guidelines, as well as health policy decisions makers should consider the addition of qHBsAg to complement existing clinical tests for HBV management.
Abbreviations- •
AASLD: American Association for the Study of Liver Diseases.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
cccDNA: covalently closed circular DNA.
- •
CHB: chronic hepatitis B.
- •
CI: confidence interval.
- •
CMIA: chemiluminescent microparticle immunoassays.
- •
DNA: deoxyribonucleic acid.
- •
ETV: entecavir.
- •
FET: Fisher’s Exact Test.
- •
HBeAb: hepatitis B e antibody.
- •
HBeAg: hepatitis B e antigen.
- •
HBsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B virus.
- •
IQR: interquartile range.
- •
LAM: lamivudine.
- •
NA: nucleoside analogue(s).
- •
NML: National Microbiology Laboratory.
- •
PCR: polymerase chain reaction.
- •
peg-IFN: pegylated interferon.
- •
qHBsAg: quantitative hepatitis B surface antigen.
- •
SD: standard deviation.
- •
TDF: tenofovir disoproxil fumarate.
- •
TE: transient elastography
Carla S Coffin is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award (Biomedical/Clinical) and a CIHR operating grant #354777.
AcknowledgementsWe would like to acknowledge the patients who participated in this project, Jacqueline Pinto, Heidi Israelson and the rest of the clinic and research staff of the Calgary Liver Unit.