Introduction and aim. Approximately 10%-15% of patients with hepatitis C genotype 1 (HCV GT1) experience virological relapse after all-oral antiviral regimen using simeprevir (SMV) and sofosbuvir (SOF). The efficacy and safety of treating such relapsers using ledipasvir/sofosbuvir (LDV/SOF) with/without ribavirin (RBV) has been limited.
Objective. Report the virological response and safety of LDV/SOF with/without RBV for 12-24 weeks in treating HCV GT1 relapsers after SMV + SOF.
Material and methods. Patients treated with standardized clinical protocol utilizing LDV/SOF with/without RBV at three transplant centers were retrospectively reviewed.
Results. Forty-five patients (29% post-LT, 82% male, 13% non-white, 73% subtype 1a, 86% IL28B CT/ TT, 78% F3-4) started LDV/SOF with/without RBV at a median of 22 weeks (range 7-55 weeks) after the last dose of SMV+SOF treatment. Thirty-seven patients received LDV/SOF for 24 weeks (24/37 patients with RBV) and eight patients received LDV/SOF for 12 weeks (5/8 patients with RBV). RBV dose was adjusted for renal function. Sixteen patients who were RBV-ineligible received LDV/SOF without RBV for 12 or 24 weeks. SVR 12 was achieved in 96% (43/45) of patients. Baseline viral load, RBV use, or GT1 subtype did not impact SVR 12. Minimal adverse events were reported in those without RBV; 45% of patients who received RBV developed significant anemia requiring RBV dose reduction and/or discontinuation. In LT recipients, minimal immunosuppression dose adjustments were required and no biopsy-proven acute rejection occurred.
Conclusions. Treatment with LDV/SOF with/without RBV for 12-24 weeks was very well tolerated and resulted in high SVR 12 rates (96%) in HCV GT1 relapsers to SMV + SOF treatment.
Approximately 3.4 to 4.9 million Americans are chronically infected with hepatitis C virus (HCV) and are at risk of developing cirrhosis, hepatocellular carcinoma (HCC) or both. The introduction of new direct acting antiviral agents (DAA) against HCV infection has dramatically altered the landscape of treatment for HCV.1,2 Several IFN-free regimens are currently approved to treat the different genotypes and are included in the treatment protocols in the United States.1 Those regimens are reported to have sustained virological response (SVR) rates well in excess of90%.3
Simeprevir (SMV), a second-generation NS3/4 protease inhibitor, and sofosbuvir (SOF), a nucleotide analogue NS5B polymerase inhibitor, were approved by the Food and Drug Administration (FDA) to be used along with Peg-IFN and/or RBV for the treatment of HCV GT1 patients. Several studies have confirmed the safety and efficacy of the combined use of SMV and SOF with/without RBV with reported SVR rates 80- 92% even in patients with compensated cirrhosis.4,5 This regimen was endorsed by American Association for the Study of Liver Diseases (AASLD)/Infectious Disease Society of America (IDSA) and European Association for the Study of Liver (EASL) in their respective guidelines.3 Subsequently, the FDA approved this regimen in November 2014 to treat non-LT patients for 12 weeks in non-cirrhotic patients with METAVIR stage F0-F3 and 24 weeks in cirrhotic patients with METAVIR stage F4.
Approximately 10%-15% of patients with HCV GT1 infection treated with this regimen experience virological relapse. Those rates can be higher in patients with decompensated cirrhosis as have been reported by our group previously.6,7
The fixed combination of ledipasvir (90 mg) and sofosbuvir (400 mg) (hereafter, LDV/SOF) was approved by the FDA for the treatment of HCV GT1 infection in October 2014. The use of this regimen with or without RBV is safe and effective with SVR rates in excess of 90%.8,9 However, there has been limited experience regarding the efficacy of this regimen in patients who relapsed on a prior IFN-free regimen.10
In this study, we report our multicenter experience using LDV/SOF with/without RBV for 12-24 weeks in treating HCV GT1 patients who relapsed after SMV + SOF treatment.
Material and MethodsStudy design and patientsA clinical treatment protocol was developed for treating HCV GT1 who previously developed virological relapse after SMV+SOF regimen at Mayo Clinic Transplant Centers in Arizona, Florida and Minnesota. This study was a retrospective review of prospectively collected data concerning the safety and efficacy of this protocol. The Mayo Clinic Institution Review Board approved the study.
The treatment regimen consisted of LDV/SOF with/ without RBV for 12-24 weeks. Our protocol was based on the available AASLD guidelines for HCV treatment at that time. Treatment decisions were guided by clinical experience, presence of cirrhosis, insurance approval, and patient ability to take RBV. Whenever possible, weight-based RBV was used in those patients. The initial dose of RBV was based on the estimated glomerular filtration rate (eGFR) and dose was adjusted depending on hemoglobin levels. All patients had Egfr > 30 mL/min at time of treatment initiation. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula,11 RBV was not used in 16 patients; 7/13 patients due to prior intolerance and 6/13 due to existing anemia at time of treatment initiation.
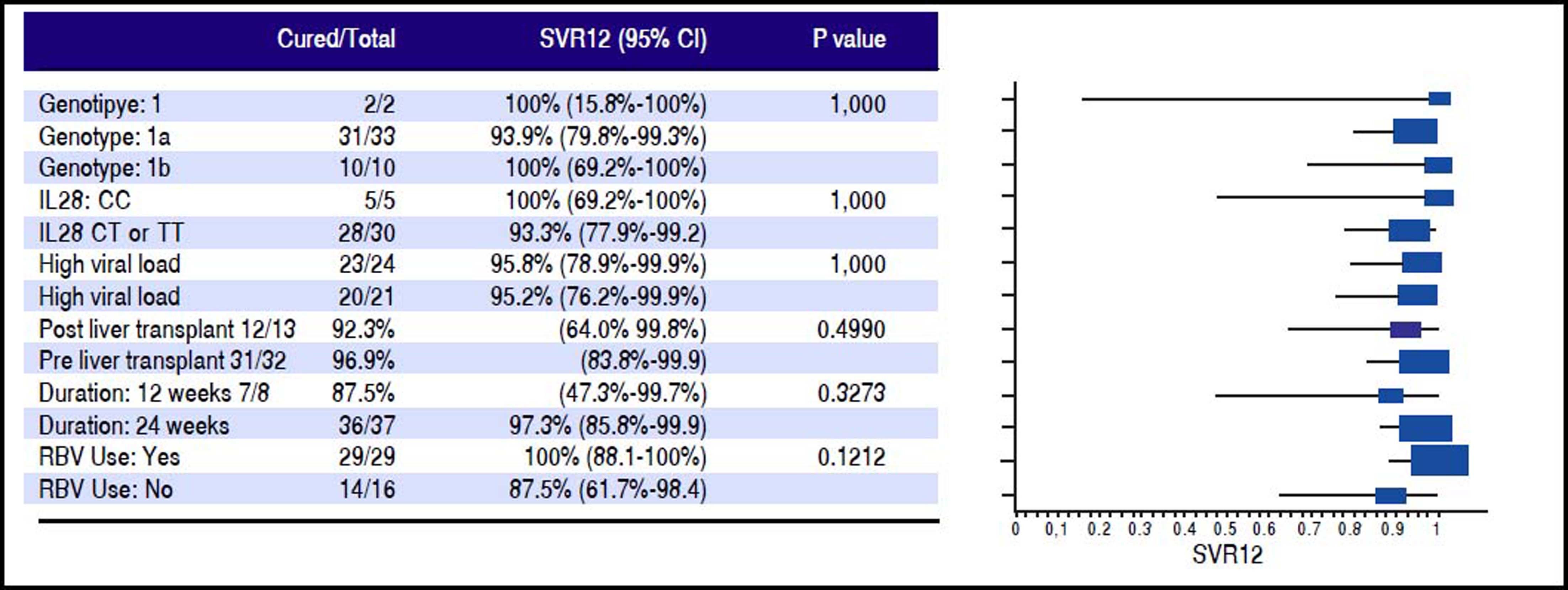
Efficacy assessmentsPlasma HCV RNA levels were quantified by COBAS TaqMan HCV assay, version 2.0 (Roche Molecular Systems, Inc.) with a lower limit of quantification (LLOQ) of 15-43 IU/mL and a lower limit of detection (LLOD) of 10 IU/mL. HCV RNA was monitored every 2 weeks until undetected, then every 4 weeks thereafter until 24 weeks after the completion of treatment. The primary endpoint was the proportion of patients who achieved undetected HCV RNA or SVR 12 weeks after completing treatment (SVR12). SVR12 results were calculated based on intention to treat (ITT) analysis. Demographics and disease characteristics for the study population were summarized in table 1. SVR negative rate at different time points by different treatment groups were calculated and summarized in Table 3. SVR12 and its 95% confidence interval were calculated using exact binomial method. The comparisons of the SVR12 by potential risk factors (genotype subtype, IL 28 B status (CC vs. non CC), baseline viral load, transplant status, treatment duration, RBV use) were done using Fisher’s exact test. The results were summarized in figure 2.
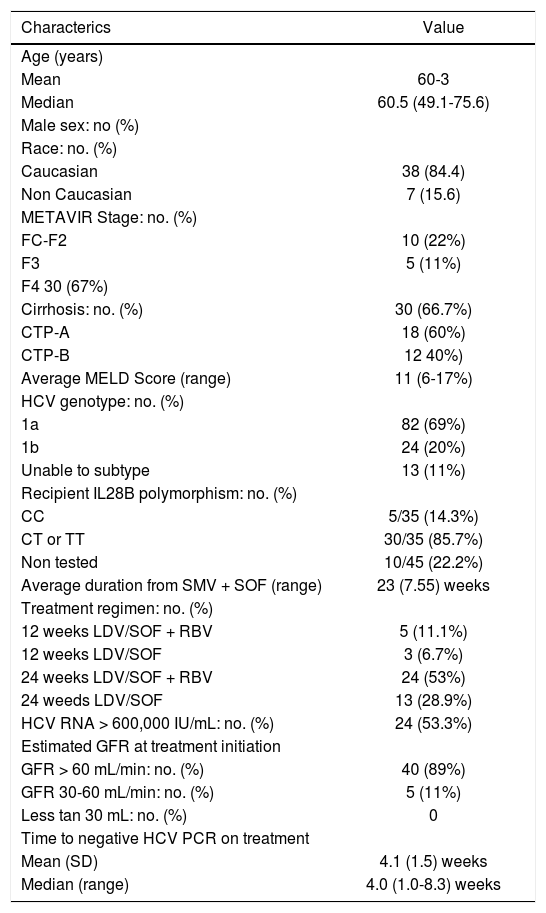
Baseline patient demographics and clinical characteristics of 45 patients.
| Characterics | Value |
|---|---|
| Age (years) | |
| Mean | 60-3 |
| Median | 60.5 (49.1-75.6) |
| Male sex: no (%) | |
| Race: no. (%) | |
| Caucasian | 38 (84.4) |
| Non Caucasian | 7 (15.6) |
| METAVIR Stage: no. (%) | |
| FC-F2 | 10 (22%) |
| F3 | 5 (11%) |
| F4 30 (67%) | |
| Cirrhosis: no. (%) | 30 (66.7%) |
| CTP-A | 18 (60%) |
| CTP-B | 12 40%) |
| Average MELD Score (range) | 11 (6-17%) |
| HCV genotype: no. (%) | |
| 1a | 82 (69%) |
| 1b | 24 (20%) |
| Unable to subtype | 13 (11%) |
| Recipient IL28B polymorphism: no. (%) | |
| CC | 5/35 (14.3%) |
| CT or TT | 30/35 (85.7%) |
| Non tested | 10/45 (22.2%) |
| Average duration from SMV + SOF (range) | 23 (7.55) weeks |
| Treatment regimen: no. (%) | |
| 12 weeks LDV/SOF + RBV | 5 (11.1%) |
| 12 weeks LDV/SOF | 3 (6.7%) |
| 24 weeks LDV/SOF + RBV | 24 (53%) |
| 24 weeds LDV/SOF | 13 (28.9%) |
| HCV RNA > 600,000 IU/mL: no. (%) | 24 (53.3%) |
| Estimated GFR at treatment initiation | |
| GFR > 60 mL/min: no. (%) | 40 (89%) |
| GFR 30-60 mL/min: no. (%) | 5 (11%) |
| Less tan 30 mL: no. (%) | 0 |
| Time to negative HCV PCR on treatment | |
| Mean (SD) | 4.1 (1.5) weeks |
| Median (range) | 4.0 (1.0-8.3) weeks |
Safety data were collected from all patients from the time of starting treatment until the assessment of the primary endpoint. Standard laboratory tests including Model for End-Stage Liver Disease (MELD) score parameters in patients with cirrhosis, and immunosuppression trough levels in patients post-LT were performed at treatment week TWs 0, 4, 8, 12, and post-treatment week 4, 8, 12 and 24. Clinic visits were conducted at approximately TW 4, end of treatment (EOT), and week 12 and 24 after treatment completion. Telephone communications by our advanced practitioners or pre-LT and post-LT nurse coordinators were conducted frequently throughout the study duration to identify any AEs. Serious AE (SAE) included urgent clinic visits and/or hospitalizations were thoroughly reviewed to identify the causal relationship with treatment regimen.
ResultsA total of 45 patients with HCV GT-1 infection who relapsed after SMV+SOF were treated per protocol with LDV/SOF with/without RBV at the three participating sites. Table 1 summarizes the baseline patient demographic and clinical characteristics. RBV was administered in 29 patients (64%). Genotype 1a was present in 33 patients (73%), and IL-28B polymorphism non-CC was present in 30/35 patients (86%). High viral load at baseline (viral load > 600,000 IU/mL) was present in 24 patients (53.3%). LDV/SOF was started at a median of 22 weeks (range 7-55 weeks) after the last dose of SMV + SOF treatment. At time of treatment, 13/45 (29%) patients were treated post-LT. Cirrhosis was present in 30/45 (67%) of patients: 18/30 (60%) had Child-Pugh-Turcott class-A (CTP-A) cirrhosis, and 12/30 (40%) had CTP class B (CTP-B) cirrhosis. One patient had HIV co-infection. RBV was used in 29/45 (64.4%) of patients. Treatment regimens for the entire cohort are outlined in table 1; 8/45 patients (18%) were treated for 12 weeks (five patients with RBV), 37/45 patients (82%) were treated with 24 weeks (24 patients received RBV). All patients completed antiviral treatment and no patients were lost to follow-up. To date, all patients have had assessment for SVR12.
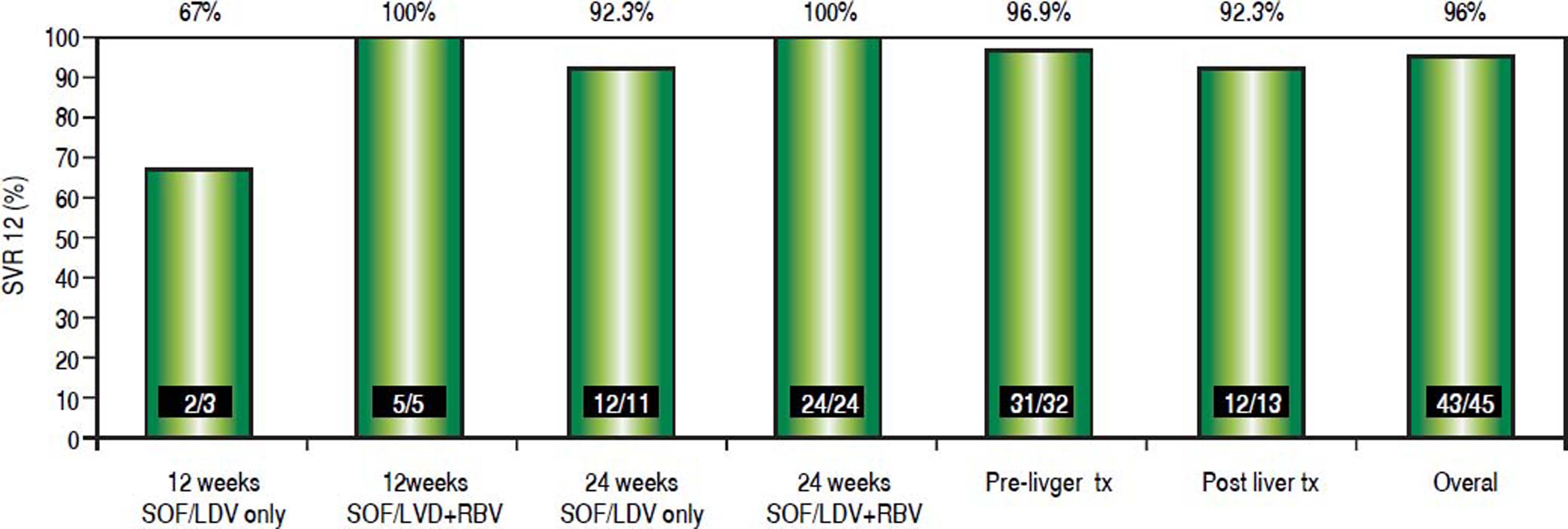
Transplantation statusDuring the study period, 13 patients (30%) were treated post-liver transplantation. Treatment was completed in all patients. SVR 12 was achieved in 12/13 patients (92.3%)(Figure 1). Those patients tolerated treatment well with minimal adverse events. All patients were on tacrolimus based immunosuppression. Tacrolimus levels were checked on a weekly basis while patients on treatment. In 9 patients (69%), adjustment of immunosuppression was needed to maintain therapeutic trough tacrolimus levels after HCV clearance. Majority of those patients (7/ 9) required an increase in the tacrolimus dose especially after achieving viral clearance. Median increase in tacrolimus dose was 3 mg/day. None of our patients developed biopsy proven acute cellular rejection while on treatment.
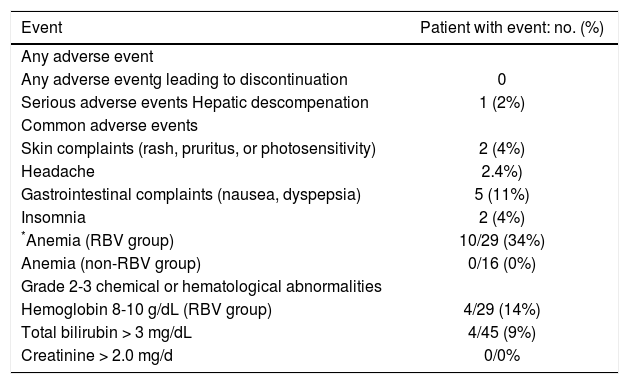
Adverse eventsTable 2 summarizes the AE reported during the antiviral treatment. Twenty-six patients (22%) had at least one AE. These AEs were in mild in severity, requiring only supportive and symptomatic treatment without interruption of antiviral treatment. None of our patients had serious AEs. Minimal adverse events were reported in patients treated without RBV. Gastrointestinal side effects (dyspepsia and nausea) were the most common AE and were seen in five patients. Anemia was seen in ten patients, all receiving RBV. RBV was discontinued in three patients and the rest were managed with RBV dose reduction and growth factor support. Grade 3-4 hyperbilirubinemia developed in 4/45 (3%) patients. One patient with CTP-B cirrhosis (MELD 16) at treatment initiation decompensated while on treatment (ascites and encephalopathy) and MELD score increased to 20. Patient completed treatment, achieved SVR12, and underwent successful liver transplant.
Adverse events and laboratory abnormalities.
| Event | Patient with event: no. (%) |
|---|---|
| Any adverse event | |
| Any adverse eventg leading to discontinuation | 0 |
| Serious adverse events Hepatic descompenation | 1 (2%) |
| Common adverse events | |
| Skin complaints (rash, pruritus, or photosensitivity) | 2 (4%) |
| Headache | 2.4%) |
| Gastrointestinal complaints (nausea, dyspepsia) | 5 (11%) |
| Insomnia | 2 (4%) |
| *Anemia (RBV group) | 10/29 (34%) |
| Anemia (non-RBV group) | 0/16 (0%) |
| Grade 2-3 chemical or hematological abnormalities | |
| Hemoglobin 8-10 g/dL (RBV group) | 4/29 (14%) |
| Total bilirubin > 3 mg/dL | 4/45 (9%) |
| Creatinine > 2.0 mg/d | 0/0% |
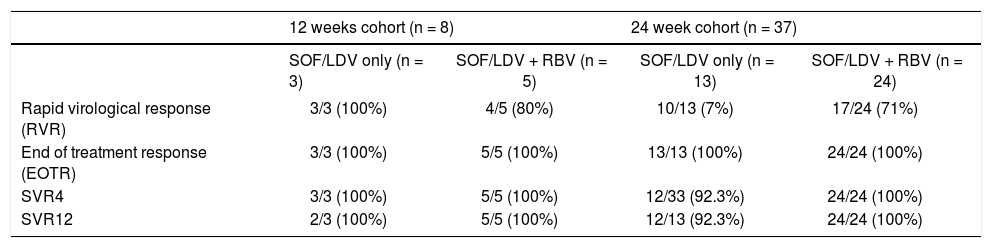
Table 3 summarizes the virological response in our cohort. All patients achieved undetected HCV RNA during treatment, at a median of 4 weeks (range 1.0-8.3 weeks). Rapid virological response (RVR) with undetected HCV RNA at TW4 was achieved in 34/45 patients. End of treatment response (EOTR) by ITT analysis was achieved in 100% of patients. On-treatment viral kinetics, SVR4, and SVR12 were similar between the 29 patients who received RBV compared with the remaining 16 patients who did not receive RBV.
Virological response.
| 12 weeks cohort (n = 8) | 24 week cohort (n = 37) | |||
|---|---|---|---|---|
| SOF/LDV only (n = 3) | SOF/LDV + RBV (n = 5) | SOF/LDV only (n = 13) | SOF/LDV + RBV (n = 24) | |
| Rapid virological response (RVR) | 3/3 (100%) | 4/5 (80%) | 10/13 (7%) | 17/24 (71%) |
| End of treatment response (EOTR) | 3/3 (100%) | 5/5 (100%) | 13/13 (100%) | 24/24 (100%) |
| SVR4 | 3/3 (100%) | 5/5 (100%) | 12/33 (92.3%) | 24/24 (100%) |
| SVR12 | 2/3 (100%) | 5/5 (100%) | 12/13 (92.3%) | 24/24 (100%) |
SVR: sustained virological response.
One patient developed virological relapse within 4 weeks after treatment completion resulting in an SVR4 rate of 98% (44/45 patients) by ITT analysis. An additional patient relapsed after achieving SVR4 yielding SVR12 rate of 96% (43/45 patients, 95% CI: 85%-99%) by ITT analysis. This patient was a LT recipient with cirrhosis who was RBV-ineligible. Though the plan was to treat for 24 weeks with LDV/SOF, he was only treated for 12 weeks due to insurance issues and he developed virological relapse between 4 and 8 weeks after treatment discontinuation. Both patients who relapsed were treated with RBV free regimen. RAV analysis after virological relapse confirmed NS5A Y93N mutation in the post-LT patient who was treated for 12 weeks of LDV/SOF This specific mutation confers resistance to LDV, daclatasvir, and ombitasvir. The second patient had compensated CTP-A cirrhosis who was treated with 24 weeks of LDV/SOF only. RAV analysis confirmed the presence of NS5A Q30H mutation (confers resistance to LDV and daclatasvir), and NS3 R155K, D168E mutations (confer resistance to paritaprevir, boceprevir and SMV). HCV NS5B mutation was not detected in either of those patients.
Factors influencing SVR12 rateFigure 1 outlines the SVR12 rates in the entire cohort and according to the different treatment regimens used. The overall SVR12 rate was high at 96%. It was the highest in the group that received LDV/SOF with RBV for 24 weeks (100%), while it was the lowest in the group who received LDV/SOF without RBV for 12 weeks (67%). Figure 2 shows the SVR12 with 95% confidence intervals (CI) based on several variables. Overall none of those variables had significant impact on SVR12. Eight patients were treated for 12 weeks; one patient relapsed resulting in an SVR12 of 87.5% (95% CI: 47.3%-99.7%). Thirty-seven patients received 24 weeks of treatment; one patient relapsed resulting in an SVR12 of 97.3% (95% CI: 85.8%-99.9%). The difference in SVR12 based on treatment duration did not reach statistical difference (p = 0.3). Sixteen patients were treated with LDV/SOF only; two patients relapsed in this cohort resulting in an SVR12 of 87.5% (95% CI: 61.7%-98.4%). Twenty-nine patients were treated with LDV/SOF+RBV, and none of those patients had virological relapse resulting in an SVR12 of 100% (95% CI: 88.1%-100%). The difference if SVR12 between the two groups based on the RBV use did not reach statistical significance (p = 0.12).
DiscussionIn this large multicenter experience, we describe the efficacy of LDV/SOF with/without RBV for 12-24 weeks treating HCV GT1 patients who experienced virological relapse after treatment with SMV + SOF. Overall, treatment was effective and very well tolerated. None of the patients discontinued treatment. For the patient cohort as a whole, the SVR rate 12 weeks after completing treatment was 96% (95% CI: 85%-99%). Patients who received LDV/ SOF with RBV achieved higher SVR12 rate (100%, CI: 88.1%-100%), compared to those patients who were treated with LDV/SOF alone (SVR12 87.5%, CI: 61.7%-98.4%). The difference between the two groups did not reach statistical difference (p = 0.12).
Virological relapse have been seen in approximately 10-15% of patient with HCV GT1 infection treated with SMV + SOF with or without RBV.4,6,7 Failure rates can be higher in patients with cirrhosis and decompensated liver disease. In most of those patients, treatment failure was associated with resistance to SMV with cross-resistance to other HCV NS3 protease inhibitors such as paritaprevir, telaprevir, and boceprevir. Some studies even suggest that cross-resistance can extend to grazoprevir in the presence of certain resistance variants at D168 and A156 positions. Sofosbuvir RAV’s have only been described in one patients treated with this regimen supporting the rare occurrence of this variant in clinical practice and supporting the use of SOF based regimens to retreat patients who failed SMV + SOF combination therapy. Given the lack of sufficient data, and the heterogeneous nature of this group, current AASLD guidelines did not have an optimal retreatment regimen for this patient population.3
Data on retreatment of SMV + SOF failures are extremely limited.12 Our study is one of the largest reports to date using LDV/SOF with or without RBV to retreat those patients. Gonzales et al recently presented some interim data from a cohort of 31 patients who failed SMV + SOF therapy and were treated with 12-24 weeks of LDV/ SOF with or without RBV. In the subset of patients in whom SVR12 data was available, SVR12 rates were seen in 85%-91% of those patients.13
LDV/SOF with or without RBV combination therapy was effective, well tolerated and associated with few adverse events. One patient with advanced liver disease experienced decompensation while on treatment and he did proceed to receive LT after completing treatment and achieving SVR12. Significant grade 3-4 hyperbilirubinemia was rare and only observed in 4 patients (9%) in our cohort. It predominantly affected patients with more advanced liver disease. Majority of AEs occurred in patients receiving RBV. Anemia was very prominent in this group and was seen in 34% of patients receiving RBV. Ribavirin was discontinued in three patients and the rest were managed by RBV dose reductions and growth factor support. Treatment was well tolerated in LT recipients and none of patients experienced SAE or biopsy-proven acute cellular rejection.
Though both relapses seen in our study population occurred in patients with genotype 1a infection, our study demonstrated LDV/SOF with or without RBV was equally effective in patients with genotype 1a, and genotype 1b infections with SVR12 of 94%, and 100% respectively (p = 1.0). RAV analysis in those two patients confirmed two different variants: NS5A Y93N mutation in the post-LT patient, who was treated for 12 weeks of LDV/SOF, and NS5A Q30H, NS3 R155K, D168E mutations in the pre-LT patient who was treated with 24 weeks of LDV/SOF. Both patients were RBV ineligible. None of those patients had evidence of NS5B mutations.
RBV use was associated with a higher SVR12 (100%, 95% CI: 88.1%-100%) when compared to patients who were ineligible to RBV (SVR12 87.5%, CI: 61.7%-98.4%). Though the difference in SVR12 did not reach statistical difference (p = 0.12) most likely related to the small sample size, the trend does support the current AASLD guidelines encouraging the use of weight-based RBV when treating GT1 HCV NS5A treatment experienced patients.3
There are few limitations that should be considered in the interpretation of this report, including the retrospective element in the design that led to the heterogeneity of the patient population. Though our study includes the largest report to date describing the outcomes of retreatment in patients who failed SMV/SOF, the sample size was still small across the different treatment groups, and thus our study was underpowered to detect a significant difference between the treatment groups even if it does exist. Furthermore, the treatment regimens were not standardized. This can be explained by the fact that at the time the study was designed and conducted, the AASLD had no recommendations regarding the best treatment regimen or duration for those patients. Therefore, we used the best available data and experience to guide or treatment regimens.
In summary, we found that LDV/SOF with/without RBV for 12-24 weeks treating HCV GT1 patients who experienced virological relapse after treatment with SMV+SOF was well tolerated and demonstrated an SVR12 rate of 96%. The use of RBV, whenever possible, should be considered in this cohort and may allow shorter treatment duration. Despite prior exposure to SOF twice, none of the patients developed SOF RAV’s which potentially limit the use of SOF-containing regimens in the future.
Abbreviations- •
AE: adverse event.
- •
CTP: Child-Pugh-Turcott.
- •
DAA: direct acting antiviral agent.
- •
eGFR: estimated glomerular filtration rate.
- •
EOTR: end of treatment response.
- •
FDA: Food and Drug Administration.
- •
HCV: hepatitis C virus.
- •
IL28b: interleukin 28b rs12979860.
- •
ITT: intention to treat.
- •
LDV: ledipasvir.
- •
LLOD: lower limit of detection.
- •
LLOQ: lower limit of quantification.
- •
LT: liver transplantation.
- •
MELD: Model For End Stage Liver Disease.
- •
Peg-IFN: peginterferon.
- •
RAV: resistance associated variants.
- •
RBV: ribavirin.
- •
SAE: serious adverse event.
- •
SMV: simeprevir.
- •
SOF: sofosbuvir.
- •
SVR: sustained virologic response.
- •
TW: treatment week.
None of authors has any financial, professional or personal conflicts to declare.
Author ContributionsBashar Aqel; study concept and design, acquisition of data, analysis and interpretation of data, writing of manuscript. Surakit Pungpapong; study concept and design, data collection, critical revision of manuscript. Nan Zhang; statistical analysis and critical revisions of the manuscript. Michael Leise, Hugo E. Vargas, Kymberly D. Watt and Andrew P. Keaveny have contributed with critical revisions to the manuscript.