Background. Conflicting data have been reported on the prevalence of liver steatosis, its risk factors and its relationship with fibrosis in patients with human immunodeficiency virus (HIV)/hepatitis C virus (HCV) co-infection or with HCV mono-infection.
Aim. The study aims were to assess steatosis prevalence and its risk factors in both HCV groups. We also evaluated whether steatosis was linked with advanced fibrosis. Sixty-eight HIV/HCV co-infected and 69 HCV mono-infected patients were consecutively enrolled. They underwent liver ultrasonography and transient elastography. Bright liver echo-pattern was used to diagnose steatosis; advanced fibrosis was defined as liver stiffness > 9.5 kPa and FIB–4 values > 3.25. The optimal stiffness cut-off according to FIB–4 > 3.25 was evaluated by ROC analysis.
Results. No significant difference was found in steatosis-prevalence between mono- and co-infected patients (46.3 vs. 51.4%). Steatosis was associated with triglycerides and impaired fasting glucose/diabetes in HCV mono-infected, with lipodystrophy, metabolic syndrome, total-cholesterol and triglycerides in co-infected patients. Stiffness > 9.5 was significantly more frequent in co-infection (P < 0.003). Advanced fibrosis wasn't significantly associated with steatosis. The area under the ROC curve was 0.85 (95% CI 0.79–0.9). On multivariate analysis steatosis was associated with triglycerides in both HCV mono- and co-infected groups (P < 0.02; P < 0.03).
Conclusion. Although steatosis was common in both HCV mono- and co-infected patients, it was not linked with advanced fibrosis. Triglycerides were independent predictors of steatosis in either of the HCV-groups. Dietary interventions and lifestyle changes should be proposed to prevent metabolic risk factors.
Hepatic steatosis is a very common feature in hepatitis C virus (HCV) infected patients. The prevalence of steatosis is estimated to range from 40 to 86% according to the HCV genotype.1 Two different types of steatosis can be observed in patients with HCV infection: viral-related steatosis (reported in patients with HCV genotype 3, which is directly linked to a cytopathic effect of the virus) and metabolic-related steatosis (found in patients infected with non-genotype 3 HCV).2 The majority of patients with fatty infiltration of the liver show simple steatosis. However, patients with non-alcoholic steatohepatitis, approximately 10% of those with chronic HCV, are at risk for progressive fibrosis, cirrhosis and hepatocellular carcinoma.3
In the last few years several studies have been conducted in human immunodeficiency virus (HIV)/ HCV co-infected patients with discrepant results both for steatosis prevalence and for its risk factors.4 In addition, it is unclear whether the prevalence of steatosis is higher or its impact on liver disease is more severe in co-infected than in HCV mono-infected patients.
Liver biopsy is the best diagnostic tool for the diagnosis of steatosis and fibrosis, even though its invasiveness limits its use in the routine assessment of liver disease.5,6 Ultrasonography (US), because of its low cost, safety and widespread availability is accepted as the most frequently-used technique for the screening of steatosis.7,8 Likewise, transient elastography (TE) (Fibro-Scan®; EchoSensTM, Paris, France) and biochemical markers have been demonstrated to be very useful methods in the non-invasive assessment of liver fibrosis in patients with HCV mono-infection or HIV/HCV co-infection.9–11
The aims of this study were to assess the prevalence of steatosis and its risk factors in Caucasian patients with chronic hepatitis C with or without HIV co-infection. In addition, we evaluated whether steatosis correlated with advanced liver fibrosis, measured by TE and biochemical markers in both HCV-groups.
Material and MethodsSubjectsThe study population was composed of Caucasian HCV mono-and HIV/HCV co-infected patients consecutively enrolled from September 2009 to August 2011, who were followed-up prospectively at the Outpatient Clinic of the Department of Internal Medicine and at the AIDS Center of the University of Palermo. All these patients underwent both US and liver TE.
Exclusion criteria were acute liver events, hepatocellular carcinoma, chronic hepatitis B. To avoid TE failure, also patients with decompensated cirrhosis and body mass index > 30 kg/m2 were excluded.
Age, gender, risk factors for HCV, HIV infections and duration of cumulative exposure to antiretroviral therapy (ART) were evaluated and recorded in a database designed for this study.
Alcohol intake > 20 g/day at the time of the study or in the past was recorded through patient interviews. Body mass index was calculated as weight (in kg) divided by height squared (m2). Diabetes mellitus or impaired fasting glucose were defined according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus criteria.12 Diabetes and impaired fasting glucose were analyzed together because of the small number of patients. Metabolic syndrome (MS) was diagnosed according to the National Cholesterol Education Program definition.13 Diagnosis of lipodystrophy was made on the basis of body fat changes, sub-cutaneous fat loss and/or increased waist or buffalo hump, recognized by the patients and confirmed by physical examination.14 Moreover, in the HIV/HCV co-infected patients CD4 T-cell counts (latest value and nadir) and plasma HIV-RNA levels were assessed. In all the HCV-infected patients HCV genotype and plasma HCV-RNA levels were also recorded. Baseline complete blood cell count and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total-cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides and glycaemia were measured in the overall population. The plasma concentration ratio of triglycerides to HDL (triglycerides/HDL ratio) was also calculated as a surrogate marker of insulin resistance.15 All the blood tests were performed on samples from fasting patients.
Ultrasound assessmentLiver US was performed in the morning after fasting for at least 10 hours by a single operator (MS), using a real-time Philips 5000 HDI apparatus with a 2–5 MHz convex multi-frequency probe.
The hepatic parenchyma echo-pattern characteristic of steatosis was defined as “bright liver” (BL) when it was characterized by numerous fine packed, uniformly-distributed echoes of high amplitude and increased echogenicity when compared to the parenchyma of the right kidney.16 BL was graded 0 (no BL) to 1 (1: BL present).
Liver fibrosis assessmentLiver fibrosis was assessed by a single certified operator (trained by the manufacturer) using TE (FibroScan®; EchoSens, Paris, France). Advanced liver fibrosis (severe fibrosis and cirrhosis) was defined as a median liver stiffness ≥ 9.5 kPa.9–11,17 Fibrosis was also assessed biologically using 2 different well-validated indices: the AST platelet ratio index (APRI) and the FIB-4 index. The APRI was calculated as follows: AST/upper limit of normal x 100/platelet count (109/L).18 The FIB–4 index was calculated as follows: age x AST [IU/L]/[(platelet count [109/L]) x (ALT [IU/L])1/2].19 We also calculated the receiver-operating characteristic (ROC) curve for FIB–4 ≥ 3.25 to evaluate the optimal cutoff of liver stiffness for advanced fibrosis diagnosis.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Patients were enrolled after written informed consent had been obtained.
Data analysesData were expressed as mean ± standard deviation when the distribution of values was Gaussian, otherwise as the median and range (min-max). Differences in values of the groups were calculated using Student’s t-test if data were normally distributed, otherwise the Mann-Whitney U test was used. Fisher’s exact and χ2tests, χ2 test of Maentel Haenszel, Spearman’s rank correlations and Pearson’s correlation were used where appropriate. The ROC curve and the corresponding area under the curve were calculated to evaluate the accuracy of liver stiffness in distinguishing between patients with or without advanced liver fibrosis according to FIB-4 ≥ 3.25 values.19 Non-parametric estimates of the area under the ROC curve and the respective standard error were applied.20 The optimal cut-off value was the one corresponding to the maximum of the Youden index.21
Multiple logistic regression analysis was performed to estimate the independence of the association between variables significant at univariate analysis and BL. Variables contributing significantly to fit the logistic equation were then selected by a step-wise procedure. P < 0.05 was considered significant.
All analyses were performed using the SPSS software package (version 16.0; Chicago, IL, USA) and MedCalc Software (Broekstraat 52, 9030 Mariakerke, Belgium).
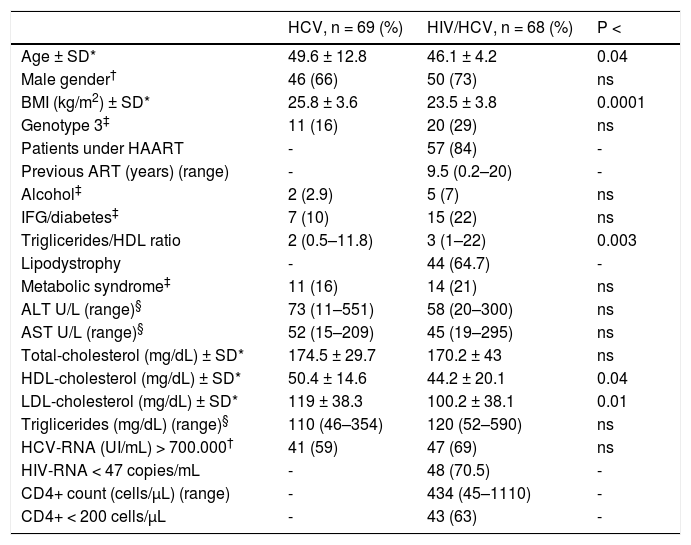
ResultsStudy populationWe enrolled 137 Caucasian patients suitable for the study, 68 of which were HIV/HCV co-infected and 69 HCV mono-infected patients. Patient characteristics at US and liver stiffness measurements are shown in table 1.
Baseline demographic, clinical and laboratory characteristics.
| HCV, n = 69 (%) | HIV/HCV, n = 68 (%) | P < | |
|---|---|---|---|
| Age ± SD* | 49.6 ± 12.8 | 46.1 ± 4.2 | 0.04 |
| Male gender† | 46 (66) | 50 (73) | ns |
| BMI (kg/m2) ± SD* | 25.8 ± 3.6 | 23.5 ± 3.8 | 0.0001 |
| Genotype 3‡ | 11 (16) | 20 (29) | ns |
| Patients under HAART | - | 57 (84) | - |
| Previous ART (years) (range) | - | 9.5 (0.2–20) | - |
| Alcohol‡ | 2 (2.9) | 5 (7) | ns |
| IFG/diabetes‡ | 7 (10) | 15 (22) | ns |
| Triglicerides/HDL ratio | 2 (0.5–11.8) | 3 (1–22) | 0.003 |
| Lipodystrophy | - | 44 (64.7) | - |
| Metabolic syndrome‡ | 11 (16) | 14 (21) | ns |
| ALT U/L (range)§ | 73 (11–551) | 58 (20–300) | ns |
| AST U/L (range)§ | 52 (15–209) | 45 (19–295) | ns |
| Total-cholesterol (mg/dL) ± SD* | 174.5 ± 29.7 | 170.2 ± 43 | ns |
| HDL-cholesterol (mg/dL) ± SD* | 50.4 ± 14.6 | 44.2 ± 20.1 | 0.04 |
| LDL-cholesterol (mg/dL) ± SD* | 119 ± 38.3 | 100.2 ± 38.1 | 0.01 |
| Triglicerides (mg/dL) (range)§ | 110 (46–354) | 120 (52–590) | ns |
| HCV-RNA (UI/mL) > 700.000† | 41 (59) | 47 (69) | ns |
| HIV-RNA < 47 copies/mL | - | 48 (70.5) | - |
| CD4+ count (cells/μL) (range) | - | 434 (45–1110) | - |
| CD4+ < 200 cells/μL | - | 43 (63) | - |
HCV: hepatitis C virus. HIV: human immunodeficiency virus. BMI: body mass index. HAART: highly-active antiretroviral therapy. ART: antiretroviral therapy. IFG: impaired fasting glucose. ALT: alanine aminotransferase. AST: aspartate aminotransferase. HDL: high-density lipoprotein. LDL: low-density lipoprotein.
The HIV/HCV co-infected patients were significantly younger than the HCV mono-infected subjects (P < 0.04). In the HCV mono-infected patients body mass index was significantly higher than in co-infected patients (P < 0.0001). No significant difference was found in the prevalence of HCV genotypes in patients with or without HIV infection. Most of the HIV/HCV co-infected patients (84%) were undergoing ART.
All the HCV patients had detectable HCV-RNA. The HCV mono-infected patients had HDL-cholesterol and LDL-cholesterol levels significantly higher than the HIV/HCV co-infected patients (P < 0.04; P < 0.01, respectively).
Prevalence of BL and association between BL and risk factorsNo significant difference was shown in BL prevalence between HIV/HCV co-infected and HCV mono-infected patients: 35/68 (51.4%) vs. 32/69 (46.3%) respectively (P = ns).
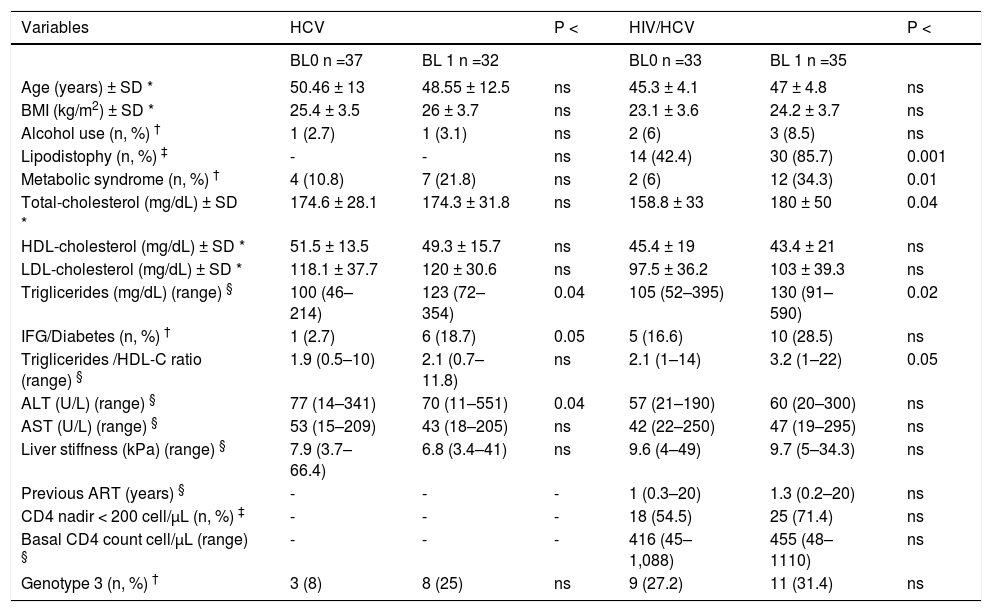
Table 2 shows the association between BL and the risk factors analyzed. HCV mono-infected patients with BL had higher triglyceride levels (P < 0.04) and IGF/diabetes (P < 0.05).
Comparison between bright liver echo-pattern (BL0/BL1) and studied risk factors in HCV patients with or without HIV co-infection.
| Variables | HCV | P < | HIV/HCV | P < | ||
|---|---|---|---|---|---|---|
| BL0 n =37 | BL 1 n =32 | BL0 n =33 | BL 1 n =35 | |||
| Age (years) ± SD * | 50.46 ± 13 | 48.55 ± 12.5 | ns | 45.3 ± 4.1 | 47 ± 4.8 | ns |
| BMI (kg/m2) ± SD * | 25.4 ± 3.5 | 26 ± 3.7 | ns | 23.1 ± 3.6 | 24.2 ± 3.7 | ns |
| Alcohol use (n, %) † | 1 (2.7) | 1 (3.1) | ns | 2 (6) | 3 (8.5) | ns |
| Lipodistophy (n, %) ‡ | - | - | ns | 14 (42.4) | 30 (85.7) | 0.001 |
| Metabolic syndrome (n, %) † | 4 (10.8) | 7 (21.8) | ns | 2 (6) | 12 (34.3) | 0.01 |
| Total-cholesterol (mg/dL) ± SD * | 174.6 ± 28.1 | 174.3 ± 31.8 | ns | 158.8 ± 33 | 180 ± 50 | 0.04 |
| HDL-cholesterol (mg/dL) ± SD * | 51.5 ± 13.5 | 49.3 ± 15.7 | ns | 45.4 ± 19 | 43.4 ± 21 | ns |
| LDL-cholesterol (mg/dL) ± SD * | 118.1 ± 37.7 | 120 ± 30.6 | ns | 97.5 ± 36.2 | 103 ± 39.3 | ns |
| Triglicerides (mg/dL) (range) § | 100 (46–214) | 123 (72–354) | 0.04 | 105 (52–395) | 130 (91–590) | 0.02 |
| IFG/Diabetes (n, %) † | 1 (2.7) | 6 (18.7) | 0.05 | 5 (16.6) | 10 (28.5) | ns |
| Triglicerides /HDL-C ratio (range) § | 1.9 (0.5–10) | 2.1 (0.7–11.8) | ns | 2.1 (1–14) | 3.2 (1–22) | 0.05 |
| ALT (U/L) (range) § | 77 (14–341) | 70 (11–551) | 0.04 | 57 (21–190) | 60 (20–300) | ns |
| AST (U/L) (range) § | 53 (15–209) | 43 (18–205) | ns | 42 (22–250) | 47 (19–295) | ns |
| Liver stiffness (kPa) (range) § | 7.9 (3.7–66.4) | 6.8 (3.4–41) | ns | 9.6 (4–49) | 9.7 (5–34.3) | ns |
| Previous ART (years) § | - | - | - | 1 (0.3–20) | 1.3 (0.2–20) | ns |
| CD4 nadir < 200 cell/μL (n, %) ‡ | - | - | - | 18 (54.5) | 25 (71.4) | ns |
| Basal CD4 count cell/μL (range) § | - | - | - | 416 (45–1,088) | 455 (48–1110) | ns |
| Genotype 3 (n, %) † | 3 (8) | 8 (25) | ns | 9 (27.2) | 11 (31.4) | ns |
BL: bright liver. HCV: hepatitis C virus. HIV: human immunodeficiency virus. BMI: body mass index. HDL: high-density lipoprotein. LDL: low-density lipoprotein. IFG: impaired fasting glucose. ALT: alanine aminotransferase. AST: aspartate aminotransferase. ART: antiretroviral therapy.
In the HIV/HCV co-infected patients BL was significantly associated with lipodystrophy (P < 0.001), metabolic syndrome (P < 0.01), triglycerides/HDL ratio (P < 0.05), higher total-cholesterol (P < 0.04) and triglyceride levels (P < 0.02). No significant association was found between BL and the duration of ART exposure in the HIV/HCV co-infected patients.
On multiple logistic regression analysis BL was associated only with metabolic syndrome in co-infected patients: OR 8.1 (95% CI 1.6–22); P < 0.02. When metabolic syndrome was removed from the model, the only variable independently associated with BL was higher triglyceride levels: OR 1.15 (95% CI 1.13–1.23); P < 0.02. In the HCV mono-infected patients higher triglyceride levels were also independently associated with BL: OR 1.16 (95% CI 1.1–1.21); P < 0.03.
Extent of liver fibrosis using TE and biochemical markersThe HIV/HCV co-infected patients had liver stiffness values higher than HCV mono-infected patients: 9.8 (4.3–48.8) vs. 7.2 (3.4–66.4) kPa, respectively (P < 0.02). The prevalence of advanced fibrosis, evaluated as liver stiffness > 9.5 kPa, was higher in the co-infected patients 36/68 (52.9%) than in those with HCV mono-infection 19/69 (27.5%); (χ2 = 8.2; P < 0.003).
The median values of APRI in HCV mono-infected and HIV/HCV co-infected patients were 0.8 (0.2–5.9) and 0.7 (0.2–19.7), respectively (P= ns). The median values of FIB-4 were 1.6 (0.3–10.4) in HCV mono-infected and 1.5 (0.6–23.8) in HIV/HCV co-infected patients (P = ns).
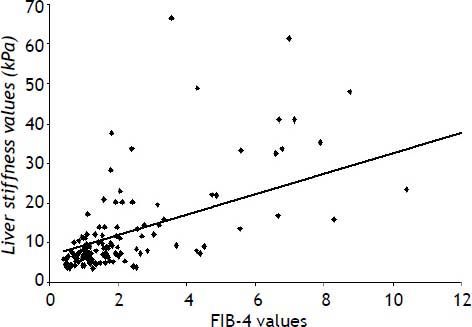
Figure 1 shows the statistically significant correlation between liver stiffness and FIB4 values (r = 0.60; P < 0.0001).
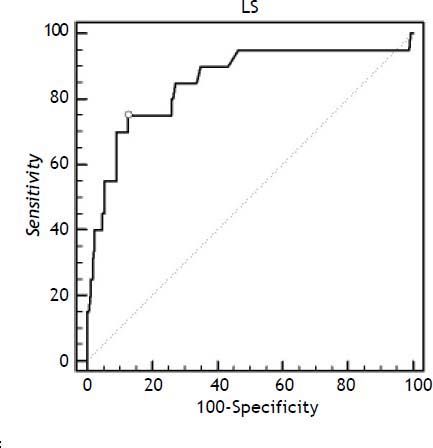
Figure 2 shows the ROC curve used to find the optimal cut-off of liver stiffness in distinguishing between patients with or without advanced liver fibrosis, according to FIB-4 values > 3.25. The liver stiffness cut-off value was computed to > 13.4 kPa. The area under the ROC curve was 0.85 ± 0.03 (95% CI 0.79–0.9); sensitivity and specificity were 75% and 87.3%, respectively.
The receiver-operating characteristic (ROC) curve of liver stiffness (LS) to discriminate advanced liver fibrosis. The area under the ROC ± SE curve was 0.85 ± 0.03. The figure reports the best discriminating value between patients with or without advanced liver fibrosis found in our population (13.4 kPa), according to FIB–4 cut off > 3.25 (see text).
No association was observed between BL and advanced fibrosis, measured as liver stiffness, and the 2 biochemical markers.
DiscussionIn the post highly-active antiretroviral therapy (HAART) era several studies on steatosis have been carried out in HIV patients with chronic hepatitis C. Discrepant results in steatosis prevalence have been reported, with a large range varying from 23 to 72%.4 Our data showed that the prevalence of steatosis was high either in HIV/HCV co-infected and HCV mono-infected patients (51.4% vs. 46.3%, respectively), although the difference was not statistically significant in both HCV groups. This result was similar to those of a meta-analysis of 12 histological studies and a recent US based study, albeit unexpected, because not only HCV infection, but also HIV infection itself or ART appear to have pro-steatogenic effects.4,22
In this regards, the natural course of HIV infection is characterized by a reduction in HDL-and LDL-cholesterol levels after HIV seroconversion, conversely increasing triglycerides levels were found in the more advanced stages.23,24 After ART start, lipid levels were showed to soon increase above pre-seroconversion levels except for HDL-cholesterol which remained persistently low.25 Interestingly, the lower HDL-cholesterol levels found in our HIV/ HCV co-infected with respect to HCV mono-infected patients suggest that HIV infection per se might be involved in dyslipidemia, thus playing a role in the steatogenesis.
Lipid abnormalities such as hypertriglyceridaemia in the majority of cases, followed by hypercholesterolaemia and mixed forms, have been previously described as ART-related effects in different studies.26 Likewise, the high serum levels of both total-cholesterol and triglycerides related to steatosis at univariate analysis in our HIV/HCV co-infected patients, may be suggestive of metabolic toxicity of ART.
Other indirect signs of HAART-related metabolic effects, such as lipodystrophy and triglycerides/HDL ratio, were associated with steatosis at univariate analysis, and appear to support that HAART exposure may have played a leading role in the development of steatosis. Nevertheless, the small study sample, the cross sectional nature of the study and the limited data on the HAART are some limitations for estimating the impact of either ART or HIV infection on steatosis among our co-infected patients. Further studies are needed to confirm this role and to evaluate the impact of HAART in the pathogenesis of steatosis and its natural history.
The link between impaired fasting glucose/diabetes and triglyceride levels and steatosis may suggest that metabolic factors were prevalent among the HCV patients uninfected with HIV. The lack of a significant association between BL and virological factors, such as HCV genotype or viral load, could be due to the small number of study patients.
Since hepatic steatosis includes a spectrum of conditions ranging from simple steatosis to steatohepatitis, which in turn may evolve to cirrhosis, and end-stage liver failure to hepatocellular carcinoma, the most important issue in the monitoring of fatty liver disease progression is the assessment of liver fibrosis.27,28 Liver biopsy is the gold standard for the diagnosis of liver fibrosis, although because of its limitations there is a growing need for non-invasive techniques in the monitoring of liver diseases.5,6 Measurement of liver stiffness by TE has been demonstrated to have a high degree of accuracy and reproducibility in predicting bridging fibrosis and cirrhosis in patients with chronic hepatitis C with or without HIV-infection, as well as in patients with non-alcoholic fatty liver disease.29–33 In the present study liver stiffness values were significantly higher in the HIV/HCV co-infected than in HCV mono-infected patients, as previously reported.11,17 Also the prevalence of advanced liver fibrosis was significantly higher in co-infected than in HCV mono-infected patients. The mechanisms of the accelerated progression of fibrosis in HIV/HCV co-infection are complex and multifactorial and are still under investigation.34 In any case, although recent conflicting data were reported, many serial liver biopsy studies have shown a fast fibrosis progression in HIV/HCV co-infected patients.35–38
Concerning the relationship between steatosis and liver fibrosis, in both our HCV-mono and co-infected patients steatosis was not associated with advanced liver fibrosis when assessed by TE and the 2 biochemical markers, the APRI and FIB–4 scores. Currently, the combination of FIB-4 with TE has resulted in a good concordance.39 To increase the diagnostic reliability of these 2 non-invasive methods for a better assessment of liver fibrosis, we evaluated the optimal cut-off for liver stiffness to identify advanced liver fibrosis, using the optimal FIB–4 values of ≥ 3.25, according to Bruno, et al.19,39 The diagnostic performance of the combination of the 2 methods was good, confirming previous reports,39 and supporting what is already known on the optimal cut-off values for the assessment of advanced liver fibrosis with both TE and FIB-4 in comparison with the gold standard.9,40 Therefore, we can reasonably state that steatosis without advanced liver fibrosis was prevalent among our HCV mono-and co-infected patients. This finding may support the recent observations reported by Sterling, et al., on HIV patients with abnormal liver enzymes without diabetes mellitus, alcohol, or viral hepatitis co-infection. The authors found that although histological steatosis was common (65%), NASH was observed in only 26% of HIV patients.41 In addition, it is known that a significant reduction in severity of steatosis is to be expected in patients with NAFLD when fibrosis tends to progress, as well as in patients with cir-rhosis.42 In the last few years many studies have suggested that non-alcoholic fatty liver disease is related to the progression of fibrosis in chronic HCV infection.43 However, this association remains rather controversial since in some studies it has not been shown or has been found only in some subgroups.37,38,44
In this respect, it has been highlighted that advanced fibrosis is not associated with steatosis but with insulin resistance in patients with HCV genotype 3.45 These data may furthermore suggest that steatosis may not be the key factor directly implicated in liver disease progression.
On multiple logistic regression analysis in co-infected patients metabolic syndrome was the variable independently associated with steatosis. When we removed this variable from the model, the only factor independently associated with steatosis was higher triglyceride levels. Individuals infected with HIV frequently have metabolic syndrome and among its components triglycerides have been found as independent factors for steatosis.46 Lipid abnormalities may be a consequence either of the HIV infection per se, of ART or traditional risk factors.47 The independent association between higher triglyceride levels and steatosis found in the HCV mono-infected patients, may suggest that the metabolic factors could be those mainly involved in steatosis promotion in these patients. In addition, this hypothesis is corroborated by the small number of patients with HCV genotype 3 infection, which is usually associated with low levels of total-cholesterol and triglycerides.48
Nevertheless, the current study has several limitations. The lack of liver biopsy to exclude a superimposed non-alcoholic fatty liver disease is a major limitation, since US cannot discriminate between metabolic and viral steatosis. Steatosis diagnosis is underestimated using BL, indeed when steatosis is less than 20% it cannot be recognized by US.49 In addition, US sensitivity in steatosis diagnosis is lower when infection with HCV genotype 1–2 occurs.50 Recently, it has been shown that the presence of steatosis may lead to overestimate the measurement of liver stiffness in co-infected patients, which could be less accurate among these patients as a result.51
Conclusion
Our findings showed that steatosis prevalence was not significantly different in both HCV monoand co-infected patients and was not linked with advanced liver fibrosis.
No optimal treatment for steatosis and non-alcoholic steatohepatitis has been found so far. The evidence for current therapies is based mostly on small trials therefore, no recommendation is available, until larger randomized trials are conducted.52 The main goal of steatosis therapy has been to promote diet and lifestyle modifications as these have been shown to improve insulin sensitivity.53,54 Also our finding regarding the independent association between triglycerides and steatosis in either of the HCV-groups, suggests that dietary interventions and lifestyle changes should be proposed to these patients in order to reduce metabolic risk factors. Anyway, whether it’s known that triglycerides and other lipid molecules participate directly to the pathogenesis of the necroinflammatory process of non-alcoholic steatohepatitis, the recent evidence that triglicerides formation is also protective against injury and inflammation, rises doubts on the current therapeutic approach for steatosis.52 Further prospective studies are needed to detect the predictor factors of the development of this increasingly prevalent disorder and their implications for the effective treatment of steatosis.
Abbreviations- •
ALT: alanine aminotransferase.
- •
ART: antiretroviral therapy
- •
AST: aspartate aminotransferase.
- •
BL: bright liver.
- •
HAART: highly-active antiretroviral therapy.
- •
HCV: hepatitis C virus.
- •
HDL: high-density lipoprotein.
- •
HIV: human immunodeficiency virus.
- •
LDL: low-density lipoprotein.
- •
ROC: receiver-operating characteristic.
- •
TE: transient elastography.
- •
US: ultrasonography.
The authors would like to thank Dr. Gaetano Leto for the statistical support.
Conflict of InterestNone.