Implementation of a one-step strategy for diagnosis of active Hepatitis C virus (HCV) infection would encourage the early diagnosis and reduce the time to access antiviral treatments. The aim of this study was to evaluate the impact of a HCV one-step diagnosis compared to the traditional two-step protocol in terms of the time required for patients to be seen by specialists and the time taken to start antiviral treatment.
Material and methodsA comparative study was carried out to assess two diagnostic algorithms (one-step and two-step) for active HCV infection. Serological markers were quantified using the same serum sample to determine both anti-HCV antibodies (HCV-Ab) and HCV core antigen (HCV-cAg) by Architect i2000 SR kit. In this period, a multidisciplinary procedure was started for telematics referral of viremic patients.
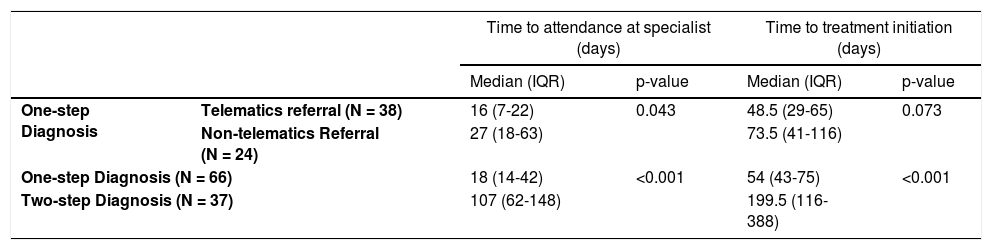
ResultsOne-step approach reduced the time required for patient HCV diagnosis, referral to a specialist, access to treatment, and eliminated the loss of patients to follow-up. Significant differences were observed between one-step and two-step diagnosis methods in the time required for patients to be seen by a specialist (18 days [Interquartile range (IQR) = 14–42] versus 107 days [IQR = 62–148]) and for the initiation of treatment (54 days [IQR = 43–75] versus 200 days [IQR = 116–388]), mainly for patients with advanced fibrosis (35 days [IQR = 116–388] versus 126 days [IQR = 152–366]).
ConclusionsUse of HCV-cAg has proven to be a useful tool for screening patients with active hepatitis C. The development of a multidisciplinary protocol for the communication of results improved the efficiency of the care process.
The World Health Organization (WHO) estimates that at least 110 million people are infected with the Hepatitis C virus (HCV), of which 70 million are chronically infected and have a high risk of liver disease [1,2]. It is estimated that 15% to 45% of individuals infected with HCV do not require any treatment to eliminate the infection. However, 55% to 85% of patients present a chronic infection that, in 30% of cases, will lead to liver cirrhosis or hepatocellular carcinoma [3,4]. According to a recent systematic review, the estimated prevalence of HCV in Spain is among the highest in Europe [5]. In addition, it has also been shown that HCV infection is the main cause of mortality due to infectious disease in Spain [6].
Rapid detection of patients with active viremia plays a crucial role in the early diagnosis of HCV. In the traditional two-step diagnosis process, anti-HCV antibodies (HCV-Ab) are first detected in serum samples and then HCV ribonucleic acid (RNA) is quantified in a second plasma sample, which creates a time lag between the collections of each sample. This strategy implies a delay in the diagnosis and loss of some patients to follow-up, thus increasing the number of late diagnoses and the corresponding increased risk of mortality [7,8].
The alternative one-step diagnosis method is based on the determination of both the HCV-Ab and the HCV-cAg in one serum sample, which allows ongoing infections to be identified, even those in the seroconversion phase [9,10,11]. Numerous studies have shown good correlation between HCV-cAg and HCV RNA levels in populations of mono-infected individuals [12,13], Human Immunodeficiency Virus (HIV)-HCV co-infected patients, transplant patients [14], and patients on haemodialysis [15]. Although its quantification is faster and cheaper than nucleic acid amplification techniques (NAATs) [16],one-step diagnosis is currently performed less frequently than would otherwise be expected [17].
The objective of this work was to compare the one-step and two-step diagnosis methods to evaluate the time required to detect active HCV viremia in each case. In addition, we also measured the time taken for patients to be referred to specialized consultations and to receive adequate treatment when the one-step or two-step protocols were applied.
2Materials and methodsWe carried out a retrospective observational study which compared two diagnostic algorithms for active HCV (one-step versus two-step). Moreover, the one-step patients‘ group was divided into two subgroups: referral without a telematics alert and referral with a telematics alert. Two, year-long periods were selected for study, one in 2018 for one-step and another in 2016 for two-step. Populations included in this study belonged to health area 9 of the Valencian Community (General University Hospital Consortium of Valencia, or the GUHCV) and included hospitalized patients as well as those treated in the hospital emergency service, primary care, penitentiary center, and addictive behavior units.
2.1Detection of anti-hepatitis C virus antibodies and the hepatitis C virus core antigen: hepatitis C virus RNA quantification and genotypingChemiluminescent Microparticle Immunoassay (CMIA) ARCHITECT Anti-HCV® and CMIA Architect HCV core Ag assay® (Abbott Diagnostics) chemiluminescence assays were used for the HCV-Ab and HCV-cAg serological tests, respectively, and the results were expressed in relative light units (RLUs). A calibration curve was used to quantify HCV-cAg and the results were interpreted according to the cut-off points indicated by the manufacturer: negative (< 3.00 fmol/L), indeterminate (≥ 3.00 to < 10.00 fmol/L), and positive (≥ 10.00 fmol/L).
The HCV RNA was extracted and amplified using the qualitative and quantitative COBAS® AmpliPrep/COBAS® TaqMan® HCV Test, v2.0 (Roche), with the lower limit of quantitation being 15 IU/mL). The viral load was determined in plasma samples before starting treatment and at 12 weeks post-treatment (Sustained Virologic Response (SVR) 12 weeks) to evaluate any sustained viral responses. The genotype study was performed on an Abbott m2000sp/rt automated platform using the Abbott Real Time Genotype II assay.
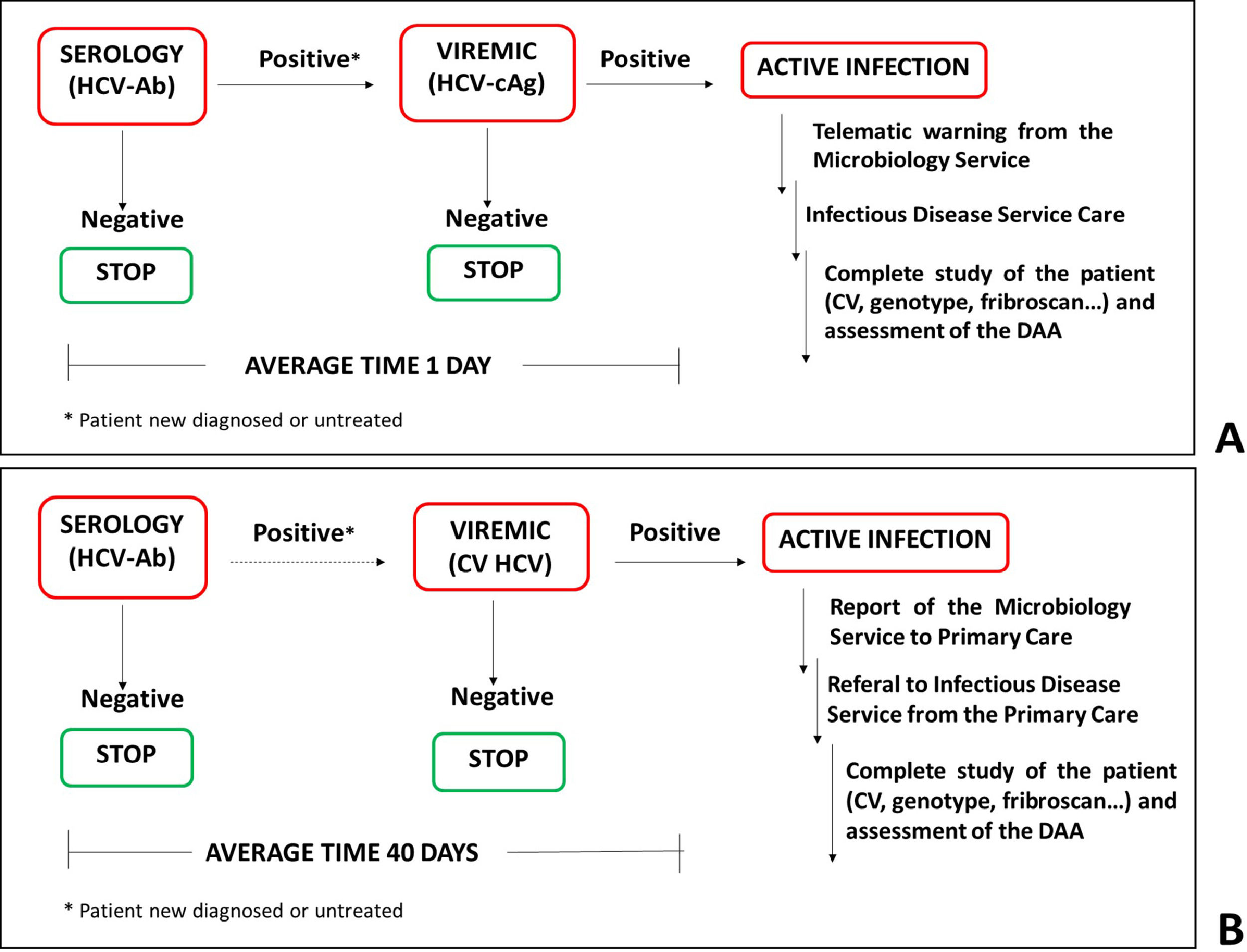
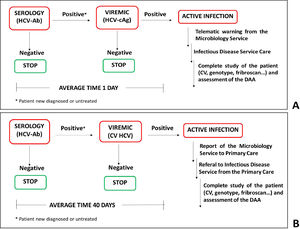
2.2One-step diagnostic algorithm with telematics referralFrom May to December 2018, 15,566 requests corresponding to 13,338 patients were processed for HCV screening. Of these, 208 patients had an immediate HCV-Ab positive test result, and the HCV-cAg test was performed on the same serum sample. The average time between performing the serology and detection of active viremia was 1 day. In patients with a negative or indeterminate HCV-cAg result, a second serum sample and plasma sample were requested to confirm the result and to quantify the HCV RNA levels (figure 1).
Diagnostic strategies for Hepatitis C infection. (A) One-step diagnosis strategy. HCV serology and viremia were diagnosed using a single serum sample. The average time between the two determinations was 1 day. (B) Two-step. HCV serology was determined in the serum sample and the viremia was assessed in a second plasma sample. The average time between the two determinations was 40 days.
In July 2018, a protocol for the telematics referral of viremic patients to the Infectious Diseases and Hepatology consultations at the GUHCV was initiated. When a positive HCV-Ab/HCV-cAg result is obtained, an alert is sent to physicians from the laboratory information system (LIS) to corporative email with the sample identification number. After that, patients are given a doctor's appointment. Thereafter, patients with the aforementioned results were submitted for microbiological and analytical studies (viral load and genotype, biochemical liver alteration parameters, and evaluation of liver fibrosis with a Fribroscan® Mini 430 ECHOSENSTM) to evaluate them for the initiation of antiviral treatments. Clinical evaluation is completed on the same day as well as DAAs initiation treatment.
2.3Two-step diagnostic algorithmFrom May to December 2016, there were 14,586 requests for 13,433 patients for HCV screening by HCV-Ab determination. A positive HCV-Ab result was obtained in 475 patients of which, 168 cases had no previous serological data indicating an HCV positive result. A second serum and plasma sample were then requested to confirm the result and to determine the viral load. These samples were only sent for analysis in 68 patient cases. Finally, the study was completed in viremic patients (figure 1).
2.4Statistical analysisQuantitative and qualitative demographic, clinical, and microbiological variables were analyzed using SPSS software (IBM Corp., Armonk, NY). The normality of the data was analyzed using a 1-sample Kolmogorov–Smirnov test. Mann–Whitney U tests were performed to compare non-parametric independent variables, pairwise between the days to appointment or days to treatment variables and the one-step and two-step conditions, telematics or non-telematics referral, and days to appointment or days to treatment, depending on the degree of patient liver fibrosis.
3Results3.1Demographic and clinical resultsThe viremia of all the HCV-Ab positive samples diagnosed by one-step was analyzed, while only 40.5% of those diagnosed as positive by two-step was analyzed because the remaining samples were not sent to the Microbiology Service. Of the 66 viremic patients diagnosed using the one-step protocol, 4 were excluded—3 because of patient death and 1 because of disagreement between the HCV-cAg value (indeterminate) and HCV-CV value (log 4). Of the remaining patients, 38 were referred to a specialist by telematics protocol while 24 were referred using the standard protocol.
Of the 68 two-step HCV-Ab positive patients, 37 had active and 31 non-active viremia. Of the remaining samples in which the viremic status could not be evaluated, there were 7 deaths, 3 patients belonged to other hospital areas where they were evaluated,32 patients had already been treated and had an undetectable viral load in the last available report, 2 decided not to seek treatment, 38 were lost to follow-up, and 18 had an unknown viremia status. These 18 latter patients were attended in other services of our hospital, but their viral load was not evaluated because they were not referred to the infectious diseases or Hepatology departments.
The sex distribution between the one-step and two-step diagnostic groups was similar (73% and 61% men and 27% and 39% women, respectively). However, in terms of ethnicity, most of the patients were European (89%) and a minority had an Asian, African, or American ethnicity. Most of the referrals had come from primary care (43% and 56%, respectively for the one-step and two-step diagnostic groups), other units in the GUHCV (48% and 31%, respectively), or the addictive behavior units and services (9% and 14%, respectively). A similar number of patients had advanced F3–F4 fibrosis between the one-step and two-step groups (36% and 27%, respectively). Regarding the microbiological results, it is worth highlighting the high percentage of patients with unknown viremia in the two-step sample (59.5%) while none of the patients in the one-step group had an unknown viremia status.
3.2Statistical analysisThe statistical results (Tables 1 and 2) showed significant differences between the types of referral in terms of the days required for the patients to be seen in specialized units, and between the one-step and two-step protocol in patients with or without F3–F4 grade fibrosis, both with respect to the days required to receive specialized care and with the time (days) to treatment initiation.
Clinical impact of telematics referral and one-step diagnosis approach in patients with active HCV.
| Time to attendance at specialist (days) | Time to treatment initiation (days) | ||||
|---|---|---|---|---|---|
| Median (IQR) | p-value | Median (IQR) | p-value | ||
| One-step Diagnosis | Telematics referral (N = 38) | 16 (7-22) | 0.043 | 48.5 (29-65) | 0.073 |
| Non-telematics Referral (N = 24) | 27 (18-63) | 73.5 (41-116) | |||
| One-step Diagnosis (N = 66) | 18 (14-42) | <0.001 | 54 (43-75) | <0.001 | |
| Two-step Diagnosis (N = 37) | 107 (62-148) | 199.5 (116-388) | |||
Non-parametric statistical analyses were performed using Mann–Whitney U tests.
The medians, IQR, and p-values were also calculated.
Abbreviations: IQR, interquartile range.
Implication of one-step HCV diagnosis strategy in patients with F3-F4 fibrosis grade.
| Time to attendance at specialist (days) | Time to treatment initiation (days) | |||
|---|---|---|---|---|
| Median (IQR) | p-value | Median (IQR) | p-value | |
| One-step Diagnosis | 15 (8-22) | <0.001 | 35 (26-49) | <0.001 |
| Two-step Diagnosis | 95.5 (35.5-308.5) | 126 (51.5-365.5) | ||
Non-parametric statistical analyses were performed using Mann–Whitney U tests. The medians, IQR, and p-values were also calculated.
Abbreviations: IQR, interquartile range.
The use of direct-acting antiviral (DAA) treatments can achieve a cure rate of up to 95% [18], and so early diagnosis and timely access to treatment are the factors that are now limiting the elimination of HCV [1,19,20]. In May 2015 in Spain, the Ministry of Health, Consumption, and Social Welfare published the Strategic Plan for the Management of Hepatitis C for the National Health System (PEACH in its Spanish acronym) with the aim of achieving the correct therapeutic approach to patients with HCV and reducing transmission of the virus [5]. In 2016, the guidelines of the European Association for the Study of the Liver (EASL), together with the WHO Global Health Strategy in its 2017 hepatitis report, included testing for the HCV-cAg as an alternative to testing HCV RNA for diagnosis confirmation [1,21].
The HCV-cAg serological marker technique has high specificity and good sensitivity and so it represents an alternative tool to NAATs which is a simpler and lower cost technique with a faster diagnostic speed [22,23]. Furthermore, in cases of acute hepatitis C, HCV-cAg is detectable in serum during the 4–6-week window when the HCV-Ab is still absent in these patients [24]. Moreover, no significant differences have been documented when using HCV-cAg or HCV RNA quantification for these cases. For example, Peterson et al. obtained similar quantifiable levels of HCV-cAg and HCV RNA in parallel determinations in 83% of the cases they examined [10].
The main drawback preventing the widespread use of the HCV-cAg test is its lower sensitivity compared to quantification of the virus RNA. Several studies have documented that this technique does not present reactivity below 5,000–10,000 IU/mL of HCV RNA [23,25] and that HCV-cAg may not be detected in up to 1% of the samples analyzed in patients with low viremia [26]. Therefore, both the WHO and several diagnostic guidelines now recommend quantification of the viral load when negative or indeterminate HCV-cAg results are obtained [1,27,28]. However, in our study, all the patients with negative HCV-cAg results also presented a negative HCV load, and only the indeterminate HCV-cAg results correlated with a low viral load (log 4). These results coincide with those published in other work in which the Pearson correlation coefficient between the HCV-cAg techniques and the quantification of HCV RNA was 0.951 [29] and less than 0.7% of viremic patients were not diagnosed with the HCV-cAg technique [28,30,31].
5ConclusionIn this current work, inclusion of HCV-cAg testing in our standard work protocols for screening patients with active HCV infection allowed us to simplify the two-step procedure we had been using to a one-step process which could more efficiently diagnose patients in the early stage of HCV viremia. Thus, the main advantages of using the one-step protocol in our hospital setting were the reduction in diagnosis times and an increase in both the number of diagnosed HCV viremic patients and in their referral to specialists. All of this made it possible to minimize patient losses to follow-up and to treat a higher percentage of patients. Moreover, joint implementation of the one-step protocol with telematics referral improved the process of patient care and reduced the waiting times required for patients to be seen in specialized consultations and for them to initiate treatment with DAAs. This reduction was especially important in patients with advanced grade F3–F4 fibrosis. Nevertheless, a possible source of bias in terms to initiate treatment could stem from the different treatment strategies. Although DAAs therapies were not carried out in all patients up to June 2016 - regardless of the degree of fibrosis- initially patients with fibrosis stages ≥F2 were prioritized in our center. Moreover, data of two-step group were collected from May to December 2016.
In closing, we have demonstrated that one-step diagnosis approach and a multidisciplinary telematics referral reduce the time required for patients to be seen by specialists and the time taken to start antiviral treatment. Moreover, missing patients to follow-up were avoided, increasing the control by physicians and the proportion of patients treated. Thus, our approach could play an important role in the process towards HCV elimination goals.
Autors´contributionConcept and design of the study: Miguel García Deltoro and María Dolores Ocete Mochón.
Methodological support: Miguel García Deltoto, María Dolores Ocete Mochón and Concepción Gimeno Cardona.
Patient enrolment and follow-up: María Dolores Ocete Mochón, Miguel García Deltoto and Moisés Diago Madrid.
Experiments and procedures: Miriam Torrecillas Muelas and María Dolores Ocete Mochón.
Data analysis and Manuscript writing: Miriam Torrecillas Muelas and Neus Gómez Muñoz.
Manuscript reviewing: María Dolores Ocete Mochón, Purificación Rubio Cuevas, Moisés Diago Madrid, Concepción Gimeno Cardona, Enrique Ortega González and Miguel García Deltoto.
Ethics approval statementThis study was approved by the Ethics Committee of Research on Drug Research (CEIm) of the General University Hospital Consortium of Valencia (Protocol Number: SEI-AAD-2020).
Informed consentNot applicable.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors would like to thank all the team at the Microbiology Department and Infectious Diseases Department at the General University Hospital Consortium of Valencia for their help with this work.